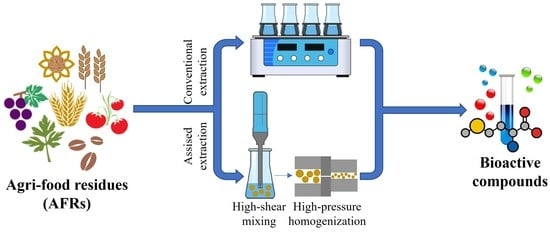
Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues
Abstract
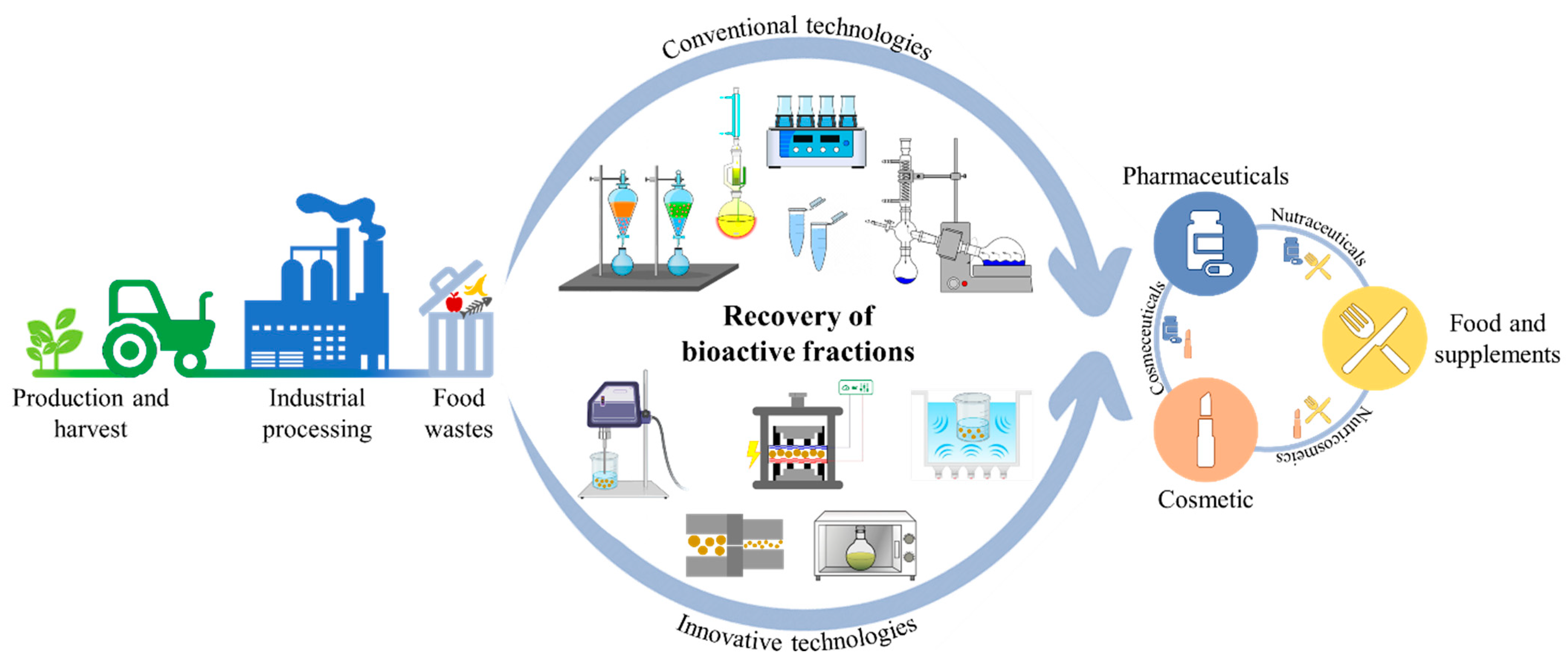
1. Introduction
2. Results and Discussion
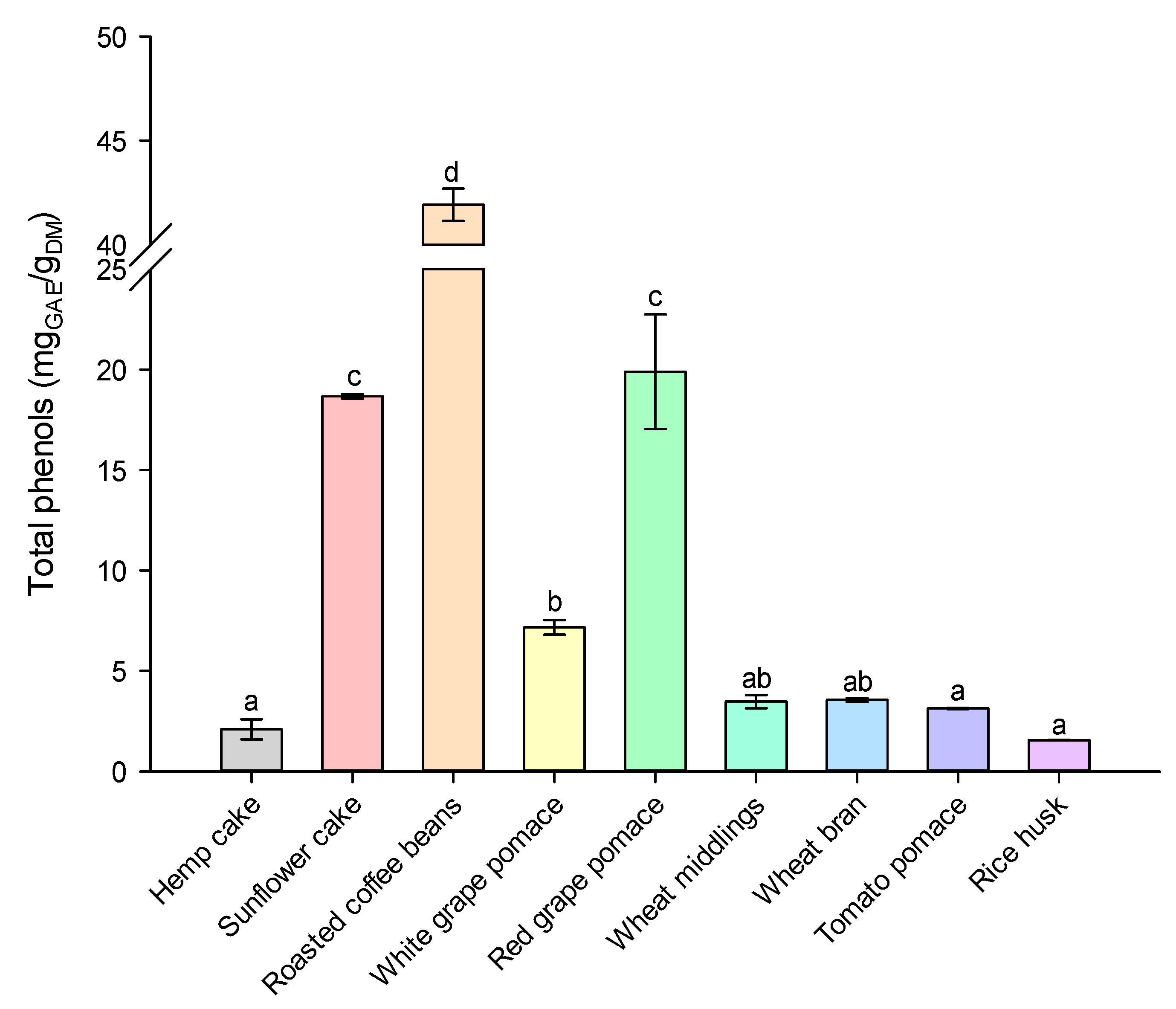
2.1. Characterization of Agri-Food Residues
2.2. Conventional SLE Extraction Process
2.3. HPH-Assisted Extraction Process
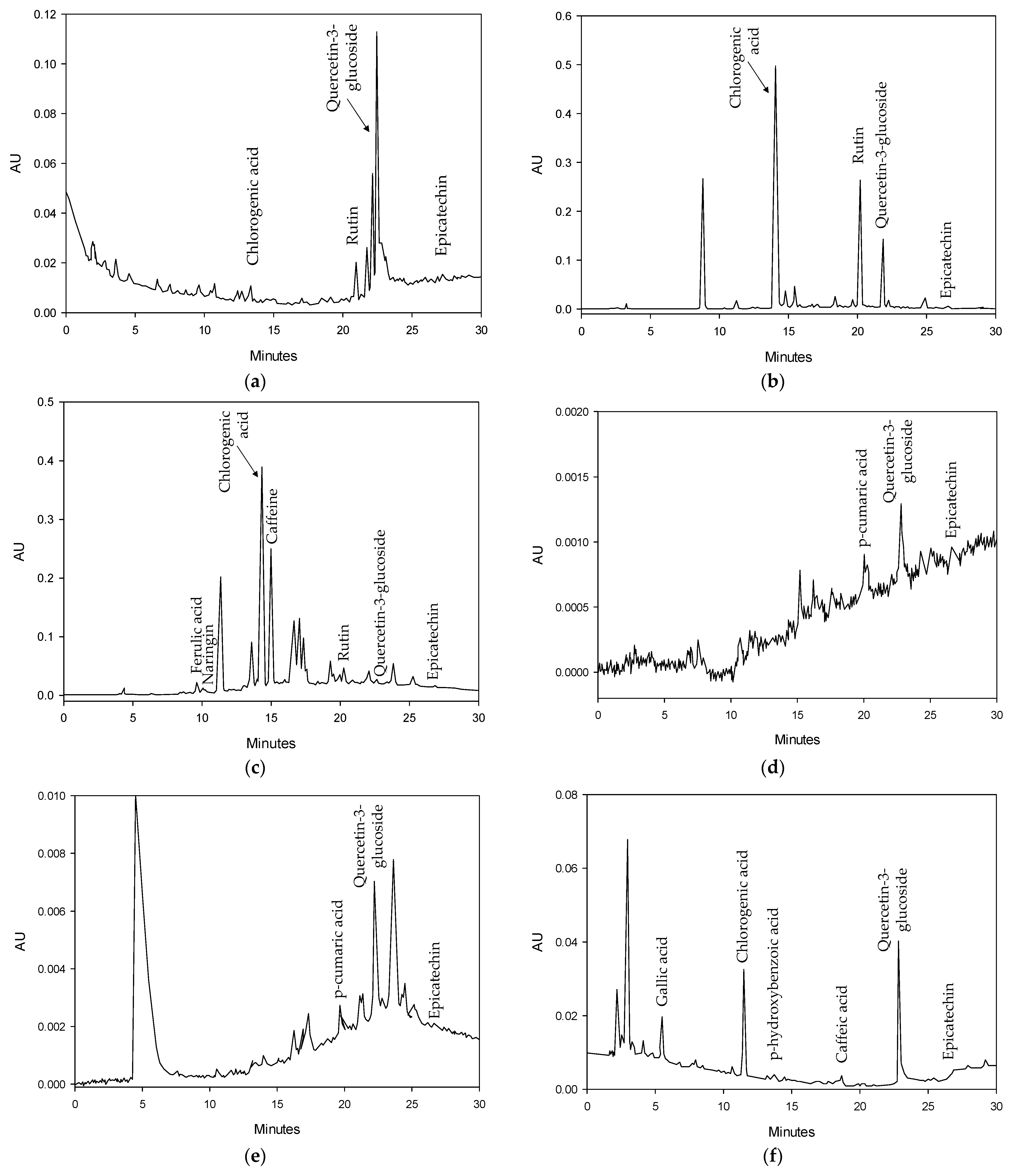
2.3.1. Effect on Bioactive Compound Extraction
2.3.2. Effect on Physical Characteristics of Agri-Food Residue Suspensions
3. Materials and Methods
3.1. Raw Materials
3.2. Chemicals
3.3. Proximate Composition Analysis of Agri-Food Residues
3.4. Conventional Solid/Liquid Extraction (SLE)
3.5. HPH-Assisted Extraction
3.6. Qualitative and Quantitative Analyses of Extracts
3.6.1. Bioactivity Determination
3.6.2. Morphological Properties
3.6.3. Particle Size Distribution
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. In Chemistry and Chemical Technologies in Waste Valorization; Topics in Current Chemistry Collections; Lin, C.S.K., Ed.; Springer: Cham, Switzerland, 2018; pp. 229–282. ISBN 978-3-319-90653-9. [Google Scholar]
- Grinberga-Zalite, G.; Zvirbule, A. Analysis of Waste Minimization Challenges to European Food Production Enterprises. Emerg. Sci. J. 2022, 6, 530–543. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- AlfredoCassano, M.; Galanakis, C. Membrane technologies for the fractionation of compounds recovered from cereal processing by-products. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 159–187. [Google Scholar]
- Narala, V.R.; Zagorska, J.; Sarenkova, I.; Ciprovica, I.; Majore, K. Acid Whey Valorization for Biotechnological Lactobionic Acid Bio-production. J. Hum. Earth Futur. 2022, 3, 46–55. [Google Scholar] [CrossRef]
- Gali, L.; Pirozzi, A.; Donsì, F. Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients. Pharmaceutics 2023, 15, 927. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Patil, S.P.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Nadar, S.S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends Food Sci. Technol. 2021, 116, 357–369. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods 2022, 11, 2454. [Google Scholar] [CrossRef]
- Mozhdeh, S.; Adel, B.; Naji-Tabasi, S. Optimizing extraction of berberine and antioxidant compounds from barberry by maceration and pulsed electric field-assisted methods. J. Berry Res. 2021, 11, 133–149. [Google Scholar] [CrossRef]
- Ghafoor, K.; Sarker, Z.I.; Al-Juhaimi, F.Y.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K.; Ahmed, I.A.M. Innovative and Green Extraction Techniques for the Optimal Recovery of Phytochemicals from Saudi Date Fruit Flesh. Processes 2022, 10, 2224. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Ultrasound-Assisted “Green” Extraction (UAE) of Antioxidant Compounds (Betalains and Phenolics) from Opuntia stricta var. Dilenii’s Fruits: Optimization and Biological Activities. Antioxidants 2021, 10, 1786. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Chen, J.; Chen, H.; Wu, Q.; Zhang, K.; Li, D.; Li, Y.; Chen, Y. Effects of high-pressure homogenization extraction on the physicochemical properties and antioxidant activity of large-leaf yellow tea polysaccharide conjugates. Process Biochem. 2022, 122, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Zhang, F.; Zheng, J. Sucrose ester alleviates the agglomeration behavior of bamboo shoot dietary fiber treated via high pressure homogenization: Influence on physicochemical, rheological, and structural properties. Food Chem. 2023, 413, 135609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, X.; Xue, F. Effects of high-pressure homogenization treatment on physiochemical properties of novel plant proteins. Appl. Food Res. 2023, 3, 100285. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Kasałka-Czarna, N.; Montowska, M.; Spychaj, A.; Mikołajczak, B. The effect of hemp cake (Cannabis sativa L.) on the characteristics of meatballs stored in refrigerated conditions. Molecules 2021, 26, 5284. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Alonso, E. Valorization of sunflower by-product using microwave-assisted extraction to obtain a rich protein flour: Recovery of chlorogenic acid, phenolic content and antioxidant capacity. Food Bioprod. Process. 2021, 125, 57–67. [Google Scholar] [CrossRef]
- Feng, X.; Sun, G.; Fang, Z. Effect of Hempseed Cake (Cannabis sativa L.) Incorporation on the Physicochemical and Antioxidant Properties of Reconstructed Potato Chips. Foods 2022, 11, 211. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A study of chemical Composition, Antioxidants, and volatile compounds in roasted Arabic coffee: Chemical Composition, Antioxidants and volatile compounds in Roasted Arabic Coffee. Saud. J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Babu, C.R.; Harsha, K.; Sheik, K.B.; Viswanatha, C.K. Wheat bran-Composition and nutritional quality: A review. Adv. Biotechnol. Microbiol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat bran phenolic acids: Bioavailability and stability in whole wheat-based foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef]
- Karami, S.; Rahimi, M.; Babaei, A. An overview on the antioxidant, anti-inflammatory, antimicrobial and anti-cancer activity of grape extract. Biomed. Res. Clin. Pract. 2018, 3, 1–4. [Google Scholar] [CrossRef][Green Version]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound extraction conditions effect on antioxidant capacity of mango by-product extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Wang, C.; Guo, J.; Wang, X.; Xu, H.; Lei, H. Synergistic effects of combinatorial Lactiplantibacillus plantarum fermentation and vegetable oils supplementation on the lycopene level, antioxidant capacities and flavor volatiles of tomato pulp. Innov. Food Sci. Emerg. Technol. 2022, 82, 103206. [Google Scholar] [CrossRef]
- Tewabe Gebeyehu, B.; Bikila, S.L. Determination of Caffeine Content and Antioxidant Activity of Coffee. Am. J. Appl. Chem. 2015, 3, 69–76. [Google Scholar] [CrossRef]
- Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and antiplatelet activities in extracts from green and fully ripe tomato fruits (Solanum lycopersicum) and pomace from industrial tomato processing. Evidence-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Kleisiaris, F.; Kadoglidou, K.; Katsantonis, D. Optimizing extraction conditions of free and bound phenolic compounds from rice by-products and their antioxidant effects. Foods 2018, 7, 93. [Google Scholar] [CrossRef]
- Lim, Y.P.; Pang, S.F.; Yusoff, M.M.; Gimbun, J. Correlation between the antioxidant, total flavonoid and total phenolic content of Phaleria Macrocarpa fruit extract. Int. J. Recent Technol. Eng. 2019, 8, 38–42. [Google Scholar]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Galvan D’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Vorobiev, E.; Louka, N.; Maroun, R.G. An Environment Friendly, Low-Cost Extraction Process of Phenolic Compounds from Grape Byproducts. Optimization by Multi-Response Surface Methodology. Food Nutr. Sci. 2013, 4, 650–659. [Google Scholar] [CrossRef]
- Coccaro, N.; Ferrari, G.; Donsì, F. Understanding the break-up phenomena in an orifice-valve high pressure homogenizer using spherical bacterial cells (Lactococcus lactis) as a model disruption indicator. J. Food Eng. 2018, 236, 60–71. [Google Scholar] [CrossRef]
- Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W pickering emulsions stabilized with cellulose nanofibrils produced through different mechanical treatments. Foods 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Wang, T.; Raddatz, J.; Chen, G. Effects of microfluidization on antioxidant properties of wheat bran. J. Cereal Sci. 2013, 58, 380–386. [Google Scholar] [CrossRef]
- Bengtsson, H.; Tornberg, E. Physicochemical characterization of fruit and vegetable fiber suspensions. I: Effect of homogenization. J. Texture Stud. 2011, 42, 268–280. [Google Scholar] [CrossRef]
- Mustafa, W.; Pataro, G.; Ferrari, G.; Donsì, F. Novel approaches to oil structuring via the addition of high-pressure homogenized agri-food residues and water forming capillary bridges. J. Food Eng. 2018, 236, 9–18. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2021, 28, 19–36. [Google Scholar] [CrossRef]
- Labaky, P.; Dahdouh, L.; Ricci, J.; Wisniewski, C.; Pallet, D.; Louka, N.; Grosmaire, L. Impact of ripening on the physical properties of mango purees and application of simultaneous rheometry and in situ FTIR spectroscopy for rapid identification of biochemical and rheological changes. J. Food Eng. 2021, 300, 110507. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC INTERNATIONAL, 22nd; Latimer, G.W., Ed.; Online Edition; AOAC Publications: New York, NY, USA, 2023; ISBN 9780197610145. [Google Scholar]
- Slinkard, K.; Singleton, V. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Fardaghi, A.A.; Es-haghi, A.; Feizy, J.; Lakshmipathy, R. Antioxidant capacity and chemical composition of different parts of saffron flowers. J. Food Bioprocess Eng. 2021, 4, 69–74. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rapisarda, P.; Tomaino, A.; Lo Cascio, R.; Bonina, F.; De Pasquale, A.; Saija, A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef] [PubMed]
- Ratnawati, R.; Wulandari, R.; Kumoro, A.C.; Hadiyanto, H. Response Surface Methodology for Formulating PVA/Starch/Lignin Biodegradable Plastic. Emerg. Sci. J. 2022, 6, 238–255. [Google Scholar] [CrossRef]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]

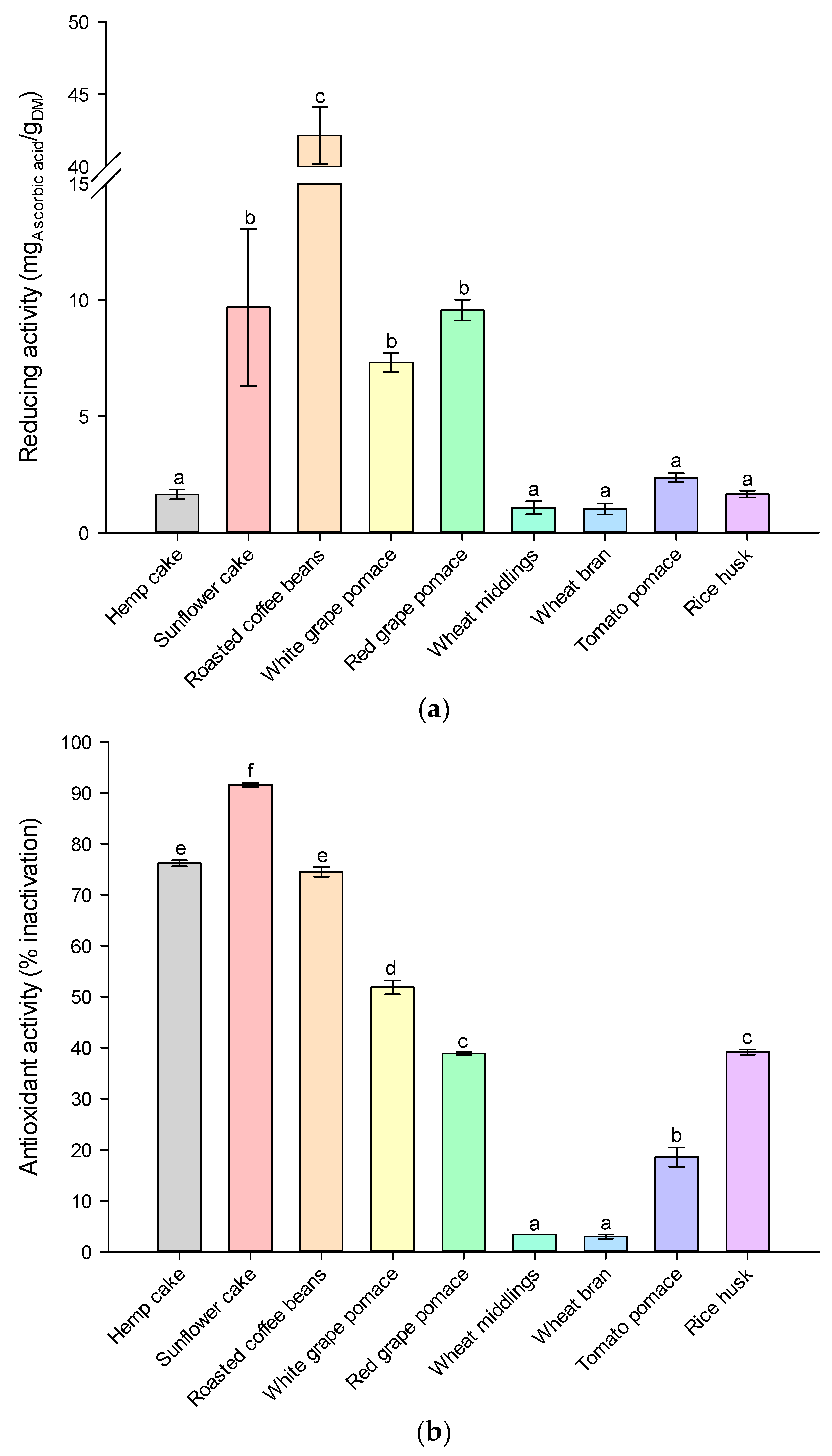
 ) SLE extraction with organic solvent at optimum operating conditions (reported in Table S3) and HPH-assisted extraction in water at (
) SLE extraction with organic solvent at optimum operating conditions (reported in Table S3) and HPH-assisted extraction in water at ( ) 5 °C, (
) 5 °C, ( ) 25 °C, and (
) 25 °C, and ( ) 50 °C. Different letters above the bars indicate significant differences among the mean values (p < 0.05).
) 50 °C. Different letters above the bars indicate significant differences among the mean values (p < 0.05).
 ) SLE extraction with organic solvent at optimum operating conditions (reported in Table S3) and HPH-assisted extraction in water at (
) SLE extraction with organic solvent at optimum operating conditions (reported in Table S3) and HPH-assisted extraction in water at ( ) 5 °C, (
) 5 °C, ( ) 25 °C, and (
) 25 °C, and ( ) 50 °C. Different letters above the bars indicate significant differences among the mean values (p < 0.05).
) 50 °C. Different letters above the bars indicate significant differences among the mean values (p < 0.05).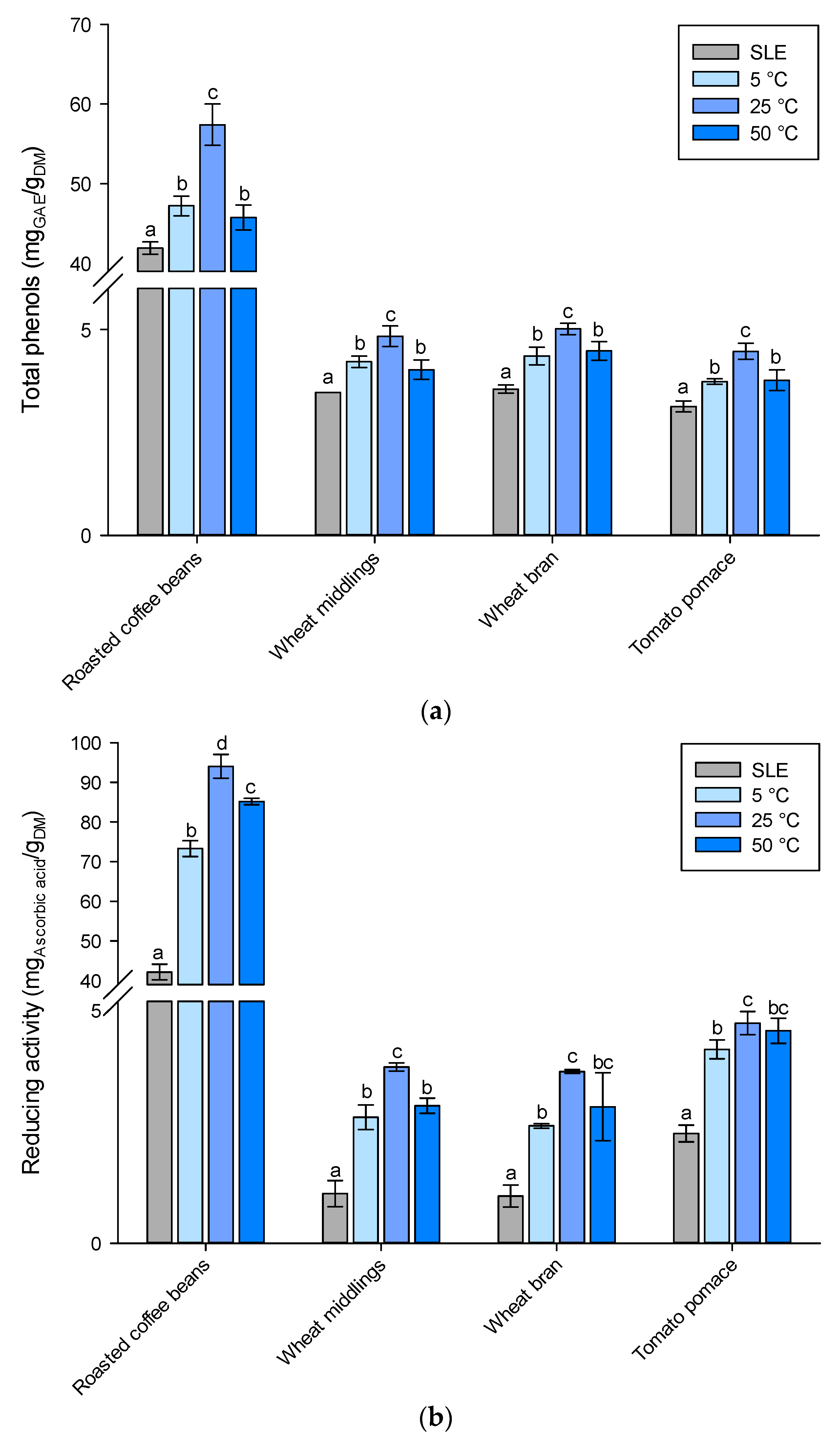
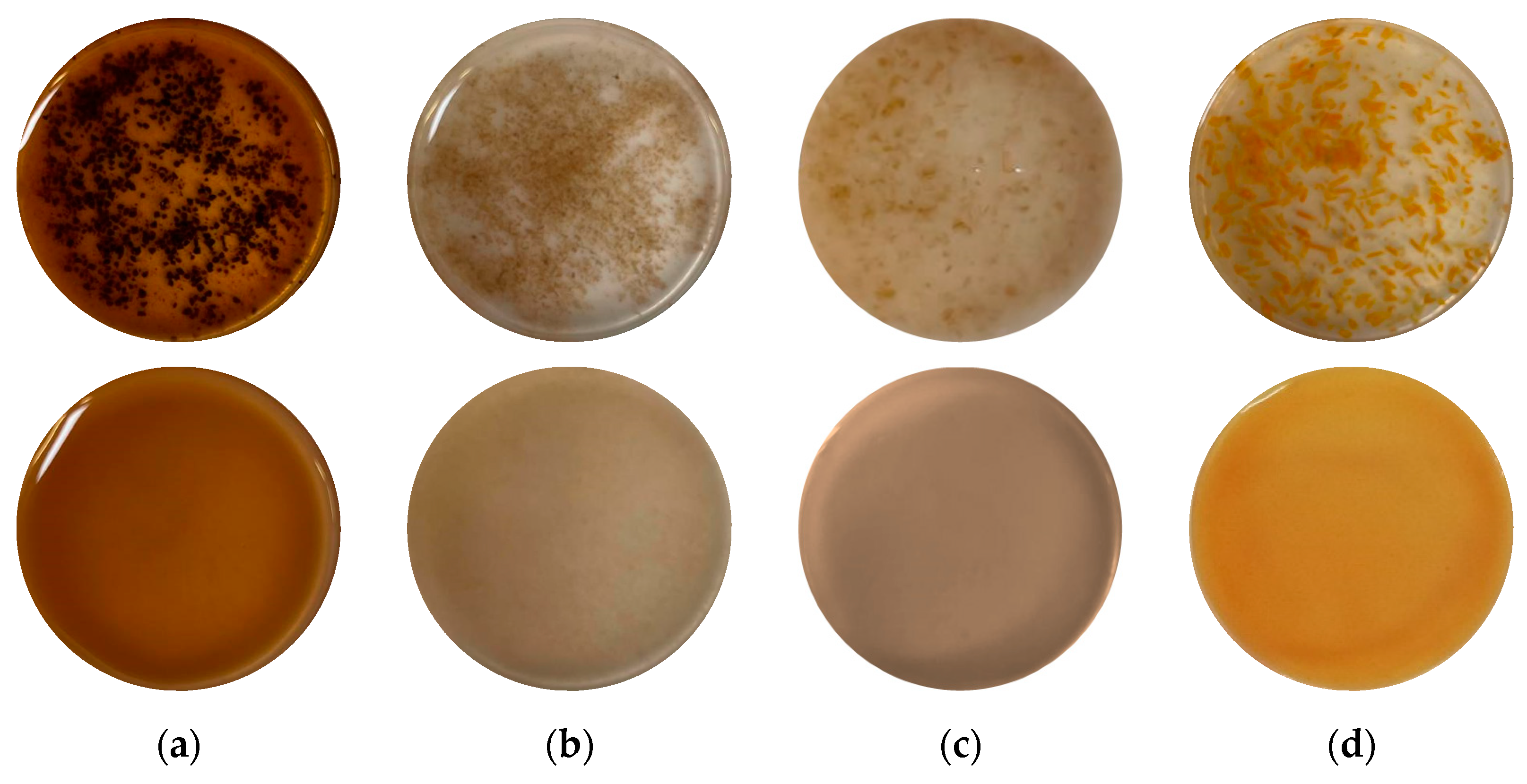
 ) HSM and (
) HSM and ( ) HPH.
) HPH.
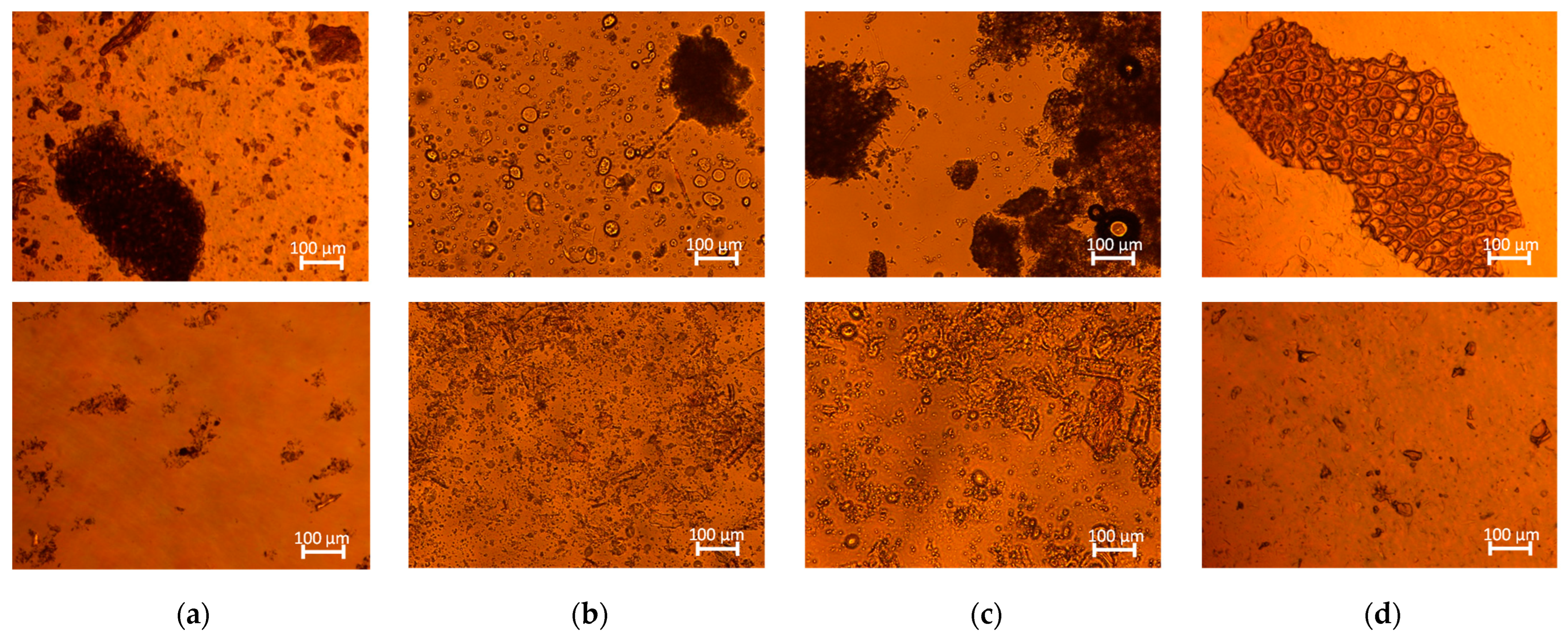
 ) HSM and (
) HSM and ( ) HPH.
) HPH.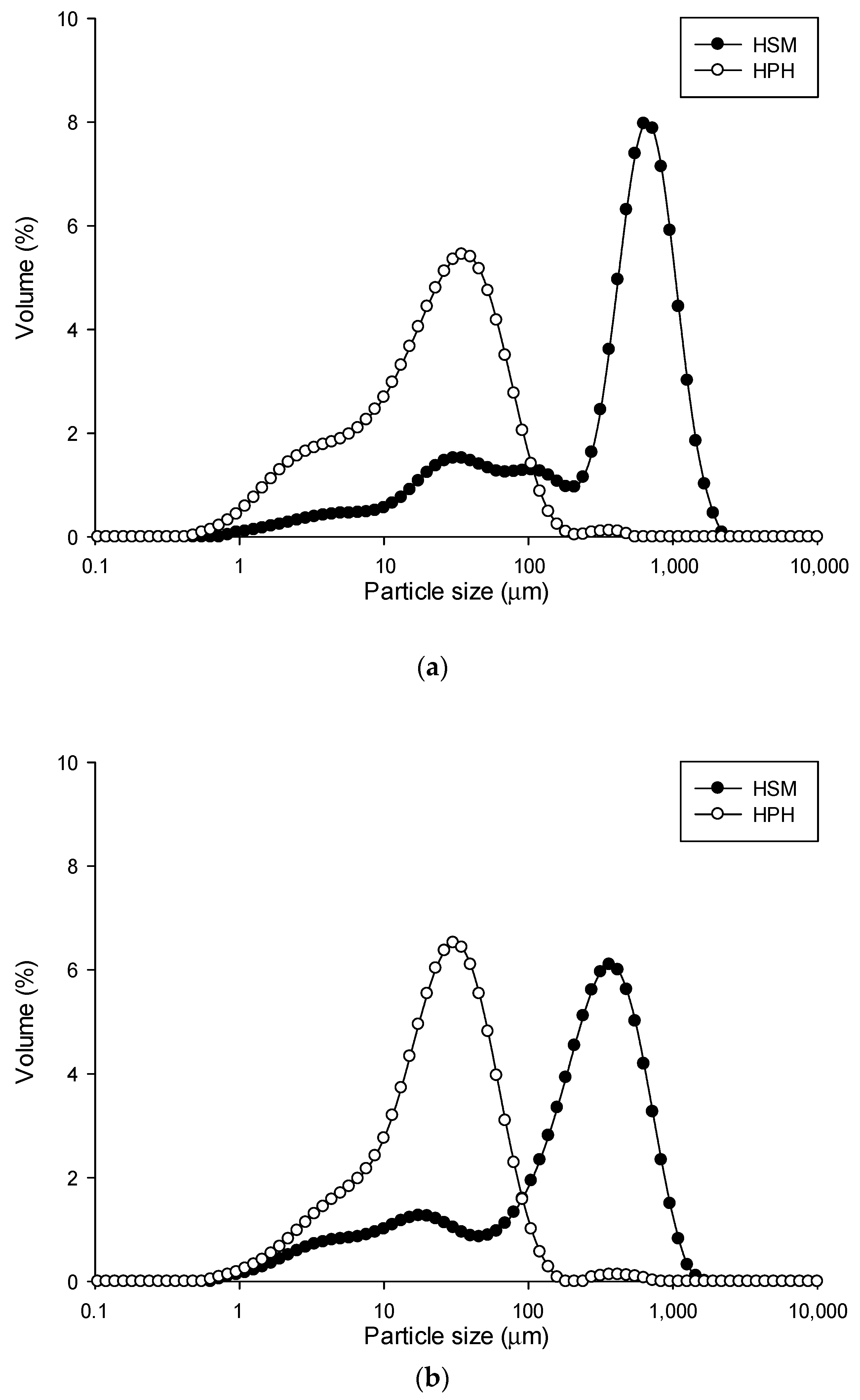

| Moisture (%) | Ash (%) | Protein (%) | Fat (%) | Carbohydrates (%) | |
|---|---|---|---|---|---|
| Hemp cake | 7.99 ± 0.06 | 6.40 ± 0.04 | 24.78 ± 0.49 | 5.30 ± 0.09 | 63.52 ± 0.49 |
| Sunflower cake | 10.75 ± 0.06 | 5.93 ± 0.03 | 24.30 ± 1.24 | 2.30 ± 0.21 | 68.47 ± 1.24 |
| Roasted coffee beans | 5.97 ± 0.04 | 5.10 ± 0.81 | 16.96 ± 0.41 | 1.00 ± 0.02 | 76.94 ± 0.91 |
| White grape pomace | 80.41 ± 1.99 | 10.52 ± 3.08 | 58.13 ± 7.95 | 1.55 ± 0.14 | 29.80 ± 9.84 |
| Red grape pomace | 62.58 ± 2.21 | 35.88 ± 5.80 | 51.88 ± 1.77 | 2.70 ± 0.33 | 9.54 ± 3.55 |
| Wheat middlings | 10.59 ± 0.01 | 3.79 ± 0.25 | 18.53 ± 1.21 | 0.79 ± 0.06 | 76.90 ± 1.23 |
| Wheat bran | 11.54 ± 0.10 | 5.83 ± 0.60 | 19.10 ± 0.40 | 0.85 ± 0.10 | 74.22 ± 0.72 |
| Tomato pomace | 80.70 ± 0.83 | 4.90 ± 0.27 | 14.65 ± 0.21 | 1.20 ± 0.14 | 79.25 ± 0.34 |
| Rice husk | 6.72 ± 0.13 | 18.71 ± 0.23 | 2.56 ± 0.25 | 0.82 ± 0.15 | 76.44 ± 0.34 |
| Treatment | d(0.1) | d(0.5) | d(0.9) | D[4,3] | D[3,2] | |
|---|---|---|---|---|---|---|
| Roasted coffee beans | HSM | 1.83 ± 0.14 | 451.45 ± 16.90 | 978.34 ± 18.41 | 468.85 ± 16.74 | 34.51 ± 2.13 |
| HPH | 2.75 ± 0.01 | 21.31 ± 0.09 | 65.03 ± 0.30 | 29.90 ± 0.42 | 7.53 ± 0.05 | |
| Wheat middlings | HSM | 8.55 ± 0.13 | 220.64 ± 3.02 | 595.37 ± 8.92 | 266.08 ± 5.17 | 21.80 ± 0.31 |
| HPH | 4.40 ± 0.05 | 22.62 ± 0.25 | 59.55 ± 0.48 | 30.68 ± 0.36 | 10.43 ± 0.14 | |
| Wheat bran | HSM | 36.28 ± 2.08 | 374.70 ± 4.63 | 898.21 ± 6.77 | 438.26 ± 3.54 | 52.85 ± 1.17 |
| HPH | 5.04 ± 0.01 | 34.41 ± 0.11 | 97.22 ± 1.30 | 47.30 ± 1.20 | 13.23 ± 0.03 | |
| Tomato pomace | HSM | 67.20 ± 0.89 | 349.43 ± 6.29 | 1089.82 ± 51.78 | 473.18 ± 16.09 | 113.74 ± 1.97 |
| HPH | 7.78 ± 0.14 | 29.97 ± 0.25 | 78.22 ± 0.50 | 38.47 ± 1.06 | 14.47 ± 0.16 |
| Run Number | Type of Solvent | Solvent–Water Mixture Ratios (% v/v) |
|---|---|---|
| 1 | Acetone (1) | 31 |
| 2 | Acetone (1) | 100 |
| 3 | Ethanol (2) | 20 |
| 4 | Ethanol (2) | 65 |
| 5 | Ethanol (2) | 59 |
| 6 | Ethanol (2) | 100 |
| 7 | Ethanol (2) | 20 |
| 8 | Ethanol (2) | 66 |
| 9 | Methanol (3) | 20 |
| 10 | Methanol (3) | 100 |
| 11 | Methanol (3) | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Donsì, F. Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues. Molecules 2023, 28, 5657. https://doi.org/10.3390/molecules28155657
Pirozzi A, Donsì F. Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues. Molecules. 2023; 28(15):5657. https://doi.org/10.3390/molecules28155657
Chicago/Turabian StylePirozzi, Annachiara, and Francesco Donsì. 2023. "Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues" Molecules 28, no. 15: 5657. https://doi.org/10.3390/molecules28155657
APA StylePirozzi, A., & Donsì, F. (2023). Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues. Molecules, 28(15), 5657. https://doi.org/10.3390/molecules28155657