Occurrence of Hydroxytyrosol, Tyrosol and Their Metabolites in Italian Cheese
Abstract
:1. Introduction
2. Results
2.1. Method Development
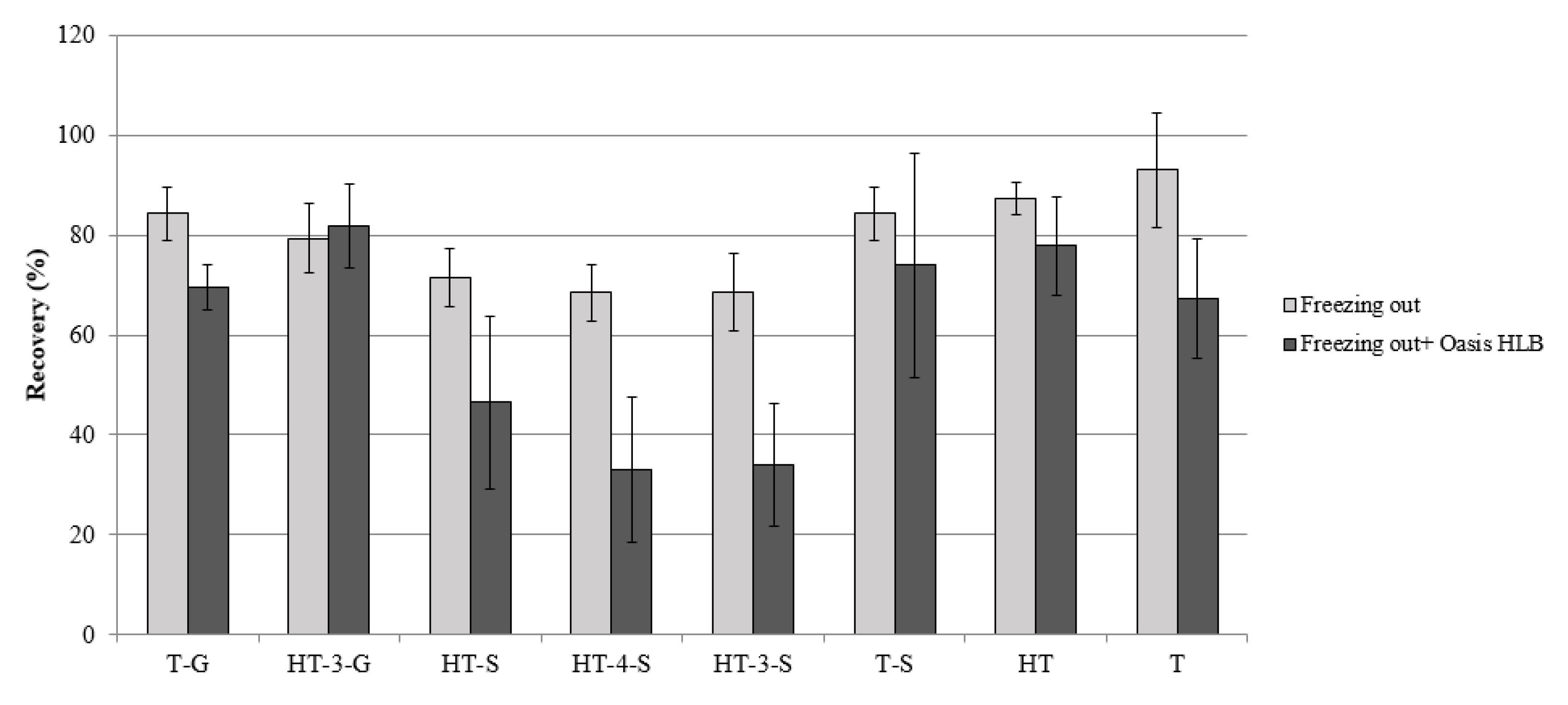
2.2. Method Validation
2.3. Analysis of Real Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. LC-MS Conditions
4.4. Validation Procedure
4.5. Analysis of Commercial Cheeses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Commission Regulation (EU) No 432/2012 of 16 May 2012. Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: http://data.europa.eu/eli/reg/2012/432/oj (accessed on 8 August 2023).
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobušic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Berbel, J.; Posadillo, A. Review and Analysis of Alternatives for the Valorisation of Agro-Industrial Olive Oil By-Products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Zorić, N.; Kosalec, I. The Antimicrobial Activities of Oleuropein and Hydroxytyrosol. In Promising Antimicrobials from Natural Products; Rai, M., Kosalec, I., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 75–89. ISBN 978-3030835033. [Google Scholar]
- Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Trabalza-Marinucci, M.; Miraglia, D.; Roila, R.; Acuti, G.; Giusepponi, D.; Dal Bosco, A.; Ranucci, D. Effects of Olive Mill Vegetation Water Phenol Metabolites Transferred to Muscle through Animal Diet on Rabbit Meat Microbial Quality. Sustainability 2021, 13, 4522. [Google Scholar] [CrossRef]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Rodríguez-Morat, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Maña, C.; Khymenets, O.; Fitó, M.; de la Torre, R. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- Hilario, M.C.; Puga, C.D.; Ocaña, A.N.; Romo, F.P. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef]
- Velázquez Vázquez, C.; Villa Rojas, M.G.; Alvarez Ramírez, C.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin-Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Servín, J.L.; Andrade-Montemayor, H.M.; Velázquez Vázquez, C.; Aguilera Barreyro, A.; García-Gasca, T.; Ferríz Martínez, R.A.; Olvera Ramírez, A.M.; de la Torre-Carbot, K. Effects of feeding system, heat treatment and season on phenolic compounds and antioxidant capacity in goat milk, whey and cheese. Small Rumin. Res. 2018, 160, 54–58. [Google Scholar] [CrossRef]
- Claps, S.; Rossi, R.; Di Trana, A.; Di Napoli, M.A.; Giorgio, D.; Sepe, L. Bioactive compounds in goat milk and cheese: The role of feeding system and breed. In Goat Science; Kukovics, S., Ed.; IntechOpen: London, UK, 2018; pp. 233–263. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kerasioti, E.; Sidiropoulos, K.; Maragou, I.; Skaperda, Z.; Kouretas, D. Nutritional habits and free grazing regimen of productive animals along with specific ingredients are influential factors for the antioxidant properties of milk: From farm to market. Biomed. Rep. 2020, 13, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Székelyhidi1, R.; Lakatos, E.; Kapcsándi, V.; Ajtony, Z. Analytical procedures for determination of phenolics active herbal ingredients in fortified functional foods: An overview. Eur. Food Res. Technol. 2022, 248, 329–344. [Google Scholar] [CrossRef]
- Vousdouka, V.I.; Papapanagiotou, E.P.; Angelidis, A.S.; Fletouris, D.J. Rapid ion-pair liquid chromatographic method for the determination of fenbendazole marker residue in fermented dairy products. Food Chem. 2017, 221, 884–890. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Analysis of oestrogenic compounds in dairy products by hollow-fibre liquid-phase microextraction coupled to liquid chromatography. Food Chem. 2014, 149, 319–325. [Google Scholar] [CrossRef]
- Veršilovskis, A.; Van Peteghem, C.; De Saeger, S. Determination of sterigmatocystin in cheese by high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2009, 26, 127–133. [Google Scholar] [CrossRef]
- Xie, J.; Peng, T.; Zhu, A.; He, J.; Chang, Q.; Hu, X.; Chen, H.; Fan, C.; Jiang, W.; Chen, M.; et al. Multi-residue analysis of veterinary drugs, pesticides and mycotoxins in dairy products by liquid chromatography–tandem mass spectrometry using low-temperature cleanup and solid phase extraction. J. Chromat. B 2015, 1002, 19–29. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Cieślik, E.; Sieja, K. Simultaneous Sample Preparation Method for Determination of 3-Monochloropropane-1,2-Diol and Polycyclic Aromatic Hydrocarbons in Different Foodstuffs. Food Anal. Methods 2016, 9, 2906–2916. [Google Scholar] [CrossRef]
- De Souza Santos Cheibub, A.M.; Bahiense de Lyra, E.S.; Pereira Netto, A.D. Development and validation of a method for simultaneous determination of trace levels of five macrocyclic lactones in cheese by HPLC-fluorescence after solid–liquid extraction with low temperature partitioning. Food Chem. 2019, 272, 148–156. [Google Scholar] [CrossRef]
- Carmical, J.; Brown, S. The impact of phospholipids and phospholipid removal on bioanalytical method performance. Biomed. Chromatogr. 2016, 30, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Goya, L.; Bravo, L. Metabolism of the Olive Oil Phenols Hydroxytyrosol, Tyrosol, and Hydroxytyrosyl Acetate by Human Hepatoma HepG2 Cells. J. Agric. Food Chem. 2005, 53, 9897–9905. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Carbot, K.; Jauregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Covas, M.I.; Casals, I.; López-Sabater, M.C. Rapid high-performance liquid chromatography-electrospray ionization tandem mass spectrometry method for qualitative and quantitative analysis of virgin olive oil phenolic metabolites in human low-density lipoproteins. J. Chromatogr. A 2006, 1116, 69–75. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.P.; Macià, A.; Valls, R.M.; Fernández, S.; Solà, R.; Motilva, M.J. Improved method for identifying and quantifying olive oil phenolic compounds and their metabolites in human plasma by microelution solid-phase extraction plate and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2009, 877, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Valls, R.M.; Romero, M.P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Macià, A.; Valls, R.M.; Catalán, Ú.; de la Torre, R.; Motilva, M.J. Application of dried spot cards as a rapid sample treatment method for determining hydroxytyrosol metabolites in human urine samples. Comparison with microelution solid-phase extraction. Anal. Bioanal. Chem. 2013, 405, 9179–9192. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Farré, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I.; Solà, R.; Farré, M.; Saez, G.; de la Torre, R. Direct analysis of glucoronidated metabolites of main olive phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubio, L.; de la Torre, R. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef]
- Khymenets, O.; Crespo, M.; Dangles, O.; Njara, R.; Andres-Lacueva, C.; Visioli, F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.J.; Kim, M.J.; Ahn, J.; Ha, T.Y.; Lee, S.H.; Jang, Y.J.; Jung, C.H. Pharmacokinetics of Tyrosol Metabolites in Rats. Molecules 2016, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, A.; Fança-Berthon, P.; Roller, M.; Zafrilla, P.; Issaly, N.; García-Conesa, M.-T. Targeted and Untargeted Metabolomics to Explore the Bioavailability of the Secoiridoids from a Seed/Fruit Extract (Fraxinus angustifolia Vahl) in Human Healthy Volunteers: A Preliminary Study. Molecules 2015, 20, 22202–22219. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo Puga, C.; Cuchillo-Hilario, M.; Navarro Ocaña, A.; Medina-Campos, O.N.; Nieto Camacho, A.; Ramírez Apan, T.; López-Tecpoyotl, Z.G.; Díaz Martínez, M.; Álvarez-Izazaga, M.A.; Cruz Martínez, Y.R.; et al. Phenolic Compounds in Organic and Aqueous Extracts from Acacia farnesiana Pods Analyzed by ULPS-ESI-Q-oa/TOF-MS. In Vitro Antioxidant Activity and Anti-Inflammatory Response in CD-1 Mice. Molecules 2018, 23, 2386. [Google Scholar] [CrossRef]
- Roca, M.; Pérez-Gálvez, A. Profile of Chlorophyll Catabolites in Senescent Leaves of Epipremnum aureum Includes a Catabolite Esterified with Hydroxytyrosol 1-O-Glucoside. J. Nat. Prod. 2020, 83, 873–880. [Google Scholar] [CrossRef]
- Kim, G.-R.; Kim, E.-N.; Park, K.J.; Kim, K.H.; Jeong, G.-S. Inhibitory Effect of LGS and ODE Isolated from the Twigs of Syringa oblata subsp. dilatata on RANKL-Induced Osteoclastogenesis in Macrophage Cells. Molecules 2021, 26, 1779. [Google Scholar] [CrossRef]
- Kołtun-Jasion, M.; Sawulska, P.; Patyra, A.; Woźniak, M.; Dudek, M.K.; Filipek, A.; Kiss, A.K. Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 3750. [Google Scholar] [CrossRef]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic Profile and Susceptibility to Fusarium Infection of Pigmented Maize Cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Rubió, L.; Valls, R.M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.P.; de la Torre, R.; Covas, M.I.; Solà, R.; Motilva, M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Rubió, L.; Kotronoulas, A.; de la Torre, R.; Solà, R.; Motilva, M.J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Ornemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Gembloux, Belgium, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- McSweeney, P.L.H.; Ottogalli, G.; Fox, P.F. Diversity and Classification of Cheese Varieties: An Overview. In Cheese: Chemistry, Physics and Microbiology; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 781–808. [Google Scholar] [CrossRef]

| Analyte 1 | Spiking Concentration (µg/kg dw) | Recovery (%) ± SD (%) | CVr (%) 2 | CVwR (%) 2 |
|---|---|---|---|---|
| HT-3-G | 15 | 73 ± 10 | 14 | 14 |
| 80 | 71 ± 6 | 8.7 | 8.7 | |
| 150 | 75 ± 8 | 9.2 | 10 | |
| 750 | 77 ± 12 | 10 | 18 | |
| HT-S | 15 | 63 ± 13 | 18 | 22 |
| 80 | 65 ± 8 | 13 | 13 | |
| 150 | 62 ± 5 | 6.0 | 8 | |
| 750 | 62 ± 7 | 8.1 | 11 | |
| T-G | 15 | 78 ± 13 | 13 | 17 |
| 80 | 76 ± 9 | 12 | 12 | |
| 150 | 73 ± 7 | 8.3 | 9.1 | |
| 750 | 76 ± 9 | 8.5 | 13 | |
| HT-4-S | 15 | 79 ± 6 | 7.7 | 7.7 |
| 80 | 72 ± 7 | 8.5 | 9.4 | |
| 150 | 70 ± 6 | 6.2 | 8.9 | |
| 750 | 68 ± 6 | 8.5 | 8.5 | |
| HT-3-S | 15 | 75 ± 5 | 5.2 | 7.2 |
| 80 | 75 ± 7 | 9.8 | 9.8 | |
| 150 | 67 ± 4 | 5.5 | 6.9 | |
| 750 | 67 ± 8 | 7.1 | 13 | |
| T-S | 15 | 81 ± 9 | 5.9 | 12 |
| 80 | 81 ± 9 | 8.2 | 12 | |
| 150 | 75 ± 7 | 6.2 | 10 | |
| 750 | 66 ± 6 | 7.6 | 8.4 | |
| HT | 15 | 86 ± 15 | 11 | 19 |
| 80 | 89 ± 7 | 5.9 | 8.6 | |
| 150 | 81 ± 10 | 7.4 | 13 | |
| 750 | 72 ± 7 | 7.6 | 10 | |
| T | 150 | 89 ± 14 | 8.7 | 17 |
| 800 | 86 ± 6 | 7.4 | 6.5 | |
| 1500 | 81 ± 6 | 6.9 | 7.9 | |
| 7500 | 73 ± 5 | 6.2 | 7.8 |
| Sample | Parameter 1 | Frequency (%) | Mean | Median 2 | Min | Max |
|---|---|---|---|---|---|---|
| Ewe cheese (19) | Moisture | - | 33.25 | 36.06 | 17.66 | 40.19 |
| HT-4-S | 100 | 279 | 213 a | 91 | 816 | |
| HT-3-S | 100 | 158 | 153 a | 75 | 263 | |
| T-S | 63 | 10 | 9 | <LOD | 33 | |
| T | 47 | 68 | 25 | <LOD | 273 | |
| Sum | - | 506 | 455 a | 224 | 1130 | |
| Goat cheese (4) | Moisture | - | 54.51 | 57.24 | 38.05 | 65.50 |
| HT-4-S | 100 | 28 | 24 ab | 12 | 52 | |
| HT-3-S | 75 | 12 | 11 ab | <LOD | 23 | |
| T-S | 100 | 11 | 11 | 7.8 | 14 | |
| T | 75 | 941 | 722 | <LOD | 2296 | |
| Sum | - | 985 | 776 ab | 40 | 2349 | |
| Cow cheese (13) | Moisture | - | 34.44 | 33.83 | 23.49 | 45.73 |
| HT-4-S | 46 | 7 | <LOD b | <LOD | 27 | |
| HT-3-S | 15 | <LOD | <LOD b | <LOD | 11 | |
| T-S | 77 | 14 | 12 | <LOD | 30 | |
| T | 54 | 277 | 60 | <LOD | 1593 | |
| Sum | - | 287 | 60 b | <5 | 1621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giusepponi, D.; Barola, C.; Bucaletti, E.; Moretti, S.; Paoletti, F.; Valiani, A.; Branciari, R.; Galarini, R. Occurrence of Hydroxytyrosol, Tyrosol and Their Metabolites in Italian Cheese. Molecules 2023, 28, 6204. https://doi.org/10.3390/molecules28176204
Giusepponi D, Barola C, Bucaletti E, Moretti S, Paoletti F, Valiani A, Branciari R, Galarini R. Occurrence of Hydroxytyrosol, Tyrosol and Their Metabolites in Italian Cheese. Molecules. 2023; 28(17):6204. https://doi.org/10.3390/molecules28176204
Chicago/Turabian StyleGiusepponi, Danilo, Carolina Barola, Elisabetta Bucaletti, Simone Moretti, Fabiola Paoletti, Andrea Valiani, Raffaella Branciari, and Roberta Galarini. 2023. "Occurrence of Hydroxytyrosol, Tyrosol and Their Metabolites in Italian Cheese" Molecules 28, no. 17: 6204. https://doi.org/10.3390/molecules28176204
APA StyleGiusepponi, D., Barola, C., Bucaletti, E., Moretti, S., Paoletti, F., Valiani, A., Branciari, R., & Galarini, R. (2023). Occurrence of Hydroxytyrosol, Tyrosol and Their Metabolites in Italian Cheese. Molecules, 28(17), 6204. https://doi.org/10.3390/molecules28176204








