Neuroprotective Iridoids and Lignans from Valeriana amurensis
Abstract
1. Introduction
2. Results and Discussion
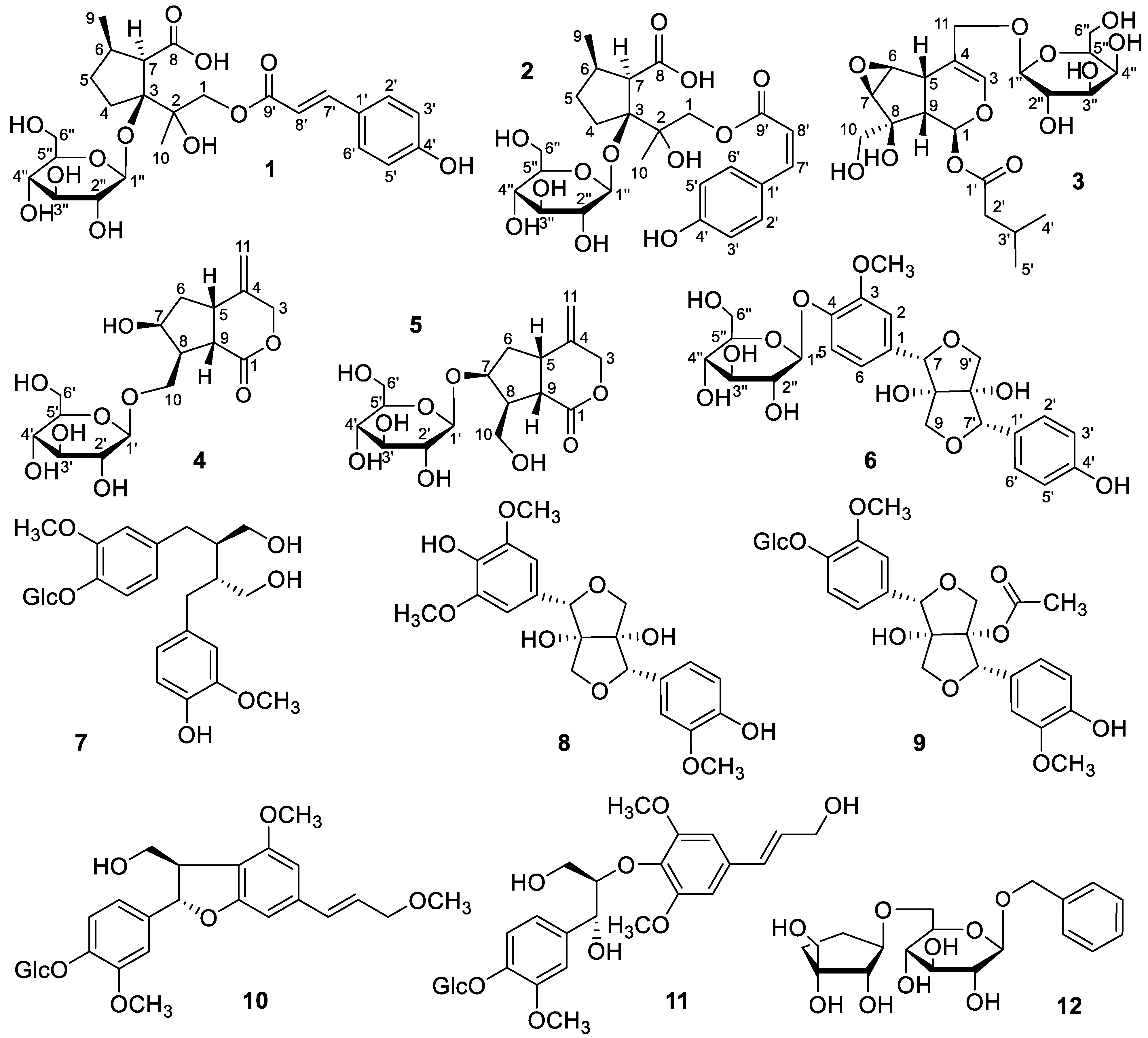
2.1. Structural Elucidation
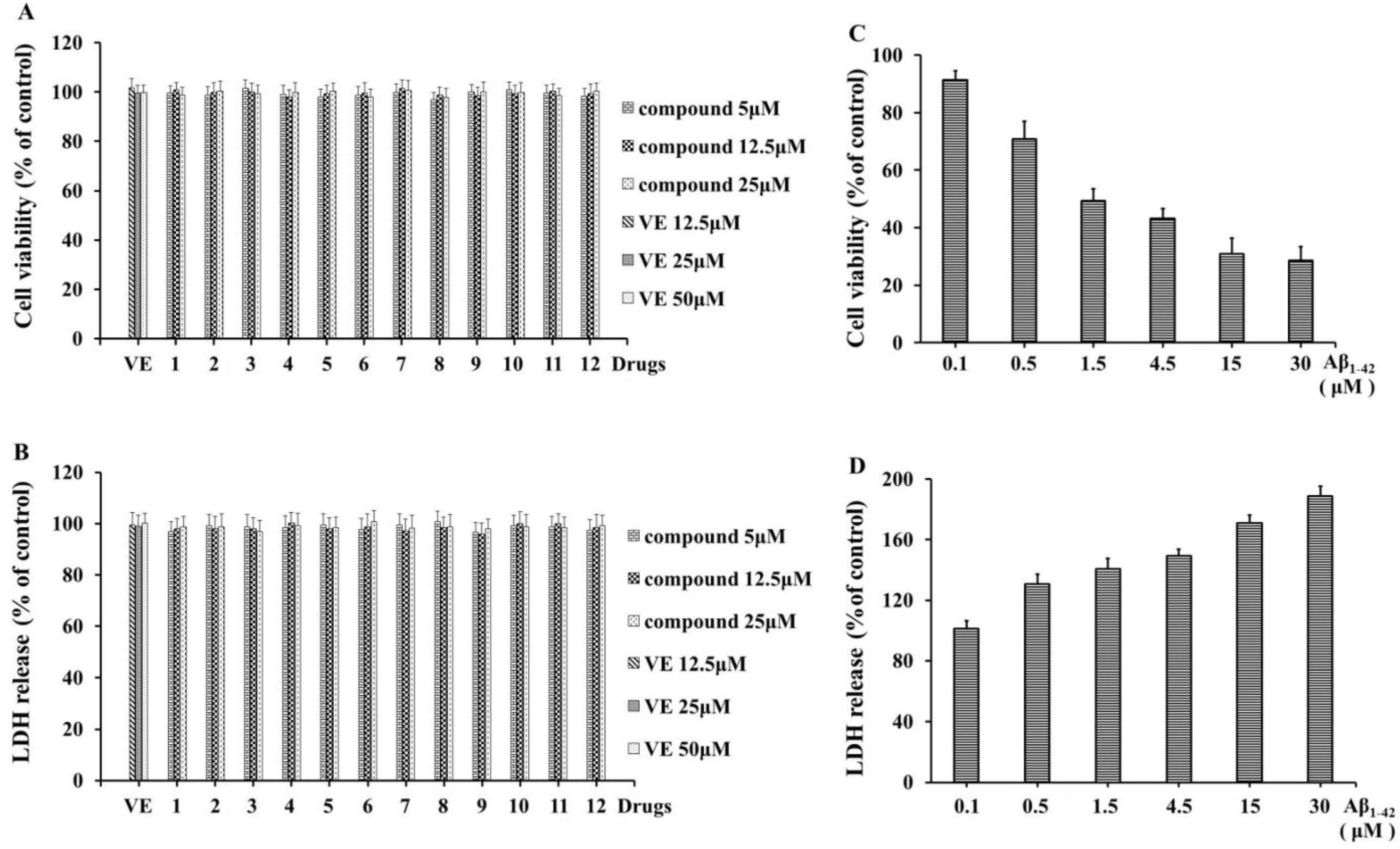
2.2. Detection of the Neuroprotective Effects
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.3.1. Xiecaoiridoidside A (1)
3.3.2. Xiecaoiridoidside B (2)
3.3.3. Xiecaoiridoidside C (3)
3.3.4. Xiecaoiridoidside D (4)
3.3.5. Xiecaoiridoidside E (5)
3.3.6. Xiecaolignanside A (6)
3.4. Monosaccharide Analysis of 1–6
3.5. Determination of the Cells’ Viability
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aramadaka, S.; Mannam, R.; Sankara, N.R.; Bansal, A.; Yanamaladoddi, V.R.; Sarvepalli, S.S.; Vemula, S.L. Neuroimaging in Alzheimer’s Disease for Early Diagnosis: A Comprehensive Review. Cureus 2023, 15, e38544. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remiao, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef] [PubMed]
- Karvandi, M.S.; Hesari, F.S.; Aref, A.R.; Mahdavi, M. The neuroprotective effects of targeting key factors of neuronal cell death in neurodegenerative diseases: The role of ER stress, oxidative stress, and neuroinflammation. Front. Cell. Neurosci. 2023, 17, 1105247. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, J.H.; Kim, B.; Yang, G.; Kim, J.U. Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 8827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Guo, H.Y.; Quan, Z.S.; Shen, Q.K.; Cui, H.; Li, X.T. Research progress of natural products and their derivatives against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2023, 38, 2171026. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Wang, C.; Zhang, M.; Ye, M.; Wang, Q. The potential of Valeriana as a traditional Chinese medicine: Traditional clinical applications, bioactivities, and phytochemistry. Front. Pharmacol. 2022, 13, 973138. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Flora of China from Chinese Academy of Sciences. Flora of China, 1st ed.; Science Press: Beijing, China, 2011; Volume 73, p. 34. [Google Scholar]
- Huang, B.K.; Zheng, H.C.; Qin, L.P.; Zheng, Q.M.; Xin, H.L. Investigation on resource of genus Valeriana in China. J. Chin. Med. Mater. 2004, 27, 632–634. [Google Scholar]
- Zhu, Y.C. Northeast Medicinal Plants, 1st ed.; Heilongjiang Science and Technology: Harbin, China, 1989; p. 145. [Google Scholar]
- Wang, Q.H.; Wang, C.F.; Shu, Z.P.; Chan, K.; Huang, S.M.; Li, Y.; Xiao, Y.; Wu, L.H.; Kuang, H.X.; Sun, X.B. Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J. Ethnopharmacol. 2014, 153, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Hassan, H.E.; Hegazy, M.F.; Tzakou, O.; Pare, P. Argolic acid A and argolic methyl Ester B, two new cyclopentano- monoterpenes diol from Nepeta argolica. Nat. Prod. Commun. 2006, 1, 523–526. [Google Scholar] [CrossRef]
- Li, N.; Di, L.; Gao, W.C.; Wang, K.J.; Zu, L.B. Cytotoxic iridoids from the roots of Patrinia scabra. J. Nat. Prod. 2012, 75, 1723–1728. [Google Scholar] [CrossRef]
- Quan, Q.L. Studies on the Secondary Metabolites and Their Bioactivities of Valeriana Jatamansi. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 1 September 2021. [Google Scholar]
- Zhao, Z.; Rao, K.R.; Zhou, Y.; Wang, Y.; Liu, D.; Li, R.T.; Li, H.M. Chemical Constituents from Processed Valeriana jatamansi and Its Anti-Inflammatory Activity. Chin. Pharm. J. 2022, 57, 539–548. [Google Scholar]
- Wang, J.L.; Li, Z.; Ruan, J.Y.; Zhang, Y.; Wang, T.; Zhang, Y. Isolation and identification of ionones and lignans from Eurycoma longifolia Jack. Chin. J. Med. Chem. 2020, 30, 101–107. [Google Scholar]
- Tsukamoto, H.; Hisada, S.; Nishibe, S.; Roux, D.G. Phenolic glucosides from Olea europaea subsp. Afr. Phytochem. 1984, 23, 2839–2841. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Gou, P.; Xie, H.H. Phenylpropanoids from Lentopodium lenotopodioides. J. Trop. Subtrop. Bot. 2016, 25, 195–201. [Google Scholar]
- Gao, Y.P.; Chen, F.Y.; Li, M.; Liu, H. Chemical Constituents of Anti-rheumatoid Arthritis Parts of Coluria longifolia Herb. J. Chin. Med. Mater. 2020, 43, 2151–2154. [Google Scholar]
- Qian, C.G.; Jin, L.; Zhu, L.P.; Zhou, Y.; Li, R.N.; Yang, D.P.; Xu, X.J.; Zhao, Z.M. Study on chemical constituents, antitumor and anti-inflammatory activities of Cinnamomi Ramulus. Chin. Tradit. Herbal Drugs 2022, 53, 31–40. [Google Scholar]
- Wang, Y.; Xiang, L.; Yi, X.; He, X. Potential Anti-inflammatory Steroidal Saponins from the Berries of Solanum nigrum L. (European Black Nightshade). J. Agric. Food Chem. 2017, 65, 4262–4272. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wen, X.; Huang, R.; He, R.; Ou, S.; Shen, W.; Huang, C.; Peng, X. Protection of feruloylated oligosaccharides from corn bran against oxidative stress in PC 12 cells. J. Agric. Food Chem. 2014, 62, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xie, H.; Zhao, T.K.; Kang, B. Catalpol regulates cholinergic nerve system function through effect on choline acetyl-transferase not M receptor affinity. Biomed. Pharmacother. 2015, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 4.26, d (11.8) 4.81, d (11.8) | 4.23, d (11.8) 4.79, d (11.8) | 6.40, brs | |||
| 2 | 7.11, brs | |||||
| 3 | 6.397, brs | 4.71, d (11.9) 4.75, d (11.9) | 4.67, d (11.7) 4.75, d (11.7) | |||
| 4 | 1.69, m 2.32, m | 1.69, m 2.32, m | ||||
| 5 | 1.48, m 1.96, m | 1.48, m 1.96, m | 3.09, brd (8.5) | 3.47, m | 3.49, m | 7.15, d (8.3) |
| 6 | 2.28, m | 2.28, m | 4.04, d (2.0) | 1.68, m 2.17, dd (13.2, 7.5) | 1.62, m 2.52, dd (13.6, 7.1) | 6.95, brd (7.6) |
| 7 | 2.94, d (4.5) | 2.89, d (4.5) | 3.37, d (2.2) | 4.41, t (3.7) | 4.38, t (3.7) | 5.01, s |
| 8 | 2.59, m | 2.56, m | ||||
| 9 | 1.21, d (6.5) | 1.20, d (6.5) | 2.05, m | 2.96, t (10.0) | 2.99, dd (9.1, 11.2) | 4.12, d (9.4) 3.99, d (9.4) |
| 10 | 1.47, s | 1.44, s | 3.69, d (3.2) | 4.15, dd (5.7, 9.6) 3.79, t (9.6) | 3.86, d (6.8) | |
| 11 | 4.21, d (11.6) 4.35, d (11.6) | 5.08, s 5.20, s | 5.08, s 5.19, s | |||
| 1′ | 4.33, d (7.8) | 4.43, d (7.7) | ||||
| 2′ | 7.42, d (8.3) | 7.62, d (8.3) | 2.18, d (6.7) | 3.20, t (8.5) | 3.18, t (8.5) | 7.25, d (8.3) |
| 3′ | 6.79, d (8.3) | 6.74, d (8.3) | 2.02, m | 3.37, m | 3.36, m | 6.78, d (8.4) |
| 4′ | 0.94, d (6.7) | 3.30, m | 3.29, m | |||
| 5′ | 6.79, d (8.3) | 6.74, d (8.3) | 0.94, d (6.7) | 3.28, m | 3.29, m | 6.78, d (8.4) |
| 6′ | 7.42, d (8.3) | 7.62, d (8.3) | 3.68, dd (4.6, 12.0) 3.86, brd (11.8) | 3.68, dd (3.6, 11.8) 3.86, brd (11.3) | 7.25, d (8.3) | |
| 7′ | 7.57, d (16.0) | 6.85, d (12.9) | 4.97, s | |||
| 8′ | 6.28, d (16.0) | 5.73, d (12.9) | ||||
| 9′ | 3.97, d (9.4) 4.10, d (9.4) | |||||
| 3-OCH3 | 3.88, s | |||||
| 1″ | 4.41, d (7.5) | 4.41, d (7.5) | 4.72, d (8.1) | 4.90, d (7.1) | ||
| 2″ | 3.20, t (8.0) | 3.20, t (8.0) | 3.35, m | 3.50, m | ||
| 3″ | 3.39, m | 3.39, m | 4.05, m | 3.40, m | ||
| 4″ | 3.32, m | 3.32, m | 3.48, dd (2.7, 9.3) | 3.41, m | ||
| 5″ | 3.30, m | 3.30, m | 3.68, m | 3.48, m | ||
| 6″ | 3.88, brd (11.6) 3.67, brd (11.9) | 3.88, brd (11.6) 3.67, brd (11.9) | 3.66, m 3.86, brd (9.8) | 3.70, brd (13.3) 3.87, m |
| Position | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 68.7, CH2 | 68.3, CH2 | 90.8, CH | 177.2, C | 177.3, C | 133.4, C |
| 2 | 87.9, C | 87.7, C | 113.8, CH | |||
| 3 | 94.6, C | 94.7, C | 142.4, CH | 72.6, CH2 | 72.8, CH2 | 150.6, C |
| 4 | 31.7, CH2 | 31.7, CH2 | 109.8, C | 144.2, C | 144.1, C | 147.8, C |
| 5 | 34.4, CH2 | 34.4, CH2 | 35.4, CH | 40.9, CH | 41.1, CH | 117.7, CH |
| 6 | 39.5, CH | 39.4, CH | 59.9, CH | 41.1, CH2 | 40.2, CH2 | 121.6, CH |
| 7 | 61.6, CH | 61.3, CH | 60.3, CH | 72.9, CH | 83.7, CH | 88.9, CH |
| 8 | 179.8, C | 179.7, C | 80.2, C | 50.5, CH | 51.8, CH | 89.1, C |
| 9 | 22.0, CH3 | 22.0, CH3 | 43.6, CH | 45.2, CH | 45.1, CH | 76.8, CH2 |
| 10 | 18.0, CH3 | 18.1, CH3 | 67.2, CH2 | 69.6, CH2 | 61.7, CH2 | |
| 11 | 69.8, CH2 | 113.9, CH2 | 114.0, CH2 | |||
| 1′ | 127.2, C | 127.6, C | 173.2, C | 104.9, CH | 105.6, CH | 129.1, C |
| 2′ | 131.4, CH | 134.0, CH | 44.3, CH2 | 75.3, CH | 75.6, CH | 130.3, CH |
| 3′ | 117.1, CH | 116.12, CH | 27.0, CH | 78.3, CH | 78.3, CH | 115.9, CH |
| 4′ | 161.5, C | 160.3, C | 22.7, CH3 | 71.7, CH | 71.7, CH | 158.5, C |
| 5′ | 117.1, CH | 116.12, CH | 22.7, CH3 | 78.2, CH | 78.2, CH | 115.9, CH |
| 6′ | 131.4, CH | 134.0, CH | 62.8, CH2 | 62.8, CH2 | 130.3, CH | |
| 7′ | 147.2, CH | 146.1, CH | 89.1, CH | |||
| 8′ | 115.0, CH | 116.09, CH | 89.4, C | |||
| 9′ | 168.6, C | 167.6, C | 77.1, CH2 | |||
| 3-OCH3 | 56.9, CH3 | |||||
| 1″ | 98.2, CH | 98.2, CH | 100.4, CH | 103.1, CH | ||
| 2″ | 75.1, CH | 75.1, CH | 72.6, CH | 75.1, CH | ||
| 3″ | 78.1, CH | 78.1, CH | 73.2, CH | 78.3, CH | ||
| 4″ | 71.7, CH | 71.7, CH | 69.2, CH | 71.5, CH | ||
| 5″ | 78.2, CH | 78.2, CH | 75.6, CH | 78.0, CH | ||
| 6″ | 62.8, CH2 | 62.8, CH2 | 63.4, CH2 | 62.7, CH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Lin, X.; Wang, Q.; Yang, B.; Wang, C. Neuroprotective Iridoids and Lignans from Valeriana amurensis. Molecules 2023, 28, 5793. https://doi.org/10.3390/molecules28155793
Ye M, Lin X, Wang Q, Yang B, Wang C. Neuroprotective Iridoids and Lignans from Valeriana amurensis. Molecules. 2023; 28(15):5793. https://doi.org/10.3390/molecules28155793
Chicago/Turabian StyleYe, Minhui, Xiaoju Lin, Qiuhong Wang, Bingyou Yang, and Changfu Wang. 2023. "Neuroprotective Iridoids and Lignans from Valeriana amurensis" Molecules 28, no. 15: 5793. https://doi.org/10.3390/molecules28155793
APA StyleYe, M., Lin, X., Wang, Q., Yang, B., & Wang, C. (2023). Neuroprotective Iridoids and Lignans from Valeriana amurensis. Molecules, 28(15), 5793. https://doi.org/10.3390/molecules28155793





