Abstract
Fixing carbon dioxide as a polymer material is an effective and environmentally beneficial approach for reducing the harm of CO2 greenhouse gas. In this paper, carbon dioxide and cyclohexene oxide were used as co-monomers, and a chiral binuclear cobalt complex with a biphenyl linker was employed as the catalyst to successfully prepare a poly(cyclohexenylene carbonate) with high stereoregularity. The influence of catalyst structure, CO2 pressure, and operating temperature on the copolymerization rate and polymer structure were systematically investigated. Optimal catalyst structure and operating conditions were determined, resulting in an excellent poly(cyclohexenylene carbonate) with a stereoregularity as high as 93.5%. Performance testing revealed that the polyester had a molecular weight of approximately 20 kg/mol, a glass transition temperature of 129.7 °C, an onset decomposition temperature of 290 °C, and a tensile strength of 42.8 MPa. These results demonstrate high thermal stability and mechanical strength, indicating the potential for expanding the applications of aliphatic polycarbonate materials.
1. Introduction
In recent years, with the excessive use of fossil resources, there has been a continuous and significant increase in CO2 emissions, leading to severe environmental issues [1,2,3]. Under this background, the reduction and capture utilization of CO2 have garnered widespread attention in the industry [4,5,6,7]. In fact, CO2 is not only a typical greenhouse gas but also a renewable C1 resource with abundant sources and a low cost, making it highly valuable for various applications. Currently, several valuable methods and technologies for CO2 utilization have been explored, including application as a supercritical solvent and refrigerant [8,9], food preservatives [10], and raw materials for preparation of urea [11], methanol [12], isocyanate [13], methane [14], CO-rich syngas [15], and polycarbonates, etc. [16]. Among these utilization pathways, the preparation of aliphatic polycarbonates using CO2 and epoxides as monomers provided a feasible approach with good application prospects for the fixation and high-value utilization of carbon dioxide [17,18]. Aliphatic polycarbonates (APC), compared to traditional aromatic polycarbonates (PC), usually have better biodegradability and biocompatibility and can be widely used as surgical sutures, bone fixation materials, and drug release carriers in biomedical emerging fields [19,20,21].
Under catalytic action, polycarbonates can be directly prepared through the alternating copolymerization of carbon dioxide and epoxides. Compared to traditional methods such as direct phosgene condensation [22], ester exchange [23], and ring-opening of cyclic carbonates [24], this approach has advantages of abundant and inexpensive raw materials, simple synthetic process, and high operational safety, making it more promising for practical application. Currently, commonly used catalysts are organic compounds of transition metals (Zn, Co, Mn, etc.), including metal alkyls [25], metal carboxylates [26], metal porphyrins [27], metal diimine complexes [28], and SalenM(III)X (M = Cr, Al, Co), etc. [29]. Among these various catalysts, SalenM(III)X has attracted more significant attention in recent years. Compared to Al and Cr ions, Co ion has a more favorable electronic structure and acidity [30], making it better suited for the copolymerization of carbon dioxide and epoxides. Among them, SalenCo(III)X catalysts have been applied in the preparation of isotactic poly(propylene carbonate) (PPC) due to their excellent catalytic performance and simple preparation process. However, being a flexible molecular structure, PPC has some limitations in practical applications, including weak mechanical strength, low glass transition temperature (Tg < 40–50 °C), and poor weather resistance [31]. Relevant studies have shown that introducing rigid cyclic groups into the main chain of polycarbonate can restrict the free movement of segments, significantly increasing the rigidity of the molecular chains and the intermolecular interaction forces, which can effectively improve the mechanical strength and thermal resistance, etc. [32,33].
From the perspective of structure-property relationships in aliphatic polycarbonates, cyclohexene oxide, with a rigid hexagonal structure, has outstanding advantages among similar epoxides. Therefore, research on the alternating copolymerization of carbon dioxide and cyclohexene oxide has been conducted to develop a new, excellent polycarbonate material named poly(cyclohexenylene carbonate) (PCHC), which is of great significance for promoting the application of aliphatic polycarbonates [34,35]. However, cyclohexene oxide exhibits relatively low reactivity compared with other epoxides, and conventional mononuclear cobalt catalysts have difficulty achieving the desired results [36,37,38]. In this study, based on the bimetallic synergistic catalytic mechanism, a novel dinuclear cobalt catalysts was prepared and used for the alternating copolymerization of carbon dioxide and cyclohexene oxide (Figure 1). A novel PCHC material with high stereoregularity was successfully synthesized. In addition, the thermodynamic and mechanical properties were also tested, and the influence of stereoregularity on these properties is preliminarily discussed based on these results.
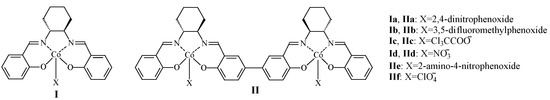
Figure 1.
Structure of mononuclear and dinuclear cobalt complexes.
2. Results and Discussion
2.1. Catalytic Activity of Catalysts
Catalysts have a significant impact on the efficiency of polymerization reactions, the molecular weight and distribution of polyesters, as well as the selectivity of their stereo structures. The catalytic results of different catalysts are shown in Table 1.

Table 1.
Result of copolymerization catalyzed by different catalysts (P = 2.0 MPa, T = 25 °C).
It can be seen from Table 1 that the catalytic activity of binuclear cobalt complexes was significantly higher than that of mononuclear cobalt complexes. A possible reason is that the rigidly connected biphenyl groups maintain an appropriate distance between the two cobalt atoms, achieving good synergistic catalytic effects [39]. Excellent catalytic activity can be realized without the use of bis(triphenylphosphine)iminium chloride (PPNCl) as a co-catalyst, which makes the catalytic system simpler. On the other hand, mononuclear cobalt complex catalysts exhibited almost no catalytic activity in the polymerization reaction without the co-catalyst PPNCl.
Axial coordinating ions have a significant impact on the electronic properties of the central cobalt ion, thereby affecting the catalytic activity [40]. When the coordinating ions were 2,4-dinitrophenoxide, 3,5-bis(trifluoromethyl)phenoxide, and trichloroacetic acid ion, etc., both mononuclear and dinuclear cobalt complexes exhibited relatively high catalytic activity and stereochemical selectivity. The reason may be that these ions all have strong electron-withdrawing abilities, which can keep the central cobalt atom in an electron-deficient state and maintain a stable Co(III) structure, resulting in a high and persistent catalytic activity. Conversely, when the weak nucleophiles ClO4− acted as an axial coordinating ion, it basically lacked the ability to catalyze the copolymerization reaction and could not even generate polymers. Although NO3− has some nucleophilic ability, its ease of losing electrons may cause part of Co(III) ions to be reduced to Co(II) ions and became inactive, leading to relatively lower catalytic activity [41].
2.2. Influence of Operation Conditions
Operating conditions, especially the pressure of CO2 and temperature, have a significant impact on the alternating copolymerization reaction rate of carbon dioxide and cyclohexene oxide, as well as the molecular weight and distribution of the polycarbonate products. In this study, the polymerization results under different reaction pressure and temperature conditions were investigated using the most effective dinuclear cobalt complex IIa as a catalyst under the same material ratio and reaction time. The results are shown in Table 2.

Table 2.
Results of copolymerization catalyzed by IIa under different pressures and temperatures.
From Table 2, it can be observed that the variations in temperature and CO2 pressure had a significant impact on the polymerization reaction. At room temperature (25 °C), increasing the CO2 pressure from 2.0 MPa to 4.0 MPa resulted in an increase in TOF from 133 h−1 to 166 h−1, indicating a significant improvement in catalyst effect. Additionally, the ee value of the polycarbonate increased from 72.5% to 93.5%, indicating an enhanced stereo-regularity and greater specificity of the polymerization reaction. As for temperature, although raising the reaction temperature can also significantly increase the TOF of the catalyst—for instance, at a CO2 pressure of 2.0 MPa, increasing the temperature from 25 °C to 45 °C could elevate the TOF to 2.16 times the original value—the stereo-regularity of the polyester inevitably decreased in some extent. This is because the energy difference between the two enantiomeric structures is small and at higher temperatures, the rate of the unfavorable reaction increases significantly, leading to increased randomness in the polycarbonate and a decrease in ee value. Overall, high pressure and low temperature conditions are more favorable for obtaining highly stereo-regular PCHC.
2.3. Analysis of the Structure and Properties of PCHC
2.3.1. Stereo-Regularity
Besides determining the ee value of the hydrolysis products of PCHC, 13C NMR is also a direct and effective means for assessing the stereo-regularity of PCHC [42]. The carbonyl carbons of PCHC in different chemical environments exhibit distinctive chemical shifts in the 13C NMR spectrum. Peaks at 153.45–153.50 ppm represents the syndiotactic structure, while the peak at 153.65–153.70 ppm belongs to the isotactic structure. Furthermore, a relatively larger peak area at 153.65–153.70 ppm indicates higher stereo-regularity of the PCHC material. Figure 2 displays the 13C NMR spectra of two representative samples, sample A (Table 1, Entry 4) and sample B (Table 2, Entry 3).
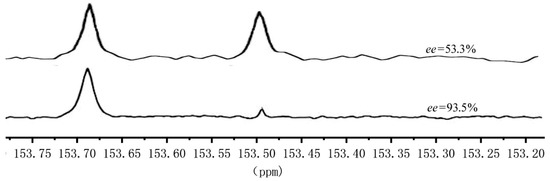
Figure 2.
Local magnification of the 13C NMR spectrum of different stereo-regularity PCHC.
From Figure 2, it can be observed that the signal strengths in 152.8–153.5 ppm and 153.65–153.70 ppm of sample A are not significantly different. As for sample B, the signal of the meso structure carbonyl peak in the range of 153.45–153.50 ppm was relatively weak, while the peak at 153.65–153.70 ppm corresponding to the isotactic structure carbonyl carbon was prominent, indicating the dominance of the isotactic structure in the molecule. After hydrolyzing the two PCHC samples and measuring the excess enantiomer content of the mixed alcohols using a chiral gas chromatography, the ee value was 53.3% and 93.5% respectively, demonstrating that catalyst IIa has a more excellent asymmetric catalytic effect.
2.3.2. Thermal Properties
To investigate the influence of stereo-regularity on PCHC material, sample A and sample B were detected, respectively. The thermal gravimetric analysis (TGA) results are shown in Figure 3.
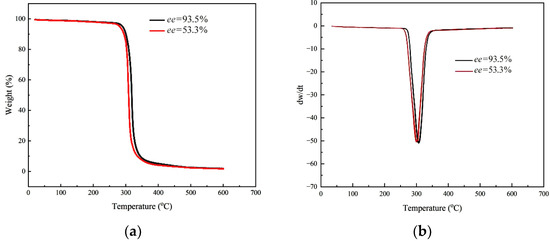
Figure 3.
TGA (a) and DTG (b) curves of two PCHC samples with different ee value.
From Figure 3, it can be observed that the two PCHC samples with different ee values exhibited different initial decomposition temperatures (T-5w%), which were 280 °C and 290 °C, respectively. These values were significantly higher than that of PPC with a similar molecular weight (217 °C). Additionally, both of them showed a narrow temperature range of thermal decomposition, approximately 10 °C, indicating high purity and low content of ether segments in the two samples. The DTG curves revealed that the maximum decomposition temperatures (T-50w%) of the two PCHC samples were 300 °C and 309 °C, respectively, with sharp exothermic peaks. These observations suggested that the PCHC materials possess better thermal stability than PPC. Furthermore, PCHC with higher stereo-regularity (ee value) exhibited superior thermodynamic properties, which can be attributed to the more ordered molecular arrangement and stronger intermolecular forces.
To validate this hypothesis, we conducted further analysis using differential scanning calorimetry (DSC) on the two samples. The results are shown in Figure 4.
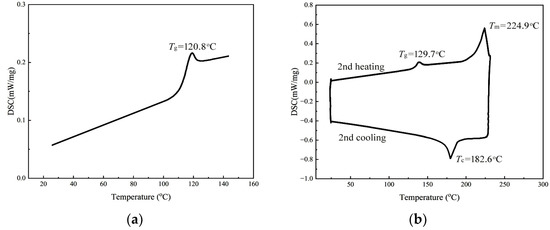
Figure 4.
DSC curves of two PCHC samples with different ee values: (a) ee = 53.3%, (b) ee = 93.5%.
According to the literature, PCHC tends to exhibit crystallization behavior when the ee value exceeds 91% [43]. The study of DSC can provide valuable insights into the crystallization behavior and thermal properties. For PCHC samples with an ee value of 53.3%, the stereo-regularity was relatively low, resulting in the absence of crystallization. Therefore, in Figure 4a, only the glass transition temperature (Tg) can be observed. From Figure 4b, it can be seen that the PCHC sample with an ee value of 93.5% indeed exhibits crystallization behavior observed from second cooling curve. No crystallization peak appears in the second heating curve; the reason is because crystallization has been induced by the first heating program, so there was no crystallization peak appeared in the second heating process, only a melting endotherm peak. However, since the stereo-regularity did not reach 96–100%, a glass transition phenomenon still occurred with a value of 129.7 °C, which is consistent with the findings reported in reference [43]. The enthalpy change of the PCHC can be obtained by integrating the corresponding DSC curve with heating time [44]. The results revealed a distinct endothermic peak at a melting point of 224.9 °C, with a melting enthalpy (∆Hm) of 20.691 J/g. During the cooling process, an exothermic peak appeared around 182.6 °C, indicating the occurrence of crystallization, with a crystallization enthalpy (∆Hc) of −17.312 J/g.
2.3.3. Mechanical Properties
Mechanical properties are important characteristics of structural materials and have a significant impact on their application fields and service life [45,46]. We conducted tensile strength and elongation at break measurements on the PCHC samples with different ee values (the samples with ee value of 53.3%, 72.5% and 93.5% were named PCHC-1, PCHC-2 and PCHC-3) and compared these values with those of the currently used PPC material. The results are shown in Figure 5.
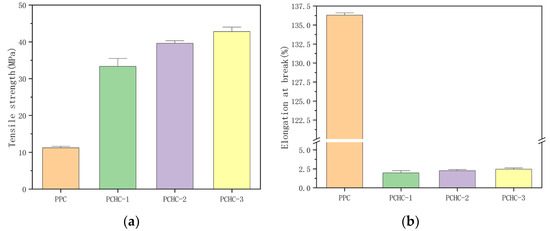
Figure 5.
Tensile strength (a) and elongation at break (b) of PCHC samples with different ee values.
From Figure 5, it can be observed that the comparative sample of PPC is a ductile material with a high elongation at break of 136.3%. However, its tensile strength is low, only 11.2 MPa, indicating a deficiency in material strength. In contrast, the PCHC material prepared in this study, with the introduction of bulky hexagonal ring structures in the main chain of polymer molecule, exhibited significantly improved strength and rigidity compared to PPC. Even the low stereo-regularity PCHC with an ee value of only 53.3% showed a high tensile strength of 33.0 MPa, nearly three times that of PPC material. Meanwhile, the tensile strength increased significantly with the increase of the ee value, eventually reaching 42.8 MPa. However, the elongation at break did not show significant improvement, which may be attributed to the rigid nature of PCHC material. So, it can be drawn that increasing the stereo-regularity of PCHC is beneficial for enhancing its mechanical properties. However, it should be noted that PCHC is a rigid material with poor deformability, exhibiting an elongation at break of only about 2.5%, and a relatively brittle texture. If the properties of PCHC and PPC can be combined, it is possible to obtain a composite polycarbonate material with both satisfactory strength and toughness.
3. Materials and Methods
3.1. Chemicals
Carbon dioxide, oxygen, and nitrogen were purchased from Henan Yuanzheng Special Gas Co., Ltd. (Zhengzhou, China). Cyclohexene oxide was provided by Henan Shenma Nylon Co., Ltd. (Pingdingshan, China). Anhydrous cobalt acetate, 3,5-bis(trifluoromethyl)nitrophenol, 2-amino-4-nitrophenol, trichloroacetic acid, and 2,4-dinitrophenol bis(triphenylphosphine)ammonium chloride were all analytical grade and purchased from Shanghai McKlin Technology Co., Ltd. (Shanghai, China). Poly(propylene carbonate) (Mn = 100 kg/mol, Tg = 49.8 °C) was obtained from Shenzhen Hongli Plastic Raw Material Co., Ltd. (Shenzhen, China). Other reagents and solvents were all analytical grade and were purchased from Tianjin ChemiO Chemical Reagent Co., Ltd. (Tianjin, China). All raw materials needed to be dried before use to reduce the moisture content. The 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl and salicylaldehyde condensation product were prepared according to references [47,48], respectively, and their structures were confirmed by FTIR and NMR characterization (Figures S1–S6). The monometallic cobalt catalysts Ia–Id used for comparison were prepared according to references [49,50].
3.2. Synthetic Route
3.2.1. Catalyst of Dinuclear Cobalt Complexes
The preparation of the bimetallic catalyst started with materials (S,S)-cyclohexane-1,2-diamine aldehyde condensation product and 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl as starting reagents, and it underwent condensation, salt formation, oxidation, and ion exchange to obtain the desired catalyst. The preparation route is shown in Figure 6.
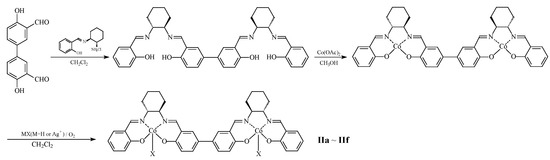
Figure 6.
Synthetic route of dinuclear cobalt complex.
3.2.2. Poly(cyclohexenylene carbonate)
The preparation of isotactic poly(cyclohexene carbonate) involved carbon dioxide and cyclohexene oxide as starting materials. Under the action of a chiral catalyst, the mixture underwent alternating copolymerization under high-pressure conditions to obtain the desired PCHC. The preparation route is shown in Figure 7.
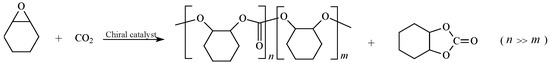
Figure 7.
Synthetic route of stereoregular PCHC.
3.3. Synthesis of Compounds
3.3.1. Salen Ligand
A 100 mL round-bottom flask equipped with a magnetic stir bar was placed in a low-temperature cooling bath, and stirring was initiated. The temperature was controlled below 0 °C. 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl (0.24 g, 1.00 mmol), condensate of (S,S)-cyclohexane-1,2-diamine hydrochloride, and salicylaldehyde (0.485 g, 2.00 mmol) were dissolved in 60 mL of CH2Cl2. Then, triethylamine (0.55 mL, 4.00 mmol) and a small amount of 5A molecular sieve were added. The mixture was allowed to react at room temperature for 24 h and then filtered under vacuum. The filter cake was washed with an appropriate amount of CH2Cl2 before collecting the filtrate. The crude product was obtained by vacuum distillation of the filtrate and purified by column chromatography (dry loading; silica gel column; eluent: petroleum ether/ethyl acetate = 10/1), resulting in a golden yellow powder compound with a yield of 85.6%. IR (KBr, cm−1) ν: 3455, 3241, 2932, 2740, 1649, 1498, 1456, 1276, 1145, 1035, 823, 759, 660; 1H NMR (CDCl3, 400 MHz) δ 13.61 (s, 2H), 13.13 (s, 2H), 8.38 (s, 2H), 8.23 (s, 2H), 7.22 (s, 2H), 7.13 (s, 2H), 6.89 (m, 2H), 6.85 (s, 2H), 6.76 (s, 2H), 3.62–3.55 (m, 2H), 3.32–3.26 (m, 2H), 2.02–1.81 (m, 4H), 1.80–1.51 (m, 4H), 1.50–1.31 (m, 12H). 13C NMR (DMSO-d6, 100 MHz) δ 162.8, 162.4, 160.8, 160.7, 132.7, 132.3, 130.5, 129.7, 120.2, 119.8, 119.6, 119.4, 118.8, 117.0, 67.6, 67.2, 32.9, 32.7, 24.2, 24.1. The spectra of IR, 1H-NMR and 13C-NMR were listed in Figures S7–S9.
3.3.2. SalenCo(II) Complex
Under N2 protection, salen ligand (0.321 g, 0.50 mmol) and 5 mL of CH2Cl2 were added to a 150 mL three-neck flask. Then, the flask was placed in a low-temperature cooling bath with magnetic stirring. The solution of 0.18 g anhydrous cobalt acetate in 30 mL of CH3OH was slowly added dropwise to the flask within 20 min and stirred for 30 min. Then, the precipitate was filtered out and washed with a small amount of CH3OH. The filter cake was vacuum dried at 60 °C for 24 h, resulting in the formation of a brick-red solid product with a yield of 93.1%. 1H NMR (CDCl3, 400 MHz) δ 8.38 (s, 2H), 8.23 (s, 2H), 7.22 (s, 2H), 7.13 (s, 2H), 6.89 (m, 2H), 6.85 (s, 2H), 6.76 (s, 2H), 3.62–3.54 (m, 2H), 3.32–3.25 (m, 2H), 2.02–1.80 (m, 4H), 1.80–1.51 (m, 8H), 1.50–1.32 (m, 4H). 13C NMR (DMSO-d6, 100 MHz) δ 164.6, 164.1, 136.4, 135.7, 132.5, 129.5, 128.7, 128.3, 128.1, 127.2, 125.1, 124.0, 117.1, 116.0, 51.7, 51.4, 30.1, 30.0, 23.8. The spectra of 1H-NMR and 13C-NMR were listed in Figures S10 and S11.
3.3.3. SalenCo(III) Complexes
The preparation processes of SalenCo(III) complexes were similar with minor variations. Taking the preparation of catalyst IIa as an example here: In a 50 mL three-neck flask, SalenCo(II) complex (0.151 g, 0.2 mmol) and 2,4-dinitrophenol (0.074 g, 0.4 mmol) were dissolved in 30 mL of purified CH2Cl2. Dry oxygen was slowly introduced, and the mixture was oxidized for 1–2 h. The solvent was then removed by rotary evaporation to obtain the crude product. Then, the crude product was taken out and dissolved in a small amount of ethyl ether, and a small amount of n-hexane was added to adjust polarity. The mixture was placed at a low temperature and kept in the dark overnight. After filtration, the precipitate was collected and vacuum dried at 60 °C for 12 h, resulting in a dark green powder with a yield of 88.2%. 1H NMR (CDCl3, 400 MHz): δ 8.76 (s, 2H), 8.29 (s, 2H), 7.83 (s, 2H), 7.51 (m, 2H), 7.38–7.21 (m, 8H), 6.98–6.88 (m, 4H), 6.55 (d, 2H), 2.96–2.76 (m, 4H), 2.01–1.83 (m, 4H), 1.75–1.56 (m, 8H), 1.53–1.37(m, 6H). 13C NMR (DMSO-d6, 100 MHz) δ 162.6, 162.2, 159.3, 141.1, 140.8, 138.9, 138.7, 136.7, 136.3, 133.2, 133.1, 131.7, 131.5, 124.7, 124.5, 122.6, 122.4, 118.4, 118.2, 117.3, 117.1, 53.2, 52.8, 52.7, 27.1, 26.9, 26.8, 23.3, 23.1. The spectra of 1H-NMR and 13C-NMR were listed in Figures S12 and S13.
3.3.4. Poly(cyclohexenylene carbonate)
The high-pressure reactor was placed in a drying oven and dried at 120 °C for 2 h. Then, it was quickly removed while still hot, and it was sealed and evacuated under vacuum. Nitrogen gas was introduced to replace the atmosphere. In a nitrogen-filled glove box, a predetermined amount of catalyst and a certain proportion of cyclohexene carbonate were weighed into a 250 mL iodine flask successively. After the catalyst dissolved, the mixture was transferred to the high-pressure reactor using a syringe. CO2 was introduced into the reactor until the set pressure was reached (2.0–4.0 MPa). The mixture was stirred violently at a specific temperature for a certain time (controlling the conversion rate at 50–60%). Then, the mixture was cooled to room temperature, and CO2 was slowly released. The mixture was transferred to a 200 mL single-neck flask, and the excessive cyclohexene was removed by vacuum distillation. The precipitated solid in the flask was dissolved in a small amount of chloroform, and a few drops of dilute hydrochloric acid (2 mol/L) were added. The solution was then added to methanol and vigorously stirred to precipitate the polymer. This process was repeated several times to remove the residue catalyst, resulting in a white powder of PCHC after vacuum drying. The spectra of 1H-NMR was listed in Figure S14.
3.4. Structure and Properties Analysis of PCHC
3.4.1. GPC Analysis
Molecular weight and its distribution of PCHC samples were analyzed using a PL-GPC50 gel permeation chromatography instrument (Agilen, Santa Clara, CA, USA). THF was used as the mobile phase at a flow rate of 1.00 mL/min, and the sample concentration was 5 mg/mL (standard sample: polystyrene).
3.4.2. TG Analysis
Thermogravimetric analysis was carried using a TGA2 thermal analyzer (Mettler-Toledo, Greifensee, Switzerland). A total of 5.0 mg of PCHC sample was put into an alumina crucible, which was heated from 25 °C to 600 °C with a heating rate of 10 °C/min and a nitrogen flow rate of 20 mL/min.
3.4.3. DSC Analysis
DSC analysis was performed using a Netzsch STA-449-F1 differential scanning calorimeter (Netzsch, Germany). The measurement was conducted under N2 atmosphere. The sample was heated from 25 °C to 235 °C at a rate of 10 °C/min and held at 235 °C for 10 min to eliminate the sample’s thermal history. Subsequently, the temperature was decreased to 25 °C at a rate of 10 °C/min. Afterward, the second heating program was performed, where the temperature was still increased from 25 °C to 235 °C at a rate of 10 °C/min but without any holding time, and decreased from 235 °C to 25 °C at a rate of 10 °C/min. The heat flow data were recorded and used to plot the heat flow curve.
3.4.4. Determination of ee Value
A total of 120 mg of PCHC sample and 15 mL THF were added to a 100 mL round-bottom flask. Then, 2 mL of methanol and 4 mL of 3 mol/L NaOH solution were added after the PCHC dissolved. The mixture was stirred at room temperature for 8 h and the pH of the solution was subsequently adjusted to neutral by adding a small amount of 2 mol/L HCl. The aqueous phase was extracted with ethyl acetate (5 mL × 3). Then, the organic phase was dried with anhydrous MgSO4 overnight. The solvent was removed by rotary evaporation, and the residue was separated by column chromatography (silica gel column, petroleum ether/ethyl acetate = 10/1). An amount of 79 mg of a mixture of chiral diols was obtained after removing the solvent. The ee value of the chiral diols mixture was analyzed using an Agilent HP 19091G-B213 chiral gas chromatograph. The operation conditions were as follows: injection temperature of 250 °C, hydrogen flame detector temperature of 250 °C, vaporizer temperature of 260 °C, and programmed heating from 100 °C to 120 °C with a heating rate of 10 °C/min. The ee values were calculated by the Equation (1).
where [S,S] and [R,R] are the mass content of (S,S)-enantiomer and (R,R)-enantiomer of 1,2-cyclohexanediol, respectively. Since the absolute correction factors for the two components are identical, the mass percentage content can be replaced by the percentage of peak area. A typical chiral gas chromatogram is shown in Figure 8.
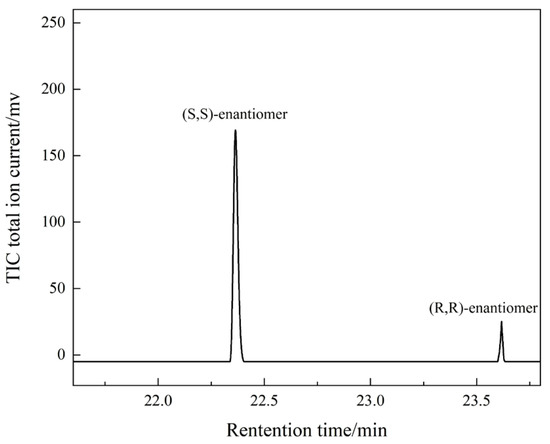
Figure 8.
The chiral chromatogram of PCHC hydrolysis products.
3.4.5. Mechanical Performance Test
The mechanical properties of polyester were tested on a CMT6103 universal material testing machine (ZWICK, Ulm, Germany) according to the reference [51]. At room temperature, the PCHC sample was fully dissolved in a small amount of CH2Cl2. The viscous liquid was then evenly spread in a rectangular mold and left to stand for the solvent to evaporate under vacuum drying at 25 °C, resulting in a thin film whose thickness was about 0.5 mm. The film was cut into uniform rectangular shapes of 1.0 cm × 6.0 cm. The clamp distance was 50 mm, the test was conducted at a speed of 100 mm/min, and the measurements were taken three times using three samples of the same specifications to obtain an average value.
4. Conclusions
Starting from the bimetallic synergistic catalytic mechanism for alternating copolymerization of carbon dioxide and epoxides, a series of chiral bimetallic cobalt complexes catalysts with different axial coordinating ions was designed and prepared for the alternating copolymerization of carbon dioxide and cyclohexene oxide to produce stereoregular PCHC in this paper. The results revealed that catalysts with strong electron-withdrawing coordinating ions exhibited better catalytic activity, enabling efficient alternating copolymerization at room temperature and ensuring a higher stereo-selectivity during the copolymerization process. It is also observed that high temperature and low pressure contribute to the improvement of isotacticity. Subsequent property tests indicated that PCHC with higher isotacticity exhibits improved thermodynamic and mechanical properties, with a glass transition temperature of over 120 °C, which significantly expanded the temperature range for the application of polycarbonates. Furthermore, the higher mechanical strength of PCHC might provide an effective approach for improving the mechanical properties of PPC. However, further in-depth research is needed to explore these aspects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28135235/s1, Figure S1: FTIR spectrum of 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl; Figure S2: 1H NMR spectrum of 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl; Figure S3: 13C NMR spectrum of 3,3′-diformyl-4,4′-dihydroxy-1,1′-biphenyl; Figure S4: FTIR spectrum of salicylaldehyde condensation product; Figure S5: 1H NMR spectrum of salicylaldehyde condensation product; Figure S6: 13C NMR spectrum of salicylaldehyde condensation product; Figure S7: FTIR spectrum of the salen ligand; Figure S8: 1H NMR spectrum of the salen ligand; Figure S9: 13C NMR spectrum of the salen ligand; Figure S10: 1H NMR spectrum of the salenCo(II) complex; Figure S11: 13C NMR spectrum of the salenCo(II) complex; Figure S12: 1H NMR spectrum of the salenCo(III) complex (IIa); Figure S13: 13C NMR spectrum of the salenCo(III) complex (IIa); Figure S14: 1H NMR spectrum of PCHC.
Author Contributions
Conceptualization, Z.L. and M.Z.; methodology, M.Z. and Z.L.; formal analysis, M.Z. and C.Z.; investigation, C.Z.; data curation, P.Z.; writing—original draft preparation, M.Z. and C.Z.; writing—review and editing, Z.L. and M.Z.; project administration, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Foundation of Henan Educational Committee (grant number 22A530009), China Scholarship Council (grant number 202007045073), and State Key Laboratory of Coking Coal Resources Development and Comprehensive Utilization (grant number 41041220201132T).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Haugan, P.M.; Drange, H. Effects of CO2 on the ocean environment. Energ. Convers. Manag. 1996, 37, 1019–1022. [Google Scholar] [CrossRef]
- Bjorkegren, A.B.; Grimmond, C.S.B.; Kotthaus, S.; Malamud, B.D. CO2 emission estimation in the urban environment: Measurement of the CO2 storage term. Atmos. Environ. 2015, 122, 775–790. [Google Scholar] [CrossRef]
- Barrera-Santana, J.; Marrero, G.A.; Puch, L.A.; Diaz, A. CO2 emissions and energy technology in Western Europe. Ser. J. Span. Econ. 2021, 12, 105–150. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.Y.W.; Ngu, L.H.; How, B.S. Review of carbon capture absorbents for CO2 utilization. Greenh. Gases 2022, 12, 394–427. [Google Scholar] [CrossRef]
- Russo, M.E.; Capasso, C.; Marzocchella, A.; Salatino, P. Imobilization of carbonic anhydrase for CO2 capture and utilization. Appl. Microbiol. Biot. 2022, 106, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tan, C.S. A review: CO2 utilization. Aerosol. Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Fu, L.P.; Ren, Z.K.; Si, W.Z.; Ma, Q.L.; Huang, W.Q.; Liao, K.L.; Huang, Z.L.; Wang, Y.; Li, J.H.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Lozowski, D. Supercritical CO2: A green solvent. Air Qual. Res. 2010, 117, 15–18. [Google Scholar]
- Li, P.H.; Chen, J.J.J.; Norris, S. Review of flow condensation of CO2 as a refrigerant. Int. J. Refrig. 2016, 72, 53–73. [Google Scholar] [CrossRef]
- Parton, T.; Bertucco, A.; Elvassore, N.; Grimolizzi, L. A continuous plant for food preservation by high pressure CO2. J. Food Eng. 2007, 79, 1410–1417. [Google Scholar] [CrossRef]
- Wang, H.; Xin, Z.; Li, Y.H. Synthesis of ureas from CO2. Top. Curr. Chem. 2017, 375, 49. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M. Methanol synthesis from CO2 hydrogenation. Chemcatchem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Broere, D.L.J.; Mercado, B.Q.; Holland, P.L. Selective Conversion of CO2 into isocyanate by low-coordinate iron complexes. Angew. Chem. Int. Edit. 2018, 57, 6507–6511. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, W.T.; Huang, K.; Guo, H.Z.; Li, Z.Y.; Fang, Y.B.; Yadav, R.M.; Shanov, V.; Ajayan, P.M.; Wang, L.; et al. Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion. Nat. Commun. 2021, 12, 5265. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Fu, X.X.; Wang, K.; Wang, L.; Zhang, H.L.; Liu, Z.Y.; Liu, B.; Li, J. Chemically bonded BiVO4/Bi19Cl3S27 heterojunction with fast hole extraction dynamics for continuous CO2 photoreduction. Adv. Powder Mater. 2023; in press. [Google Scholar] [CrossRef]
- Taherimehr, M.; Pescarmona, P.P. Green polycarbonates prepared by the copolymerization of CO2 with epoxides. J. Appl. Polym. Sci. 2014, 131, 41141. [Google Scholar] [CrossRef]
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Fukuoka, S.; Fukawa, I.; Adachi, T.; Fujita, H.; Sugiyama, N.; Sawa, T. Industrialization and expansion of green sustainable chemical process: A review of non-phosgene polycarbonate from CO2. Org. Process. Res. Dev. 2019, 23, 145–169. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2017, 5, 9–21. [Google Scholar] [CrossRef]
- Qin, Y.S.; Sheng, X.F.; Liu, S.J.; Ren, G.J.; Wang, X.H.; Wang, F.S. Recent advances in carbon dioxide based copolymers. J. CO2 Util. 2015, 11, 3–9. [Google Scholar] [CrossRef]
- Ji, Y.; Kim, M. Preparation of 3d printing scaffold using aliphatic polycarbonate as a bioink and evaluation of biocompatibility. Tissue Eng. Part A 2022, 28, 322–323. [Google Scholar]
- Degee, P.; Jerome, R.; Teyssie, P. Synthesis and characterization of halato-telechelic bisphenol A polycarbonates. Polymer 1994, 35, 371–376. [Google Scholar] [CrossRef]
- Sun, J.J.; Aly, K.I.; Kuckling, D. A novel one-pot process for the preparation of linear and hyperbranched polycarbonates of various diols and triols using dimethyl carbonate. RSC Adv. 2017, 7, 12550–12560. [Google Scholar] [CrossRef]
- Mei, L.L.; Yan, G.P.; Yu, X.H.; Cheng, S.X.; Wu, J.Y. Ring-opening copolymerization and properties of polycarbonate copolymers. J. Appl. Polym. Sci. 2008, 108, 93–98. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coordin. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Mo, W.J.; Zhuo, C.W.; Cao, H.; Liu, S.J.; Wang, X.H.; Wang, F.S. Facile aluminum porphyrin complexes enable flexible terminal epoxides to boost properties of CO2-polycarbonate. Macromol. Chem. Phys. 2021, 13, 2100403. [Google Scholar]
- Moore, D.R.; Cheng, M.; Lobkovsky, E.B.; Caates, G.W. Mechanism of the alternating copolymerization of epoxides and CO2 using beta-diiminate zinc catalysts: Evidence for a bimetallic epoxide enchainment. J. Am. Chem. Soc. 2003, 125, 11911–11924. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ulusoy, M.; Karroonnirum, O.; Poland, R.R.; Reibenspies, J.H.; Cetinkaya, B. Highly Selective and Reactive (salan)CrCl Catalyst for the Copolymerization and Block Copolymerization of Epoxides with Carbon Dioxide. Macromolecules 2009, 42, 6992–6998. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.L.; Wang, Z.M.; Wang, Z.; Liu, Y.J.; Sun, W.W.; Lai, J.W.; Vajtai, R.; Ajayan, P.M.; Tour, J.M.; et al. Revealing the effect of phosphorus doping on Co@carbon in boosting oxygen evolution catalytic activity. J. Alloys Compd. 2020, 843, 156001. [Google Scholar] [CrossRef]
- Wang, S.J.; Huang, Y.H.; Liao, B.; Lin, G.; Cong, G.M.; Chen, L.B. Structure and properties of poly(propylene carbonate). Adv. Polym. Sci. 1997, 3, 141–143. [Google Scholar] [CrossRef]
- Thorat, S.D.; Phillips, P.J.; Semenov, V.; Gakh, A. Physical properties of aliphatic polycarbonates made from CO2 and epoxides. J. Appl. Polym. Sci. 2003, 89, 1163–1176. [Google Scholar] [CrossRef]
- Liu, Y.F.; Huang, K.L.; Peng, D.M.; Liu, S.Q.; Wu, H. Synthesis and properties of novel aliphatic polycarbonate from carbon dioxide with 1,2-butylene oxide and epsilon-caprolactone. Chin. Chem. Lett. 2007, 18, 209–212. [Google Scholar] [CrossRef]
- Van Meerendonk, W.J.; Duchateau, R.; Koning, C.E.; Gruter, G.J.M. High-throughput automated parallel evaluation of zinc-based catalysts for the copolymerization of CHO and CO2 to polycarbonates. Macromol. Rapid Comm. Sci. 2004, 25, 382–386. [Google Scholar] [CrossRef]
- Plommer, H.; Stein, L.; Murphy, J.N.; Ikpo, N.; Mora-Diez, N.; Kerton, F.M. Copolymerization of CHO/CO2 catalyzed by a series of aluminum amino-phenolate complexes and insights into structure-activity relationships. Dalton Trans. 2020, 49, 6884–6895. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Mackiewica, R.M.; Phelps, A.L.; Billodeaux, D.R. Copolymerization of CO2 and epoxides catalyzed by metal salen complexes. Acc. Chem. Res. 2004, 37, 836–844. [Google Scholar] [CrossRef]
- Ren, W.M.; Liu, Z.W.; Wen, Y.Q.; Zhang, R.; Lu, X.B. Mechanistic aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt(III). J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef]
- Decortes, A.; Haak, R.M.; Martin, C.; Belmonte, M.M.; Martin, E.; Benet-Buchholz, J.; Kleij, A.W. Copolymerization of CO2 and cyclohexene oxide mediated by Yb(salen)-based complexes. Macromolecules 2015, 48, 8197–8207. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.Y.; Liu, Y.; Ren, W.M.; Lu, X.B. Binuclear chromium-salan complex catalyzed alternating copolymerization of epoxides and cyclic anhydrides. Polym. Chem. 2013, 4, 1439–1444. [Google Scholar] [CrossRef]
- Cohen, C.T.; Chu, T.; Coates, G.W. Cobalt catalysts for the alternating copolymerization of propylene oxide and carbon dioxide: Combining high activity and selectivity. J. Am. Chem. Soc. 2005, 127, 10869–10878. [Google Scholar] [CrossRef]
- Qin, Y.S.; Chen, L.J.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Alternating copolymerization of cyclohexene oxide and carbon dioxide under cobalt porphyrin catalyst. Chin. J. Polym. Sci. 2011, 29, 602–608. [Google Scholar] [CrossRef]
- Guerin, W.; Diallo, A.K.; Kirilov, E.; Helou, M.; Slawinski, M.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. Enantiopure isotactic PCHC synthesized by ring-opening polymerization of cyclohexene carbonate. Macromolecules 2014, 47, 4230–4235. [Google Scholar] [CrossRef]
- Wu, G.P.; Ren, W.M.; Luo, Y.; Li, B.; Zhang, W.Z.; Lu, X.B. Enhanced asymmetric induction for the copolymerization of CO2 and cyclohexene oxide with unsymmetric enantiopure salenCo(III) complexes: Synthesis of crystalline CO2-based polycarbonate. J. Am. Chem. Soc. 2012, 134, 5682–5688. [Google Scholar] [CrossRef]
- Lu, X.B.; Shi, L.; Wang, Y.M.; Zhang, R.; Zhang, Y.J.; Peng, X.J.; Zhang, Z.C.; Li, B. Design of highly active binary catalyst systems for CO2/epoxide copolymerization: Polymer selectivity, enantioselectivity, and stereochemistry control. J. Am. Chem. Soc. 2006, 128, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Rouabah, F.; Fois, M.; Ibos, L.; Boudenne, A.; Picard, C.; Dadache, D.; Haddaoui, N. Mechanical and thermal properties of polycarbonate, part 1: Influence of free quenching. J. Appl. Polym. Sci. 2008, 109, 1505–1514. [Google Scholar] [CrossRef]
- Bahar, A.; Belhabib, S.; Guessasma, S.; Benmahiddine, F.; Hamami, A.E.; Belarbi, R. Mechanical and thermal properties of 3D printed polycarbonate. Energies 2022, 15, 3686. [Google Scholar] [CrossRef]
- Campbell, E.J.; Nguyen, S.T. Unsymmetrical salen-type ligands: High yield synthesis of salen-type Schiff bases containing two different benzaldehyde moieties. Tetrahedron Lett. 2001, 42, 1221–1225. [Google Scholar] [CrossRef]
- Paul, S.; Gupta, M.; Gupta, R. Vilsmeier reagent for formylation in solvent-free conditions using microwaves. Synlett 2000, 8, 1115–1118. [Google Scholar]
- Wang, Z.; Mu, Y. Chiral salenCo(iii) complexes with bulky substituents as catalysts for stereoselective alternating copolymerization of racemic propylene oxide with carbon dioxide and succinic anhydride. Polym. Chem. 2021, 12, 1776–1786. [Google Scholar] [CrossRef]
- Xu, K.; Chen, J.G.; Wang, K.; Liu, W.Z.; Jiang, J.Q.; Liu, Z.T. perfectly alternating copolymerization of propylene oxide and CO2 over salenco/salencr complexes. J. Macromol. Sci. A 2014, 51, 589–597. [Google Scholar] [CrossRef]
- Liang, Z.; Li, X.; Li, M.; Hong, Y.L. Study on the preparation and properties of jute microcrystalline cellulose membrane. Molecules 2023, 28, 1783. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).