Diurnal Variations in High Time-Resolved Molecular Distributions and Formation Mechanisms of Biogenic Secondary Organic Aerosols at Mt. Huang, East China
Abstract
1. Introduction
2. Results and Discussion
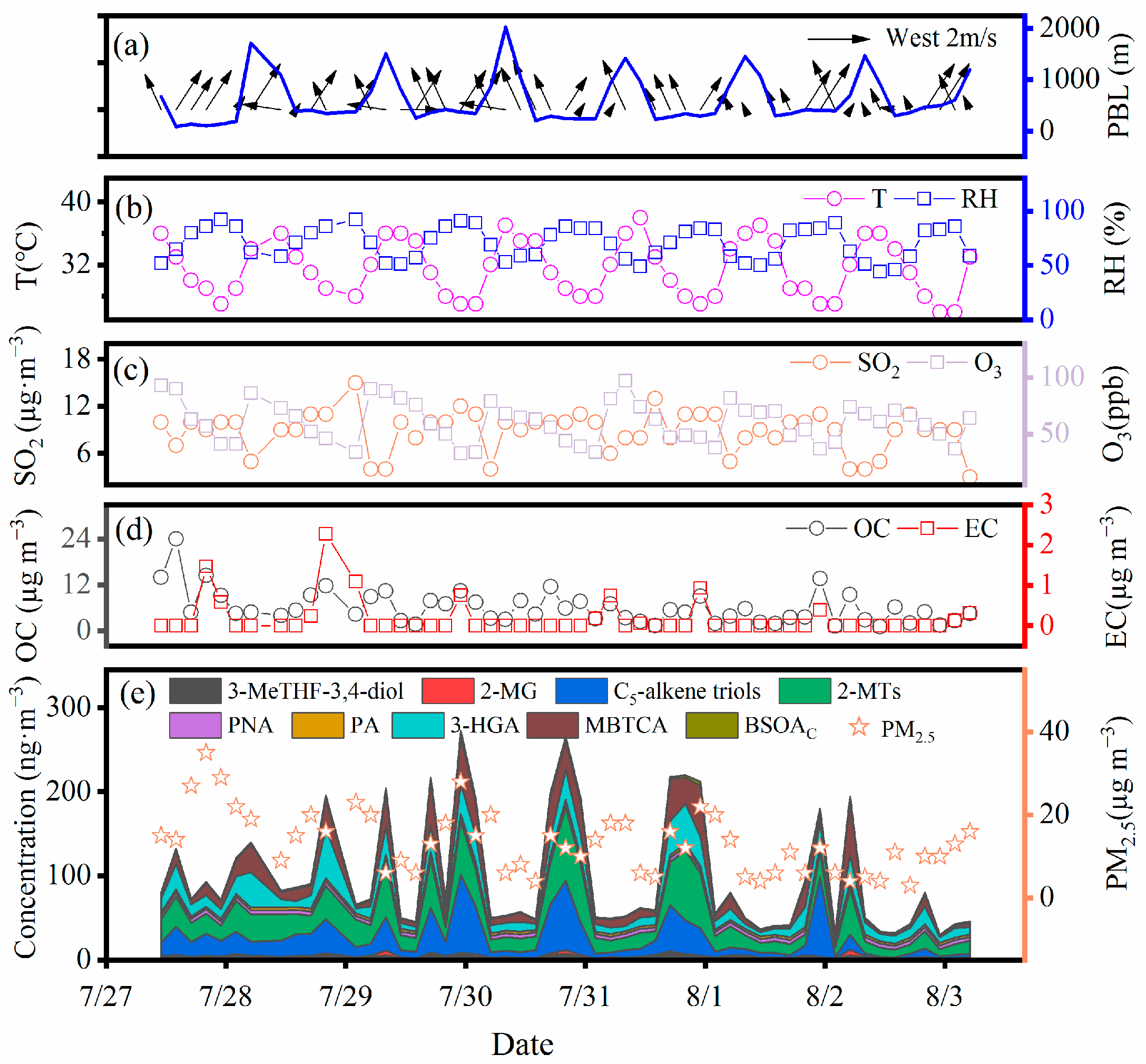
2.1. Concentrations and Major Chemical Compounds of PM2.5
2.2. Diurnal Variations in BSOA Tracers
2.2.1. Isoprene SOA Tracers
2.2.2. Monoterpene SOA Tracers
2.2.3. β-Caryophyllene SOA Tracers
2.3. Comparison of BSOA Tracers with Other Sites
2.4. Effects of Anthropogenic Sources and Meteorological Parameters on BSOA Formation
2.5. Source Apportionment
3. Materials and Methods
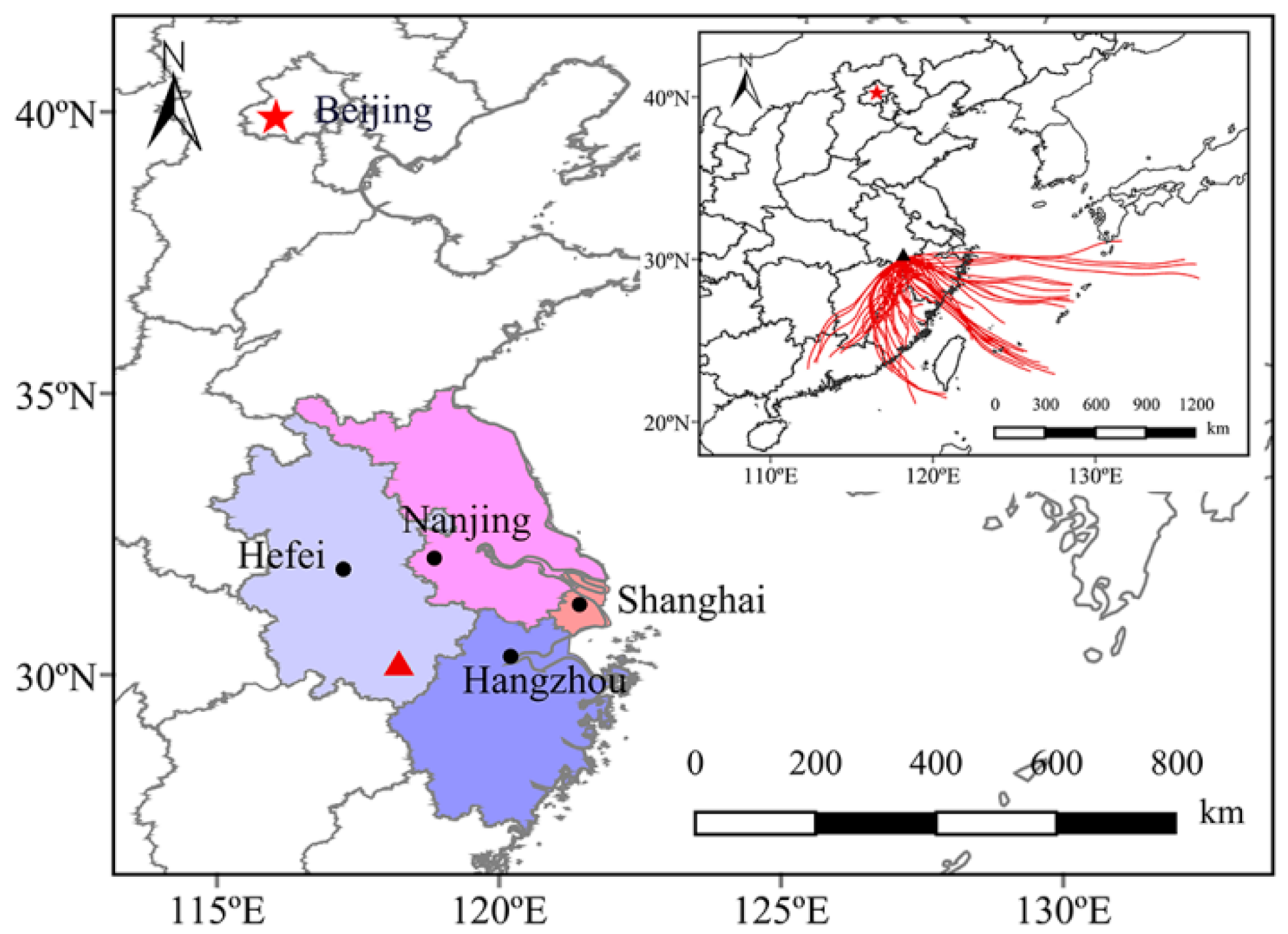
3.1. Sample Collection
3.2. Sample Analyses
3.2.1. Organic Compounds
3.2.2. Organic Carbon (OC), Elemental Carbon (EC), and Inorganic Ions
3.2.3. Quality Assurance and Quality Control
3.3. Aerosol Liquid Water Content and Particle In Situ pH
3.4. Backward Trajectories
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- An, Z.S.; Huang, R.J.; Zhang, R.Y.; Tie, X.F.; Li, G.H.; Cao, J.J.; Zhou, W.J.; Shi, Z.G.; Han, Y.M.; Gu, Z.L.; et al. Severe haze in Northern China: A synergy of anthropogenic emissions and atmospheric processes. Proc. Natl. Acad. Sci. USA 2019, 116, 8657–8666. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Zhang, Y.L.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.M.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.; Zhang, Q.; Kroll, J.H.; Decarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Mahilang, M.; Deb, M.K.; Pervez, S. Biogenic secondary organic aerosols: A review on formation mechanism, analytical challenges and environmental impacts. Chemosphere 2021, 262, 127771. [Google Scholar] [CrossRef]
- Ding, X.; He, Q.F.; Shen, R.Q.; Yu, Q.Q.; Wang, X.M. Spatial distributions of secondary organic aerosols from isoprene, monoterpenes, β-caryophyllene, and aromatics over China during summer. J. Geophys. Res. Atmos. 2014, 119, 11877–11891. [Google Scholar] [CrossRef]
- Yi, Y.N.; Liu, X.D.; Fu, M.X.; Li, Z.; Zhou, R.W.; Wang, Y.C.; Wei, B.J.; Meng, J.J.; Hou, Z.F.; Yan, L. Molecular Characteristics and Formation Mechanisms of Biogenic Secondary Organic Aerosols in the Summer Atmosphere at Mt. Tai on the North China Plain. Aerosol Air Qual. Res. 2019, 9, 2671–2682. [Google Scholar] [CrossRef]
- Mahilang, M.; Deb, M.K.; Pervez, S.; Tiwari, S.; Jain, V.K. Biogenic secondary organic aerosol formation in an urban area of eastern central India: Seasonal variation, size distribution and source characterization. Environ. Res. 2021, 195, 110802. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Wang, X.Q.; Guo, X.; Dai, W.T.; Liu, S.X.; Shen, M.X.; Liu, Y.L.; Zhang, Y.F.; Cao, Y.; Qi, W.N.; Li, L.; et al. Diurnal variations of isoprene, monoterpenes, and toluene oxidation products in aerosols at a rural site of Guanzhong Plain, northwest China. Atmosphere 2022, 13, 634. [Google Scholar] [CrossRef]
- Yuan, Q.; Lai, S.C.; Song, J.W.; Ding, X.; Zheng, L.S.; Wang, X.M.; Zhao, Y.; Zheng, J.Y.; Yue, D.L.; Zhong, L.J.; et al. Seasonal cycles of secondary organic aerosol tracers in rural Guangzhou, Southern China: The importance of atmospheric oxidants. Environ. Pollut. 2018, 240, 884–893. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Chen, D.H.; Ding, X.; Li, J.; Zhang, T.; Wang, J.Q.; Cheng, Q.; Jiang, H.; Song, W.; Ou, Y.B.; et al. Impact of anthropogenic emissions on biogenic secondary organic aerosol: Observation in the Pearl River Delta, southern China. Atmos. Chem. Phys. 2019, 19, 14403–14415. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Wang, G.H.; Tao, J.; Zhang, Z.S.; Wu, C.; Wang, J.Y.; Li, J.J.; Wei, J.; Li, H.; Meng, F. Seasonal characteristics of biogenic secondary organic aerosols at Mt. Wuyi in Southeastern China: Influence of anthropogenic pollutants. Environ. Pollut. 2019, 252, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, G.H.; Wang, X.M.; Cao, J.; Sun, T.; Cheng, C.; Meng, J.; Hu, T.; Liu, S. Abundance, composition and source of atmospheric PM2.5 at a remote site in the Tibetan Plateau, China. Tellus B 2013, 65, 20281. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K.; Chen, J.; Miyazaki, Y. Secondary production of organic aerosols from biogenic VOCs over Mt. Fuji, Japan. Environ. Sci. Technol. 2014, 48, 8491–8497. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K.; Chen, J.; Barrie, A.L. Isoprene, Monoterpene, and Sesquiterpene Oxidation Products in the High Arctic Aerosols during Late Winter to Early Summer. Environ. Sci. Technol. 2009, 43, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, G.H.; Cao, J.J.; Wang, X.M.; Zhang, R.J. Observation of biogenic secondary organic aerosols in the atmosphere of a mountain site in central China: Temperature and relative humidity effects. Atmos. Chem. Phys. 2013, 13, 11535–11549. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kawamura, K.; Miyazaki, Y.; Wada, R.; Takahashi, Y.; Saigusa, N.; Tani, A. Secondary formation of oxalic acid and related organic species from biogenic sources in a larch forest at the northern slope of Mt. Fuji. Atmos. Environ. 2017, 166, 255–262. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, G.H.; Zhang, Q.; Li, J.; Wu, C.; Jiang, W.Q.; Zhu, T.; Zeng, L.M. Molecular characteristics and diurnal variations of organic aerosols at a rural site in the North China Plain with implications for the influence of regional biomass burning. Atmos. Chem. Phys. 2019, 19, 10481–10496. [Google Scholar] [CrossRef]
- Hong, Z.Y.; Zhang, H.; Zhang, Y.R.; Xu, L.L.; Liu, T.T.; Xiao, H.; Hong, Y.W.; Chen, J.S.; Li, M.R.; Deng, J.J.; et al. Secondary organic aerosol of PM2.5 in a mountainous forest area in southeastern China: Molecular compositions and tracers implication. Sci. Total Environ. 2019, 653, 496–503. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, L.X.; Kawamura, K.; Chen, J.M.; Ono, K.; Wang, X.F.; Xue, L.K.; Wang, W.X. Contributions and source identification of biogenic and anthropogenic hydrocarbons to secondary organic aerosols at Mt. Tai in 2014. Environ. Pollut. 2017, 220, 863–872. [Google Scholar] [CrossRef]
- Yuan, Q.; Qi, B.; Hu, D.; Wang, J.; Zhang, J.; Yang, H.; Zhang, S.; Liu, L.; Xu, L.; Li, W. Spatiotemporal variations and reduction of air pollutants during the COVID-19 pandemic in a megacity of Yangtze River Delta in China. Sci. Total Environ. 2021, 751, 141820. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Jia, H.L.; Sha, T.; An, J.L.; Tian, R. Spatial and seasonal characteristics of particulate matter and gaseous pollution in China: Implications for control policy. Environ. Pollut. 2019, 248, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.J.; Wang, G.H.; Hou, Z.F.; Liu, X.D.; Wei, B.J.; Wu, C.; Cao, C.; Wang, J.Y.; Li, J.J.; Cao, J.J.; et al. Molecular distribution and stable carbon isotopic compositions of dicarboxylic acids and related SOA from biogenic sources in the summertime atmosphere of Mt. Tai in the North China Plain. Atmos. Chem. Phys. 2018, 18, 15069–15086. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Ren, H.; Kawamura, K.; Du, H.Y.; Chen, X.H.; Yue, S.Y.; Xie, Q.R.; Wei, L.F.; Li, P.; Zeng, X.; et al. Vertical distribution of particle-phase dicarboxylic acids, oxoacids and α-dicarbonyls in the urban boundary layer based on the 325 m tower in Beijing. Atmos. Chem. Phys. 2020, 20, 10331–10350. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Sullivan, A.P.; Collett, J.L., Jr.; Allen, L.R. Levoglucosan stability in biomass burning particles exposed to hydroxyl radicals. Geophys. Res. Lett. 2010, 37, L09806. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K.; Chen, J.; Li, J.; Sun, Y.L.; Liu, Y.; Tachibana, E.; Aggarwal, S.G.; Okuzawa, K.; Tanimoto, H.; et al. Diurnal variations of organic molecular tracers and stable carbon isotopic composition in atmospheric aerosols over Mt. Tai in the North China Plain: An influence of biomass burning. Atmos. Chem. Phys. 2012, 12, 8359–8375. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, G.H.; Wu, C.; Cao, C.; Ren, Y.Q.; Wang, J.Y.; Li, J.; Cao, J.J.; Zeng, L.M.; Zhu, T. Characterization of isoprene-derived secondary organic aerosols at a rural site in North China Plain with implications for anthropogenic pollution effects. Sci. Rep. 2018, 8, 535. [Google Scholar] [CrossRef]
- Hong, Y.W.; Xu, X.B.; Liao, D.; Liu, T.T.; Ji, X.T.; Xu, K.; Liao, C.Y.; Wang, T.; Lin, C.S.; Chen, J.S. Measurement report: Effects of anthropogenic emissions and environmental factors on the formation of biogenic secondary organic aerosol (BSOA) in a coastal city of southeastern China. Atmos. Chem. Phys. 2022, 22, 7827–7841. [Google Scholar] [CrossRef]
- Ding, X.; He, Q.F.; Shen, R.Q.; Yu, Q.Q.; Zhang, Y.Q.; Xin, J.Y.; Wen, T.X.; Wang, X.M. Spatial and seasonal variations of isoprene secondary organic aerosol in China: Significant impact of biomass burning during winter. Sci. Rep. 2016, 6, 20411. [Google Scholar] [CrossRef]
- Lin, Y.H.; Zhang, Z.F.; Docherty, K.S.; Zhang, H.F.; Budisulistiorini, S.H.; Rubitschun, C.L.; Shaw, S.L.; Knipping, E.M.; Edgerton, E.S.; Kleindienst, T.E.; et al. Isoprene epoxydiols as precursors to secondary organic aerosol formation: Acid-catalyzed reactive uptake studies with authentic compounds. Environ. Sci. Technol. 2012, 46, 250–258. [Google Scholar] [CrossRef]
- Surratt, J.D.; Chan, A.W.; Eddingsaas, N.C.; Chan, M.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Q.; Wang, G.H.; Li, J.J.; Wu, C.; Cao, C.; Wang, J.Y.; Zhang, L.; Meng, F.; Li, H. Seasonal variation and size distribution of biogenic secondary organic aerosols at urban and continental background sites of China. J. Environ. Sci. 2018, 71, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Surratt, J.D.; Lin, Y.H.; Bapat, J.; Kamens, R.M. Effect of relative humidity on SOA formation from isoprene/no photooxidation: Enhancement of 2-methylglyceric acid and its corresponding oligoesters under dry conditions. Atmos. Chem. Phys. 2011, 11, 6411–6424. [Google Scholar] [CrossRef]
- Müller, L.; Reinnig, M.C.; Naumann, K.H.; Saathoff, H.; Mentel, T.F.; Donahue, N.M.; Hoffmann, T. Formation of 3-methyl-1,2,3-butanetricarboxylic acid via gas phase oxidation of pinonic acid—A mass spectrometric study of SOA aging. Atmos. Chem. Phys. 2012, 11, 19443–19476. [Google Scholar] [CrossRef]
- Haque, M.M.; Kawamura, K.; Kim, Y. Seasonal variations of biogenic secondary organic aerosol tracers in ambient aerosols from Alaska. Atmos. Environ. 2016, 130, 95–104. [Google Scholar] [CrossRef]
- Offenberg, J.H.; Lewis, C.W.; Lewandowski, M.; Jaoui, M.; Kleindienst, T.E.; Edney, E.O. Contributions of toluene and alpha-pinene to SOA formed in an irradiated toluene/alpha-pinene/NO(x)/air mixture: Comparison of results using 14C content and SOA organic tracer methods. Environ. Sci. Technol. 2007, 41, 3972–3976. [Google Scholar] [CrossRef]
- Lewandowski, M.; Piletic, I.R.; Kleindienst, T.E.; Offenberg, J.H.; Beaver, M.R.; Jaoui, M.; Docherty, K.S.; Edney, E.O. Secondary organic aerosol characterization at field sites across the United States during the spring-summer period. Int. J. Environ. Anal. Chem. 2013, 93, 1084–1103. [Google Scholar] [CrossRef]
- Li, L.; Lai, W.; Pu, J.G.; Mo, H.Q.; Dai, D.J.; Wu, G.L.; Deng, S.H. Polar organic tracers in PM2.5 aerosols from an inland background area in Southwest China: Correlations between secondary organic aerosol tracers and source apportionment. J. Environ. Sci. 2018, 69, 281–293. [Google Scholar] [CrossRef]
- Man, B.; Xiang, X.; Luo, Y.; Mao, X.; Zhang, C.; Sun, B.; Wang, X. Characteristics and influencing factors of soil fungal community of typical vegetation types in Mount Huangshan, East China. Mycosystema 2021, 40, 2735–2751. [Google Scholar]
- Yi, Y.N.; Meng, J.J.; Hou, Z.F.; Wang, G.H.; Zhou, R.W.; Li, Z.; Li, Y.Y.; Chen, M.; Liu, X.D.; Li, H.J.; et al. Contrasting compositions and sources of organic aerosol markers in summertime PM2.5 from urban and mountainous regions in the North China Plain. Sci. Total Environ. 2020, 766, 144187. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K. Diurnal variations of polar organic tracers in summer forest aerosols: A case study of a Quercus and Picea mixed forest in Hokkaido, Japan. Geochem. J. 2011, 45, 297–308. [Google Scholar] [CrossRef]
- Lyu, X.P.; Guo, H.; Cheng, H.R.; Wang, X.M.; Ding, X.; Lu, H.X.; Yao, D.W.; Xu, C. Observation of SOA tracers at a mountainous site in Hong Kong: Chemical characteristics, origins and implication on particle growth. Sci. Total Environ. 2017, 605, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.Q.; Ding, X.; He, Q.F.; Cong, Z.Y.; Yu, Q.Q.; Wang, X.M. Seasonal variation of secondary organic aerosol tracers in Central Tibetan Plateau. Atmos. Chem. Phys. 2015, 15, 8781–8793. [Google Scholar] [CrossRef]
- Wang, W.; Wu, M.H.; Li, L.; Zhang, T.; Liu, X.D.; Feng, J.L.; Li, H.J.; Wang, Y.J.; Sheng, G.Y.; Claeys, M.; et al. Polar organic tracers in PM2.5 aerosols from forests in eastern China. Atmos. Chem. Phys. 2008, 8, 7507–7518. [Google Scholar] [CrossRef]
- Yang, C.; Hong, Z.Y.; Chen, J.S.; Xu, L.L.; Zhuang, M.Z.; Huang, Z. Characteristics of secondary organic aerosols tracers in PM2.5 in three central cities of the Yangtze river delta, China. Chemosphere 2022, 293, 133637. [Google Scholar] [CrossRef]
- Ren, H.; Hu, W.; Wei, L.F.; Yue, S.Y.; Zhao, J.; Li, L.J.; Wu, L.B.; Zhao, W.; Ren, L.J.; Kang, M.J.; et al. Measurement report: Vertical distribution of biogenic and anthropogenic secondary organic aerosols in the urban boundary layer over Beijing during late summer. Atmos. Chem. Phys. 2021, 21, 12949–12963. [Google Scholar] [CrossRef]
- Li, L.J.; Ren, L.J.; Ren, H.; Yue, S.Y.; Xie, Q.R.; Zhao, W.Y.; Kang, M.J.; Li, J.; Wang, Z.F.; Sun, Y.L.; et al. Molecular characterization and seasonal variation in primary and secondary organic aerosols in Beijing, China. J. Geophys. Res.-Atmos. 2018, 123, 12394–12412. [Google Scholar] [CrossRef]
- Fan, J.W.; Zhang, R.Y. Atmospheric Oxidation Mechanism of Isoprene. Environ. Chem. 2004, 1, 140–149. [Google Scholar] [CrossRef]
- Warneke, W.; Gouw, J.A.d.; Goldan, P.D.; Kuster, W.C.; Williams, E.J.; Lerner, B.M.; Jakoubek, R.; Brown, S.S.; Stark, H.; Aldener, M.; et al. Comparison of daytime and nighttime oxidation of biogenic and anthropogenic VOCs along the New England coast in summer during New England Air Quality Study 2002. J. Geophys Res. 2004, 109, D10309. [Google Scholar] [CrossRef]
- Edwards, P.M.; Aikin, K.C.; Dube, W.P.; Fry, J.L.; Gilman, J.B.; Gouw, J.A.d.; Graus, M.G.; Hanisco, T.F.; Holloway, J.; Hübler, G.; et al. Transition from high- to low-NOx control of night-time oxidation in the southeastern US. Nat. Geosci. 2017, 10, 490–495. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.M.; Gao, B.; Fu, X.X.; He, Q.F.; Zhao, X.Y.; Yu, J.Z.; Zheng, M. Tracer-based estimation of secondary organic carbon in the Pearl River Delta, south China. J. Geophys. Res.-Atmos. 2012, 117, D05313. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Lewis, C.W.; Bhave, P.V.; Edney, E.O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41, 8288–8300. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, N.; Zhou, L.; Yang, F.; Qiu, Y.; Chen, J.; Han, L.; Li, J. Component characteristics and source apportionment of volatile organic compounds during summer and winter in downtown Chengdu, southwest China. Atmos. Environ. 2021, 258, 118485. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.-L.; Fan, M.; Cao, F.; Lin, Y.-C. Characteristics of summertime ambient VOCs and their contributions to O3 and SOA formation in a suburban area of Nanjing, China. Atmos. Res. 2020, 240, 104923. [Google Scholar] [CrossRef]
- Xu, L.; Guo, H.Y.; Boyd, C.M.; Klein, M.; Bougiatioti, A.; Cerully, K.M.; Hite, J.R.; Isaacman-VanWertz, G.; Kreisberg, N.M.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl. Acad. Sci. USA 2015, 112, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Zhang, R.Y.; Gomez, M.E.; Yang, L.X.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.F.; Guo, S.; Meng, J.J. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Russell, L.M.; Lee, A.K.Y.; McKinney, K.A.; Surratt, J.D.; Ziemann, P.J. Observational evidence for pollution-influenced selective uptake contributing to biogenic secondary organic aerosols in the southeastern U.S. Geophys. Res. Lett. 2017, 44, 8056–8064. [Google Scholar] [CrossRef]
- Priyadharshini, B.; Verma, S.; Chatterjee, A.; Sharma, S.K.; Mandal, T.K. Chemical characterization of fine atmospheric particles of water-soluble ions and carbonaceous species in a tropical urban atmosphere over the eastern Indo-Gangetic Plain. Aerosol. Air Qual. Res. 2019, 19, 129–147. [Google Scholar] [CrossRef]
- Zhu, C.M.; Kawamura, K.; Fu, P.Q. Seasonal variations of biogenic secondary organic aerosol tracers in Cape Hedo, Okinawa. Atmos. Environ. 2016, 130, 113–119. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, M.; Wang, Y.C.; Huang, T.; Wang, G.; Li, Z.; Li, J.; Meng, J.; Hou, Z. Seasonal characteristics and provenance of organic aerosols in the urban atmosphere of Liaocheng in the North China Plain: Significant effect of biomass burning. Particuology 2023, 75, 185–198. [Google Scholar] [CrossRef]
- Zhang, T.; Claeys, M.; Cachier, H.; Dong, S.P.; Wang, W.; Maenhaut, W.; Liu, X.D. Identification and estimation of the biomass burning contribution to Beijing aerosol using levoglucosan as a molecular marker. Atmos. Environ. 2008, 42, 7013–7021. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Engling, G.; Lin, C.Y.; Chou, C.C.K.; Lung, S.-C.C.; Chang, S.-Y.; Fan, S.J.; Chan, C.Y.; Zhang, Y.H. Chemical speciation, transport and contribution of biomass burning smoke to ambient aerosol in Guangzhou, a mega city of China. Atmos. Environ. 2010, 44, 3187–3195. [Google Scholar] [CrossRef]
- El Haddad, I.; Marchand, N.; Temime-Roussel, B.; Wortham, H.; Piot, C.; Besombes, J.L.; Baduel, C.; Voisin, D.; Armengaud, A.; Jaffrezo, J.L. Insights into the secondary fraction of the organic aerosol in a Mediterranean urban area: Marseille. Atmos. Chem. Phys. 2011, 11, 2059–2079. [Google Scholar] [CrossRef]
- Yttri, K.E.; Simpson, D.; Nojgaard, J.K.; Kristensen, K.; Genberg, J.; Stenström, K.; Swietlicki, E.; Hillamo, R.; Aurela, M.; Bauer, H.; et al. Source apportionment of the summer time carbonaceous aerosol at Nordic rural background sites. Atmos. Chem. Phys. 2011, 11, 13339–13357. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, X.R.; Tang, G.Q.; Wang, L.L.; Lv, B.; Guo, X.Q.; Wang, Y.S. Secondary organic aerosols in Jinan, an urban site in North China: Significant anthropogenic contributions to heavy pollution. J. Environ. Sci. 2019, 80, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.F.; Huang, Z.S.; Qiao, T.; Zhang, Y.K.; Xiu, G.D.; Yu, J.Z. Chemical characterization, the transport pathways and potential sources of PM2.5 in Shanghai: Seasonal variations. Atmos. Res. 2015, 158, 66–78. [Google Scholar] [CrossRef]




| Daytime (n = 26) | Nighttime (n = 27) | Total (n = 53) | |
|---|---|---|---|
| I. Meteorological parameters and gaseous pollutants | |||
| T (°C) | 33.0 ± 3.7 | 30.3 ± 2.7 | 31.7 ± 3.5 |
| RH (%) | 64.9 ± 14.9 | 75.6 ± 12.2 | 70.3 ± 14.6 |
| Wind speed (m·s−1) | 2.0 ± 1.2 | 1.5 ± 0.6 | 1.7 ± 0.7 |
| PBL (m) | 923.1 ± 467.1 | 301.0 ± 106.5 | 612.1 ± 457.5 |
| O3 (ppb) | 72.8 ± 10.2 | 51.7 ± 12.4 | 62.3 ± 17.4 |
| NO2 (µg m−3) | 9.9 ± 1.5 | 10.1 ± 1.4 | 9.9 ± 1.7 |
| SO2 (µg m−3) | 7.2 ± 2.7 | 10.2 ± 1.0 | 8.8 ± 2.5 |
| II. Inorganic ions (µg m−3) | |||
| SO42− | 1.4 ± 1.0 | 2.2 ± 1.4 | 1.8 ± 1.3 |
| NO3− | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.5 ± 0.2 |
| NH4+ | 0.7 ± 0.4 | 0.8 ± 0.7 | 0.8 ± 0.6 |
| SIA | 2.5 ± 1.2 | 3.5 ± 1.9 | 3.0 ± 1.6 |
| III. Other species | |||
| OC (µg m−3) | 4.9 ± 3.1 | 7.2 ± 4.9 | 6.1 ± 4.3 |
| EC (µg m−3) | 0.4 ± 0.3 | 1.0 ± 0.7 | 0.7 ± 0.4 |
| OC/EC | 17.7 ± 11.9 | 18.4 ± 12.5 | 18.1 ± 12.2 |
| * SOC (µg m−3) | 2.4 ± 1.3 | 6.2 ± 3.3 | 4.3 ± 3.3 |
| pHis | 5.4 ± 2.4 | 4.4 ± 2.1 | 4.9 ± 2.3 |
| LWC (µg m−3) | 3.1 ± 3.0 | 4.0 ± 3.8 | 3.6 ± 3.5 |
| Levoglucosan (ng m−3) | 1.6 ± 1.3 | 3.2 ± 3.3 | 2.4 ± 2.6 |
| PM2.5 (µg m−3) | 12.3 ± 6.3 | 14.5 ± 8.1 | 13.3 ± 7.3 |
| Daytime (n = 26) | Night-time (n = 27) | Total (n = 53) | |
|---|---|---|---|
| I. Isoprene-derived SOA (ng m−3) | |||
| 3-MeTHF-3,4-diols | 3.0 ± 1.2 | 4.1 ± 1.9 | 3.5 ± 1.7 |
| 2-Methylglyceric acid | 1.4 ± 1.5 | 1.2 ± 0.9 | 1.3 ± 1.2 |
| C5-alkene triols | 18.6 ± 18.1 | 47.6 ± 39.6 | 33.1 ± 32.9 |
| 2-Methyltetrols | 23.6 ± 13.5 | 34.1 ± 23.6 | 28.8 ± 19.9 |
| Subtotal | 46.6 ± 32.2 | 87.0 ± 61.2 | 66.8 ± 52.9 |
| II. Monoterpene-derived SOA (ng m−3) | |||
| cis-Pinic acid | 3.2 ± 0.2 | 3.3 ± 0.3 | 3.2 ± 0.2 |
| cis-Pinonic acid | 4.8 ± 0.3 | 4.8 ± 0.2 | 4.8 ± 0.2 |
| 3-Hydroxyglutaric acid | 13.9 ± 10.0 | 22.1 ± 14.0 | 17.8 ± 12.8 |
| MBTCA | 13.0 ± 12.7 | 21.7 ± 18.7 | 17.3 ± 16.3 |
| Subtotal | 34.8 ± 24.1 | 51.9 ± 30.8 | 43.2 ± 28.9 |
| III. β-Caryophyllene-derived SOA (ng m−3) | |||
| β-Caryophyllinic acid | 1.7 ± 1.2 | 2.6 ± 1.6 | 2.2 ± 1.5 |
| Total BSOA tracers | 83.1 ± 54.8 | 141.6 ± 89.4 | 112.3 ± 37.8 |
| Ratios | |||
| P/M | 1.1 ± 0.6 | 0.8 ± 0.6 | 1.0 ± 0.8 |
| 3-HGA/MBTCA | 1.6 ± 0.9 | 1.7 ± 1.1 | 1.7 ± 1.1 |
| BSOA/OC (%) | 1.9 ± 0.8 | 2.3 ± 1.3 | 2.1 ± 1.1 |
| IV. Estimated OC mass concentration (µgC·m−3) | |||
| Isoprene SOC | 0.30 ± 0.21 | 0.56 ± 0.39 | 0.43 ± 0.34 |
| α-Pinene SOC | 0.15 ± 0.10 | 0.22 ± 0.13 | 0.19 ± 0.12 |
| β-Caryophyllene SOC | 0.08 ± 0.05 | 0.11 ± 0.07 | 0.09 ± 0.06 |
| Biomass burning OC | 0.02 ± 0.01 | 0.04 ± 0.03 | 0.03 ± 0.03 |
| Sum of biogenic SOC | 0.53 ± 0.34 | 0.90 ± 0.57 | 0.71 ± 0.40 |
| Subtotal | 0.55 ± 0.11 | 0.93 ± 0.23 | 0.74 ± 0.18 |
| VI. Percentage in OC (%) | |||
| Isoprene SOC | 6.8 ± 2.8 | 8.8 ± 5.8 | 7.8 ± 4.4 |
| α-Pinene SOC | 3.6 ± 1.7 | 3.7 ± 2.1 | 3.7 ± 1.9 |
| β-Caryophyllene SOC | 1.8 ± 0.9 | 2.0 ± 1.4 | 1.9 ± 1.2 |
| Biomass burning OC | 0.5 ± 0.5 | 0.6 ± 0.4 | 0.6 ± 0.5 |
| Sum of biogenic SOC | 12.2 ± 5.0 | 14.5 ± 8.5 | 13.4 ± 6.9 |
| Subtotal | 12.7 ± 6.0 | 15.1 ± 8.4 | 13.9 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hou, Z.; Wang, Y.; Huang, T.; Wang, Y.; Ma, J.; Chen, X.; Chen, A.; Chen, M.; Zhang, X.; et al. Diurnal Variations in High Time-Resolved Molecular Distributions and Formation Mechanisms of Biogenic Secondary Organic Aerosols at Mt. Huang, East China. Molecules 2023, 28, 5939. https://doi.org/10.3390/molecules28165939
Li Y, Hou Z, Wang Y, Huang T, Wang Y, Ma J, Chen X, Chen A, Chen M, Zhang X, et al. Diurnal Variations in High Time-Resolved Molecular Distributions and Formation Mechanisms of Biogenic Secondary Organic Aerosols at Mt. Huang, East China. Molecules. 2023; 28(16):5939. https://doi.org/10.3390/molecules28165939
Chicago/Turabian StyleLi, Yuanyuan, Zhanfang Hou, Yachen Wang, Tonglin Huang, Yanhui Wang, Jiangkai Ma, Xiuna Chen, Aimei Chen, Min Chen, Xiaoting Zhang, and et al. 2023. "Diurnal Variations in High Time-Resolved Molecular Distributions and Formation Mechanisms of Biogenic Secondary Organic Aerosols at Mt. Huang, East China" Molecules 28, no. 16: 5939. https://doi.org/10.3390/molecules28165939
APA StyleLi, Y., Hou, Z., Wang, Y., Huang, T., Wang, Y., Ma, J., Chen, X., Chen, A., Chen, M., Zhang, X., & Meng, J. (2023). Diurnal Variations in High Time-Resolved Molecular Distributions and Formation Mechanisms of Biogenic Secondary Organic Aerosols at Mt. Huang, East China. Molecules, 28(16), 5939. https://doi.org/10.3390/molecules28165939









