Oxygen Vacancies Defective La2Ti2O7 Nanosheets Enhanced Photocatalytic Activity of Hydrogen Evolution under Visible Light Irradiation
Abstract
1. Introduction
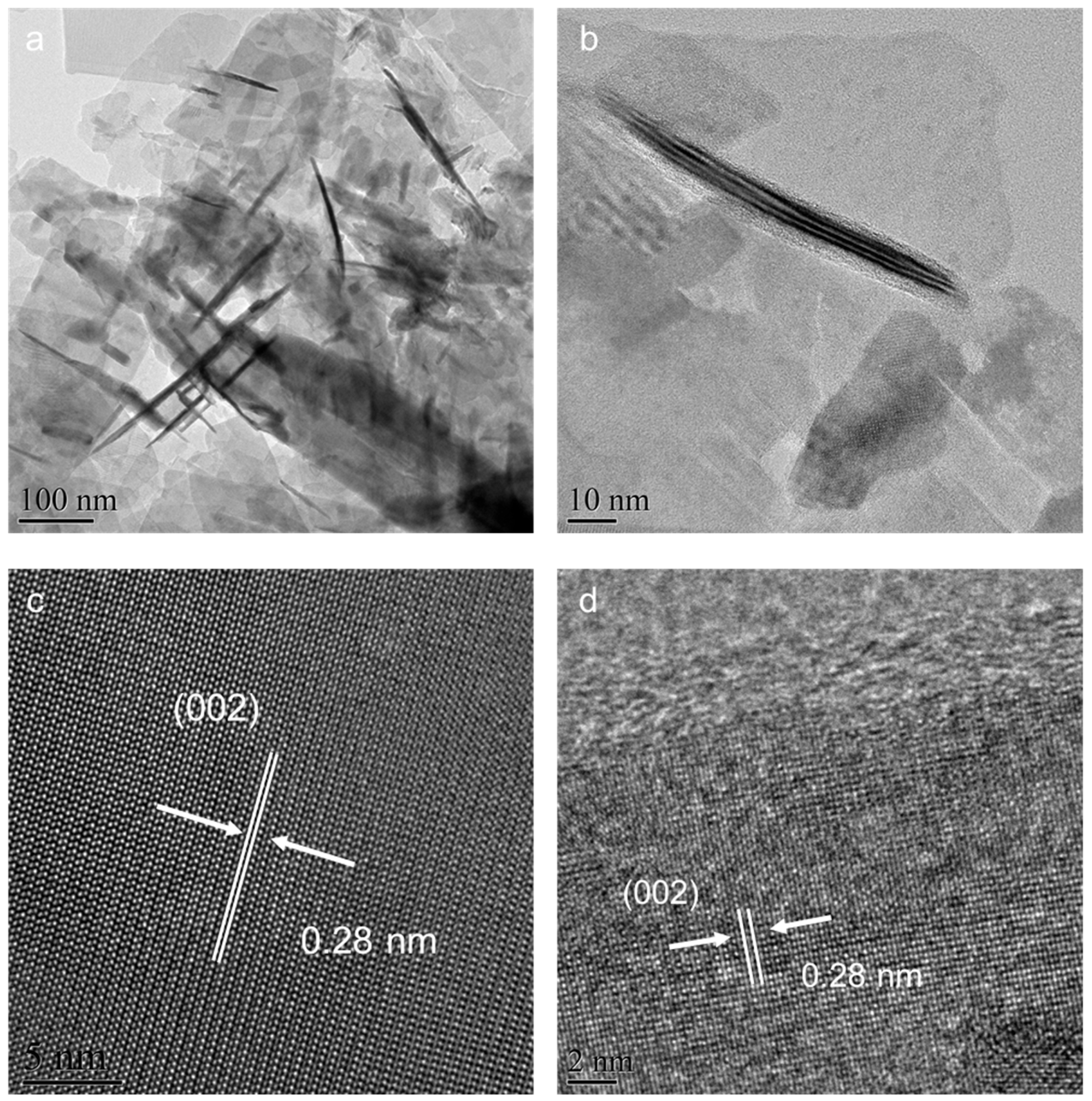
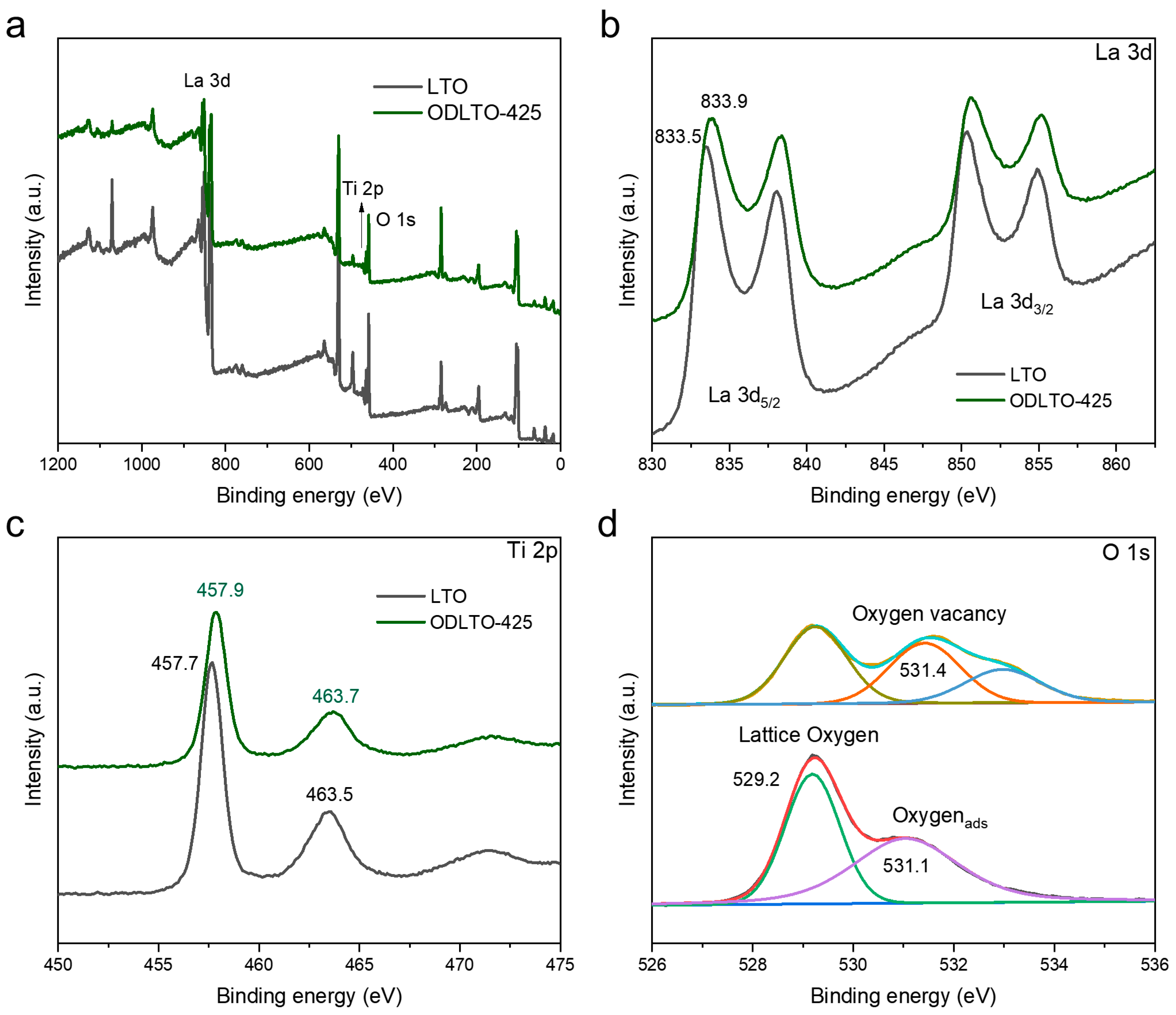
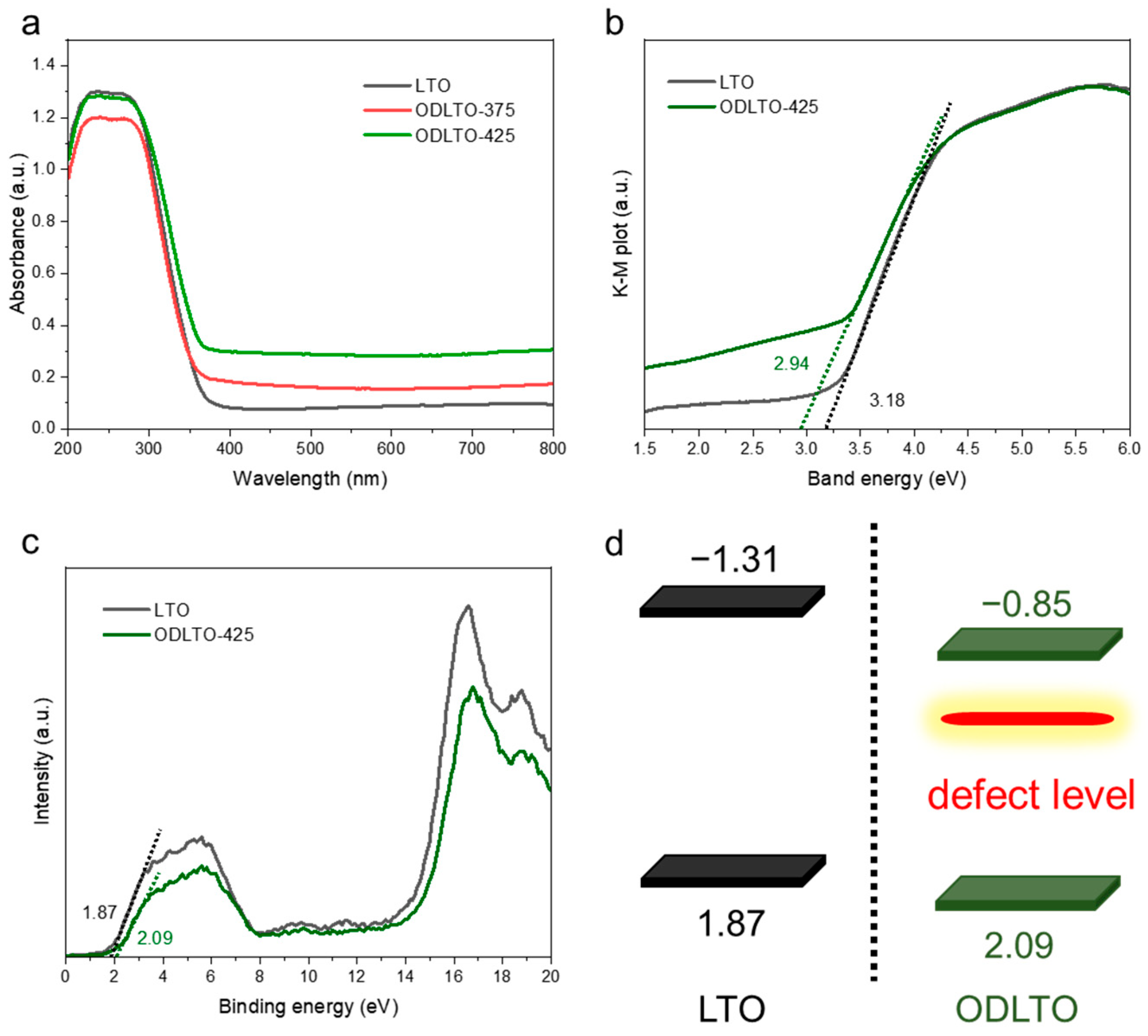
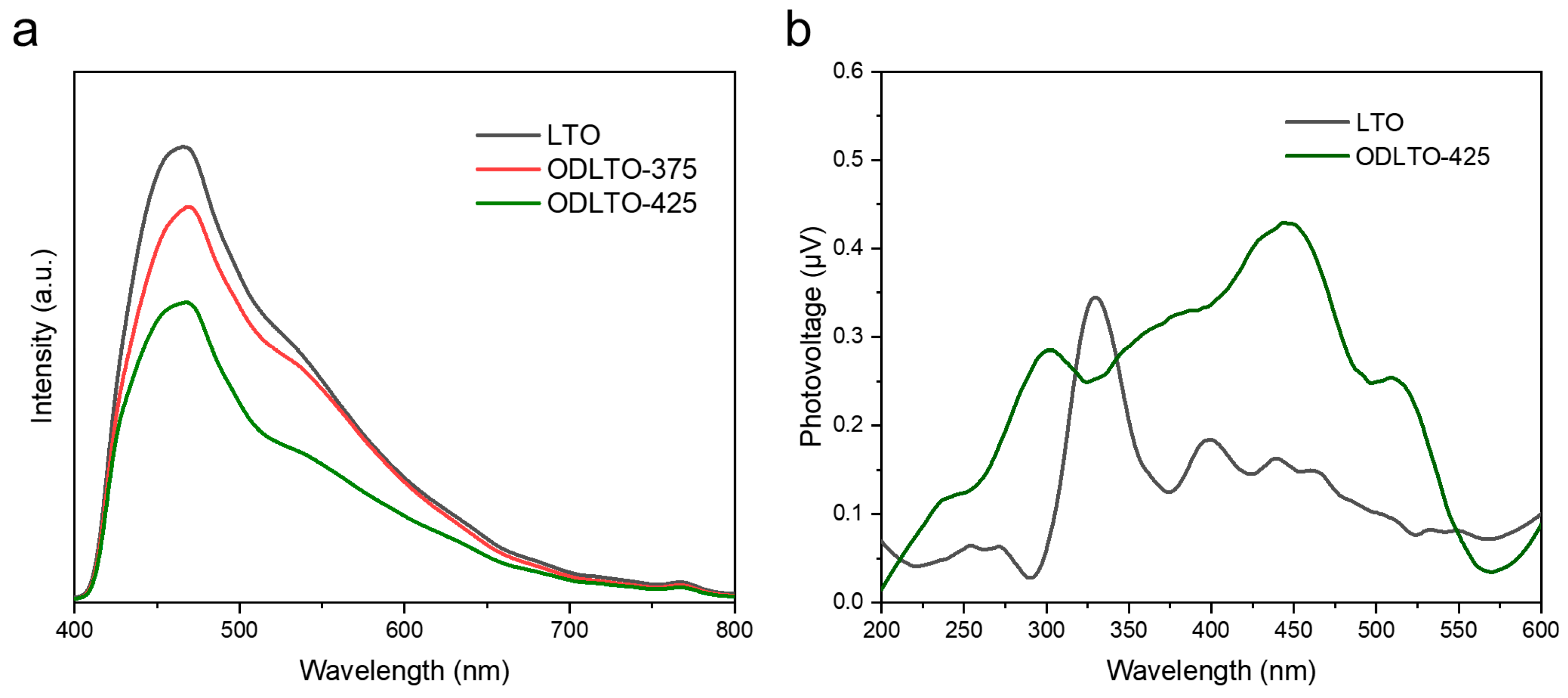
2. Results and Discussion
3. Experimental
3.1. Synthesis of Catalysts
3.2. Characterization
3.3. Photocatalytic H2 Evolution Measurements
3.4. Photocatalytic H2 Evolution Measurements
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Che, H.; Wang, J.; Gao, X.; Chen, J.; Wang, P.; Liu, B.; Ao, Y. Regulating directional transfer of electrons on polymeric g-C3N5 for highly efficient photocatalytic H2O2 production. J. Colloid Interface Sci. 2022, 627, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, L.; Xin, T.; Li, Y.; Wu, X.; Zhang, G. Single-atom V-N charge-transfer bridge on ultrathin carbon nitride for efficient photocatalytic H2 production and formaldehyde oxidation under visible light. Chem. Eng. J. 2022, 429, 132229. [Google Scholar] [CrossRef]
- Bao, H.; Wang, L.; Li, G.; Zhou, L.; Xu, Y.; Liu, Z.; Wu, M. Carrier engineering of carbon nitride boosts visible-light photocatalytic hydrogen evolution. Carbon 2021, 179, 80–88. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Chen, K.; Li, Z.; Hu, B.; Wang, L.; Wu, M. Unravelling the Role of Strong Metal-Support Interactions in Boosting the Activity toward Hydrogen Evolution Reaction on Ir Nanoparticle/N-Doped Carbon Nanosheet Catalysts. ACS Appl. Mater. Interfaces 2021, 13, 22448–22456. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, H.; Cheng, Q.; Gao, C.; Wang, G.; Wu, X. Molecular-functionalized engineering of porous carbon nitride nanosheets for wide-spectrum responsive solar fuel generation. J. Colloid Interface Sci. 2022, 607, 1061–1070. [Google Scholar] [CrossRef]
- Grochowska, K.; Harynski, L.; Karczewski, J.; Jurak, K.; Siuzdak, K. Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light. Materials 2023, 16, 2572. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, K.; Wu, X.; Zhang, G.; Yin, S. Amorphous Bimetallic Cobalt Nickel Sulfide Cocatalysts for Significantly Boosting Photocatalytic Hydrogen Evolution Performance of Graphitic Carbon Nitride: Efficient Interfacial Charge Transfer. ACS Appl. Mater. Interfaces 2019, 11, 26898–26908. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Deng, F.; Huang, J.; Han, L.; He, C.; Chen, Z.; Luo, Y.; Zhu, Y. Double-defect-induced polarization enhanced OV-BiOBr/Cu2-xS high-low junction for boosted photoelectrochemical hydrogen evolution. Appl. Catal. B 2022, 314, 121502. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, G.; Wang, J.; Wang, K.; Yan, S.; Su, Y. Nitrogen vacancy induced in situ g-C3N4 p-n homojunction for boosting visible light-driven hydrogen evolution. J. Colloid Interface Sci. 2021, 587, 110–120. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, J.; Fujitsuka, M.; Majima, T. Graphitic-C3N4 hybridized N-doped La2Ti2O7 two-dimensional layered composites as efficient visible-light-driven photocatalyst. Appl. Catal. B 2017, 202, 191–198. [Google Scholar] [CrossRef]
- Meng, F.; Li, J.; Hong, Z.; Zhi, M.; Sakla, A.; Xiang, C.; Wu, N. Photocatalytic generation of hydrogen with visible-light nitrogen-doped lanthanum titanium oxides. Catal. Today 2013, 199, 48–52. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, M.; Elbanna, O.A.; Fujitsuka, M.; Kim, S.; Mao, L.; Zhang, J.; Majima, T. Au Nanorod Photosensitized La2Ti2O7 Nanosteps: Successive Surface Heterojunctions Boosting Visible to Near-Infrared Photocatalytic H2 Evolution. ACS Catal. 2017, 8, 122–131. [Google Scholar] [CrossRef]
- Meng, F.; Cushing, S.K.; Li, J.; Hao, S.; Wu, N. Enhancement of Solar Hydrogen Generation by Synergistic Interaction of La2Ti2O7 Photocatalyst with Plasmonic Gold Nanoparticles and Reduced Graphene Oxide Nanosheets. ACS Catal. 2015, 5, 1949–1955. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, X.; Fujitsuka, M.; Zhang, J.; Majima, T. Au/La2Ti2O7 Nanostructures Sensitized with Black Phosphorus for Plasmon-Enhanced Photocatalytic Hydrogen Production in Visible and Near-Infrared Light. Angew. Chem. Int. Ed. 2017, 56, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Lukong, V.T.; Ukoba, K.; Jen, T.-C. Review of self-cleaning TiO2 thin films deposited with spin coating. Int. J. Adv. Manuf. Technol. 2022, 122, 3525–3546. [Google Scholar] [CrossRef]
- Wang, K.; Peng, L.; Shao, X.; Cheng, Q.; Wang, J.; Li, K.; Wang, H. Nb–O–C Charge Transfer Bridge in 2D/2D Nb2O5/g-C3N4 S-Scheme Heterojunction for Boosting Solar-Driven CO2 Reduction: In Situ Illuminated X-ray Photoelectron Spectroscopy Investigation and Mechanism Insight. Solar RRL 2022, 6, 2200434. [Google Scholar] [CrossRef]
- Kedves, E.Z.; Bardos, E.; Ravasz, A.; Toth, Z.R.; Mihalydeakpal, S.; Kovacs, Z.; Pap, Z.; Baia, L. Photoinhibitive Properties of alpha-MoO3 on Its Composites with TiO2, ZnO, BiOI, AgBr, and Cu2O. Materials 2023, 16, 3621. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Deng, F.; Liu, J.; Gao, X.; Huang, J.; Xu, J.; Feng, Z.; Chen, Z.; Han, L. Novel organic/inorganic PDI-Urea/BiOBr S-scheme heterojunction for improved photocatalytic antibiotic degradation and H2O2 production. Chin. Chem. Lett. 2022, 33, 5200–5207. [Google Scholar] [CrossRef]
- Wang, H.; Niu, R.; Liu, J.; Guo, S.; Yang, Y.; Liu, Z.; Li, J. Electrostatic self-assembly of 2D/2D CoWO4/g-C3N4 p-n heterojunction for improved photocatalytic hydrogen evolution: Built-in electric field modulated charge separation and mechanism unveiling. Nano Res. 2022, 15, 6987–6998. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, J.; Yang, J.; Wang, Z.; Wang, G.; Wang, K.; Wu, X.; Li, J. Photocatalytic hydrogen evolution coupled with tetracycline photodegradation over S-scheme BaTiO3/Ag2S dual-function nanofibers: Performance and mechanism. Appl. Surf. Sci. 2023, 635, 157760. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Z.; Wang, L.; Wu, X.; Hu, B.; Liu, Z.; Wu, M. Boron Nanosheet-Supported Rh Catalysts for Hydrogen Evolution: A New Territory for the Strong Metal-Support Interaction Effect. Nano-Micro Lett. 2021, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, K.; Peng, L.; Li, K.; Wen, H.; Le, X.; Wu, X.; Wang, G. In-situ irradiated XPS investigation on 2D/1D Cd0.5Zn0.5S/Nb2O5 S-scheme heterojunction photocatalysts for simultaneous promotion of antibiotics removal and hydrogen evolution. Colloids Surfaces A 2022, 652, 129846. [Google Scholar] [CrossRef]
- Du, Y.; Chen, W.; Zhong, Z.; Wang, S.; Zhou, L.; Xiong, D.; Liu, Y.; Liu, Z.; Wang, K. Bifunctional oxygen electrocatalysts with WN@Ni nanostructures implanted on N-doped carbon nanorods for rechargeable Zn-Air batteries. J. Alloys Compd. 2023, 960, 170789. [Google Scholar] [CrossRef]
- Jiang, S.; Yin, M.; Ren, H.; Qin, Y.; Wang, W.; Wang, Q.; Li, X. Novel CuMgAlTi-LDH Photocatalyst for Efficient Degradation of Microplastics under Visible Light Irradiation. Polymers 2023, 15, 2347. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Li, Y.; Wu, X.; Hu, H.; Wang, G.; Xiao, Y.; Chou, S.; Zhang, G. Atomic-level insight of sulfidation-engineered Aurivillius-related Bi2O2SiO3 nanosheets enabling visible light low-concentration CO2 conversion. Carbon Energy 2022, 5, e264. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhao, W.; Wang, G.; Wang, K.; Wu, X.; Li, J. 0D/1D Cu2-xS/TiO2 S-scheme heterojunction with enhanced photocatalytic CO2 reduction performance via surface plasmon resonance induced photothermal effects. Appl. Surf. Sci. 2023, 613, 156083. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Li, J.; Xiang, Q.; Liu, Z.; Liu, B. Identification of the Active Sites on Metallic MoO2-x Nano-Sea-Urchin for Atmospheric CO2 Photoreduction Under UV, Visible, and Near-Infrared Light Illumination. Angew. Chem. Int. Ed. 2023, 62, e202213124. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.; Wang, Z.; Wang, X.; Zhang, G. Boosting full-spectrum light driven surface lattice oxygen activation of ZnMn2O4 by facet engineering for highly efficient photothermal mineralization of toluene. Appl. Catal. B 2023, 324, 122274. [Google Scholar] [CrossRef]
- Cheng, Q.; Kang, K.; Li, Y.; Wang, J.; Wang, Z.; Selishchev, D.; Wang, X.; Zhang, G. Achieving efficient toluene mineralization over ordered porous LaMnO3 catalyst: The synergistic effect of high valence manganese and surface lattice oxygen. Appl. Surf. Sci. 2023, 615, 156248. [Google Scholar] [CrossRef]
- Coderch, G.; Cordoba, A.; Ramirez, O.; Bonardd, S.; Leiva, A.; Haring, M.; Diaz Diaz, D.; Saldias, C. Effects of the Solvent Vapor Exposure on the Optical Properties and Photocatalytic Behavior of Cellulose Acetate/Perylene Free-Standing Films. Polymers 2023, 15, 2787. [Google Scholar] [CrossRef] [PubMed]
- Coromelci, C.G.; Turcu, E.; Doroftei, F.; Palamaru, M.N.; Ignat, M. Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics. Polymers 2023, 15, 2805. [Google Scholar] [CrossRef]
- Yang, G.-C.; Pan, Q.-Y.; Yang, P.; Liu, Y.-S.; Du, Y.; Wang, K. Heteropolyacids supported on hierarchically macro/mesoporous TiO2: Efficient catalyst for deep oxidative desulfurization of fuel. Tungsten 2021, 4, 28–37. [Google Scholar] [CrossRef]
- Wang, K.; Qin, H.; Shao, X.; Jiang, L.; Li, K.; Wang, J.; Zhou, L.; Cheng, Q.; Wang, G.; Wang, H. Unveiling S-Scheme Charge Transfer Pathways in In2S3/Nb2O5 Hybrid Nanofiber Photocatalysts for Low-Concentration CO2 Hydrogenation. Solar RRL 2022, 7, 2200963. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Li, J.; Wang, K.; Zhang, G. Z-scheme g-C3N4@CsxWO3 heterostructure as smart window coating for UV isolating, Vis penetrating, NIR shielding and full spectrum photocatalytic decomposing VOCs. Appl. Catal. B 2018, 229, 218–226. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; Rodriguez-Lorenzo, L.; El Hamidi, A.; Santaballa, J.A.; Canle, M. Cobalt Impregnation on Titania Photocatalysts Enhances Vis Phenol Photodegradation. Materials 2023, 16, 4134. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, G.; Chen, H.; Li, J.; Wang, K.; Pan, Y.; Zhao, Y.; Sun, Y.; Xie, Y. Photocatalytic CO2 Conversion of M0.33WO3 Directly from the Air with High Selectivity: Insight into Full Spectrum-Induced Reaction Mechanism. J. Am. Chem. Soc. 2019, 141, 5267–5274. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, C.-Y.; Lee, P.-C.; Chen, G.-C.; Yang, Z.-R.; Lin, C.-B. AgCl-based selective laser melting photocatalytic module for degradation of azo dye and E. coli. Int. J. Adv. Manuf. Technol. 2021, 115, 1127–1138. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, D.; Hu, Y.; Guo, T.; Liu, C.; Liang, C.; Du, W.; Li, X.; Liu, W. Surface-iodination-induced efficient charge separation in bismuth oxysulfide crystals for enhanced photocatalytic CO2 conversion. Chem. Eng. J. 2023, 453, 139848. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Deng, F.; Han, L.; Gao, X.; Xu, J.; Hou, X.; Feng, Z.; Chen, Z.; et al. BiOBr with oxygen vacancies capture 0D black phosphorus quantum dots for high efficient photocatalytic ofloxacin degradation. Appl. Surf. Sci. 2022, 593, 153422. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.B.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Ling, J.; Wang, K.; Wang, Z.; Huang, H.; Zhang, G. Enhanced piezoelectric-induced catalysis of SrTiO3 nanocrystal with well-defined facets under ultrasonic vibration. Ultrason. Sonochem 2020, 61, 104819. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qin, H.; Li, J.; Cheng, Q.; Zhu, Y.; Hu, H.; Peng, J.; Chen, S.; Wang, G.; Chou, S.; et al. Metallic AgInS2 nanocrystals with sulfur vacancies boost atmospheric CO2 photoreduction under near-infrared light illumination. Appl. Catal. B 2023, 332, 122763. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yu, H.; Liu, Z. Oxygen Vacancies Defective La2Ti2O7 Nanosheets Enhanced Photocatalytic Activity of Hydrogen Evolution under Visible Light Irradiation. Molecules 2023, 28, 5792. https://doi.org/10.3390/molecules28155792
Wang Z, Yu H, Liu Z. Oxygen Vacancies Defective La2Ti2O7 Nanosheets Enhanced Photocatalytic Activity of Hydrogen Evolution under Visible Light Irradiation. Molecules. 2023; 28(15):5792. https://doi.org/10.3390/molecules28155792
Chicago/Turabian StyleWang, Zhigang, Hongliang Yu, and Zhuoyuan Liu. 2023. "Oxygen Vacancies Defective La2Ti2O7 Nanosheets Enhanced Photocatalytic Activity of Hydrogen Evolution under Visible Light Irradiation" Molecules 28, no. 15: 5792. https://doi.org/10.3390/molecules28155792
APA StyleWang, Z., Yu, H., & Liu, Z. (2023). Oxygen Vacancies Defective La2Ti2O7 Nanosheets Enhanced Photocatalytic Activity of Hydrogen Evolution under Visible Light Irradiation. Molecules, 28(15), 5792. https://doi.org/10.3390/molecules28155792




