Validation of an Eco-Friendly Automated Method for the Determination of Glucose and Fructose in Wines
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Parameters and Measurement Ranges
3.2. Samples
3.3. Chemicals
3.4. Enzymatic Method
3.4.1. Operating Modes
Reference Method OIV-MA-AS311-02 (Manual Method)
Automated Method OIV-MA-AS311-02
3.5. Method Validation
- The range of acceptability = C ± (0.056 × C)
- C = nominal concentration of the standard
- (0.056 × C) = method repeatability.
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barbosa, C.; Ramalhosa, E.; Vasconcelos, I.; Reis, M.; Mendes-Ferreira, A. Machine Learning Techniques Disclose the Combined Effect of Fermentation Conditions on Yeast Mixed-Culture Dynamics and Wine Quality. Microorganisms 2022, 10, 107. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 607/2009 Laying down certain detailed rules for the implementation of Council Regulation (EC) No 479/2008 as regards protected designations of origin and geographical indications, traditional terms, labelling and presentation of certain wine sector products. J. Eur. Union 2009, 22, 60–139.
- Vyshkvarkova, E.; Rybalko, E.; Marchukova, O.; Baranova, N. Assessment of the Current and Projected Conditions of Water Availability in the Sevastopol Region for Grape Growing. Agronomy 2021, 11, 1665. [Google Scholar] [CrossRef]
- Ubeda, C.; Hornedo-Ortega, R.; Cerezo, A.B.; Garcia-Parrilla, M.C.; Troncoso, A.M. Chemical hazards in grapes and wine, climate Change and challenges to face. Food Chem. 2020, 314, 126222. [Google Scholar] [CrossRef]
- Beech, N.; Hewer, M.J. A Climate Change Impact Assessment (CCIA) of Key Indicators and Critical Thresholds for Viticulture and Oenology in the Fraser Valley, British Columbia, Canada. Weather Clim. Soc. 2021, 13, 687–705. [Google Scholar] [CrossRef]
- Neethling, E.; Petitjean, T.; Quénol, H.; Barbeau, G. Assessing local climate vulnerability and winegrowers’ adaptive processes in the context of climate change. Mitig. Adapt. Strateg. Glob. Chang. 2017, 22, 777–803. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press: Waltham, MA, USA, 2008; p. 751. [Google Scholar]
- Sereni, A.; Osborne, J.; Tomasino, E. Exploring Retro-Nasal Aroma’s Influence on Mouthfeel Perception of Chardonnay Wines. Beverages 2016, 2, 7. [Google Scholar] [CrossRef]
- Portaro, L.; Maioli, F.; Canuti, V.; Picchi, M.; Lencioni, L.; Mannazzu, I.; Domizio, P. Schizosaccharomyces japonicus/Saccharomyces cerevisiae mixed starter cultures: New perspectives for the improvement of Sangiovese aroma, taste, and color stability. LWT 2022, 156, 113009. [Google Scholar] [CrossRef]
- Straathof, A.J. Modelling of end-product inhibition in fermentation. Biochem. Eng. J. 2023, 191, 108796. [Google Scholar] [CrossRef]
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Tronchoni, J.; Gamero, A.; Arroyo-López, F.N.; Barrio, E.; Querol, A. Differences in the glucose and fructose consumption profiles in diverse Saccharomyces wine species and their hybrids during grape juice fermentation. Int. J. Food Microbiol. 2009, 134, 237–243. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W., AOAC International, Eds.; AOAC International: Gaithersburg, MD, USA, 2019; Volume 3. [Google Scholar]
- Parpinello, G.P.; Rombolà, A.D.; Simoni, M.; Versari, A. Chemical and sensory characterisation of Sangiovese red wines: Comparison between biodynamic and organic management. Food Chem. 2015, 167, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Seccia, S.; Senatore, A.; Coppola, D.; Morelli, E. Development and Validation of an Analytical Method for Total Polyphenols Quantification in Extra Virgin Olive Oils. Food Anal. Methods 2019, 13, 457–464. [Google Scholar] [CrossRef]
- Dini, I.; Di Lorenzo, R.; Senatore, A.; Coppola, D.; Laneri, S. Comparison between Mid-Infrared (ATR-FTIR) Spectroscopy and Official Analysis Methods for Determination of the Concentrations of Alcohol, SO2, and Total Acids in Wine. Separations 2021, 8, 191. [Google Scholar] [CrossRef]
- Cosenza, G.; Pauciullo, A.; Mancusi, A.; Nicodemo, D.; Di Palo, R.; Zicarelli, L.; Di Berardino, D.; Ramunno, L. Mediterranean river buffalooxytocin-neurophysin I (OXT) gene: Structure, promoter analysis and allele detection. Ital. J. Anim. Sci. 2007, 6, 303–306. [Google Scholar] [CrossRef]
- International Code of Oenological Practices. Available online: https://www.oiv.int/public/medias/3570/e-code-ii-358.pdf (accessed on 10 March 2022).
- Montone, A.M.I.; Capuano, F.; Mancusi, A.; Di Maro, O.; Peruzy, M.F.; Proroga, Y.T.R.; Cristiano, D. Exposure to Bacillus cereus in Water Buffalo Mozzarella Cheese. Foods 2020, 9, 1899. [Google Scholar] [CrossRef]
- Dini, I.; Senatore, A.; Coppola, D.; Mancusi, A. Validation of a rapid test to dose SO2 in vinegar. AIMS Agric. Food. 2023, 8, 1–24. [Google Scholar] [CrossRef]
- Ghernaout, D.; Aichouni, M.; Alghamdi, A. Overlapping ISO/IEC 17025: 2017 into big data: A review and perspectives. Inter. J.Sci. Qual. Anal. 2018, 4, 83–92. [Google Scholar]
- Dini, I.; Di Lorenzo, R.; Senatore, A.; Coppola, D.; Laneri, S. Validation of Rapid Enzymatic Quantification of Acetic Acid in Vinegar on Automated Spectrophotometric System. Foods 2020, 9, 761. [Google Scholar] [CrossRef]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Standard Organization (ISO): Geneva, Switzerland, 2005.
- Misra, S.; Huddy, J.; Hanna, G.; Oliver, N. Validation and regulation of point of care devices for medical applications. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–44. [Google Scholar]
- Best, H.; Wolf, C.; Meuleman, B.; Loosveldt, G.; Emonds, V. Regression Analysis: Assumptions and Diagnostics; SAGE: Newcastle upon Tyne, UK, 2014; pp. 83–110. [Google Scholar]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
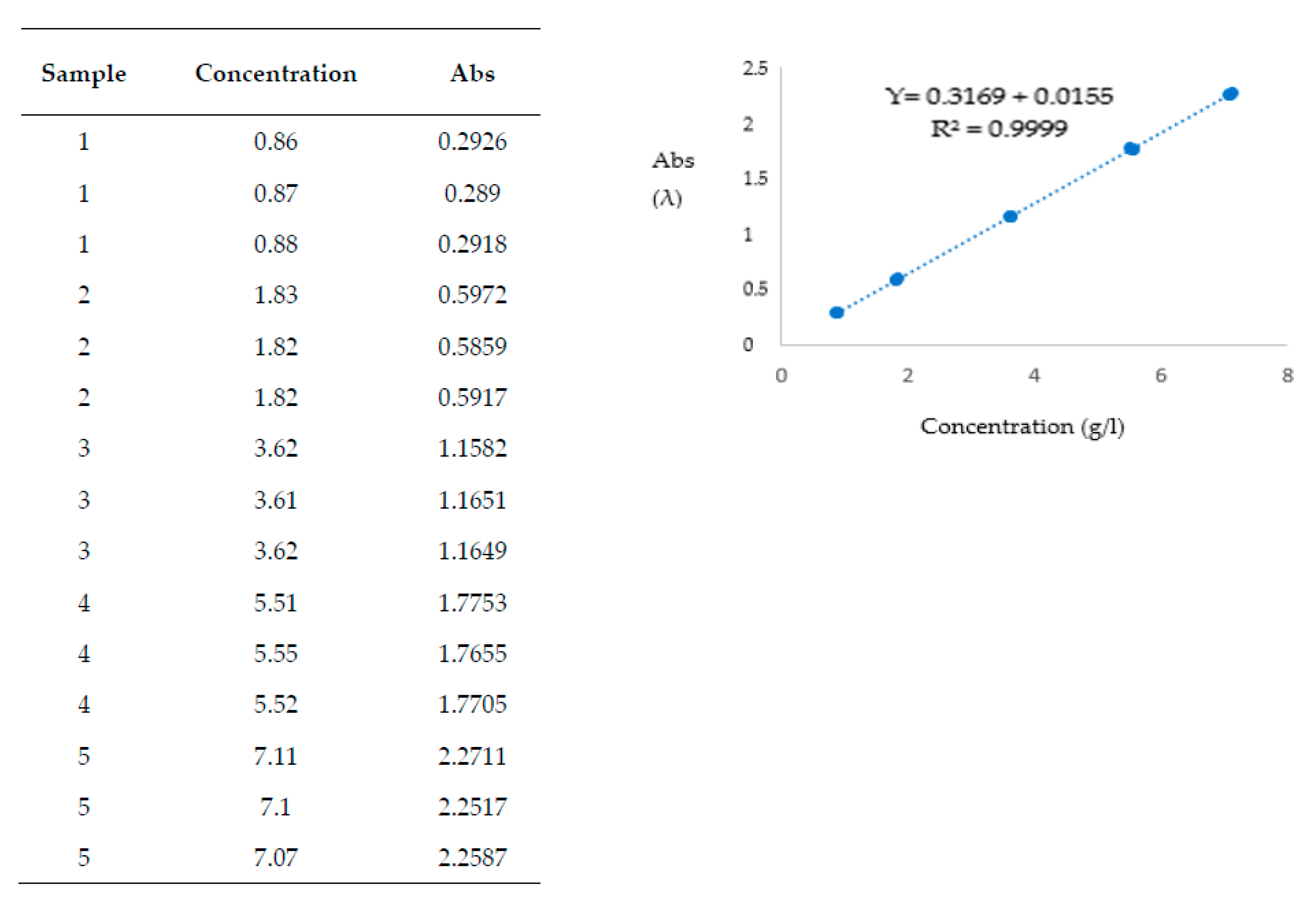
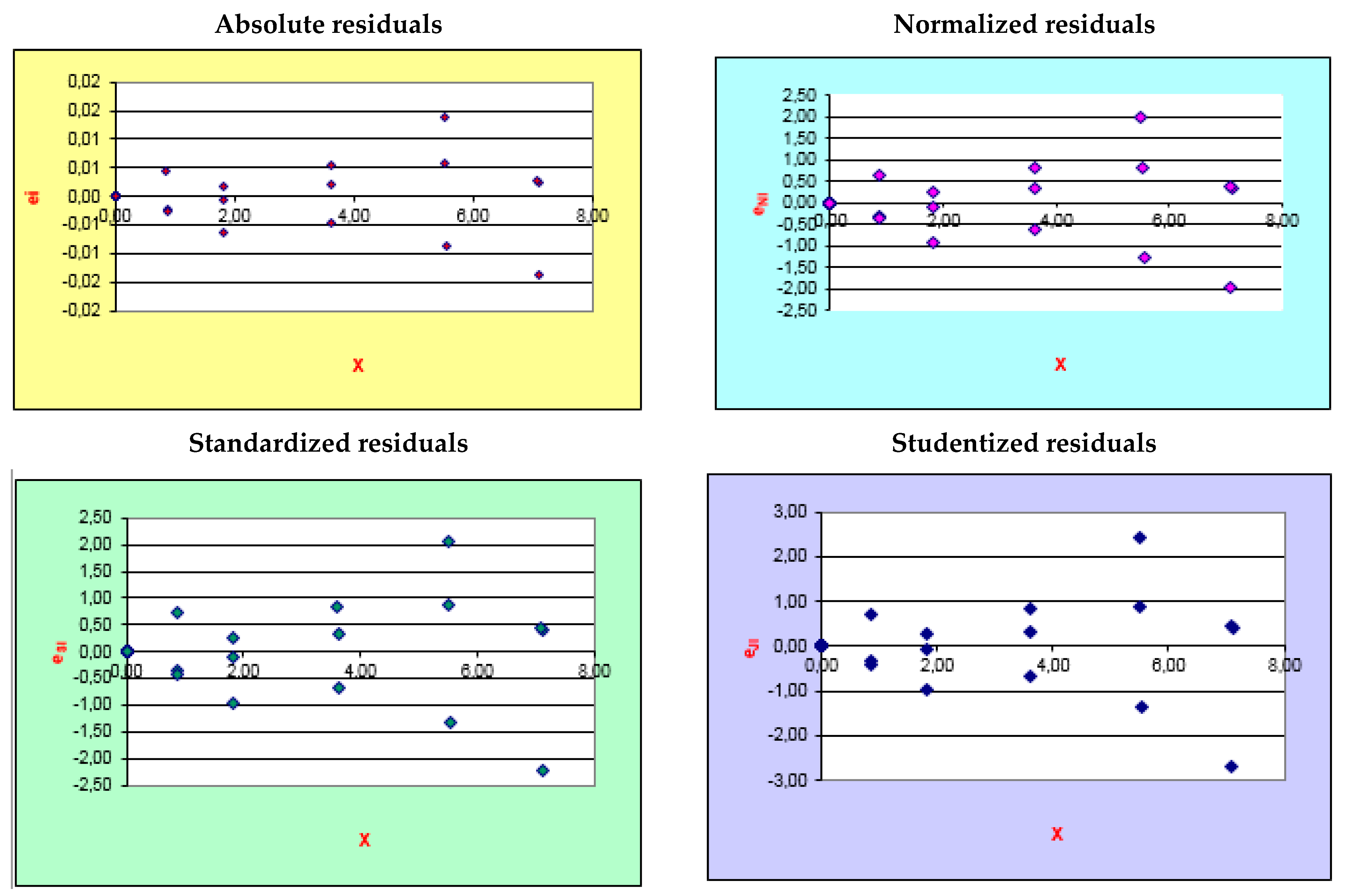
| DRY RED WINE | ||
|---|---|---|
| Automated method Abs | Manual method Abs | |
| 2.64 | 2.447 | |
| 2.655 | 2.48 | |
| 2.621 | 2.44 | |
| 2.624 | 2.435 | |
| 2.643 | 2.402 | |
| 2.652 | 2.355 | |
| 2.622 | 2.434 | |
| 2.579 | 2.373 | |
| 2.603 | 2.398 | |
| 2.587 | 2.405 | |
| Automated method | Manual method | |
| Average (xm) | 2.623 | 2.417 |
| Standard deviation | 0.026 | 0.037 |
| Repeatability | 0.105 | |
| Degrees of freedom | 18 | |
| Method repeatability | 0.135 | |
| r/r(M) | 0.779 | |
| Method reproducibility | 0.304 | |
| Uncertainty | 0.429 | |
| T experimental | 13.534 | |
| P | 0.000 | |
| T critical | 2.100922 | |
| DRY WHITE WINE | ||
| Automated method Abs | Manual method Abs | |
| 1.678 | 1.679 | |
| 1.658 | 1.66 | |
| 1.651 | 1.661 | |
| 1.652 | 1.639 | |
| 1.65 | 1.725 | |
| 1.637 | 1.623 | |
| 1.64 | 1.726 | |
| 1.636 | 1.745 | |
| 1.663 | 1.756 | |
| 1.64 | 1.738 | |
| Automated method | Manual method | |
| Average (xm) | 1.651 | 1.695 |
| Standard deviation | 0.013 | 0.048 |
| Repeatability | 0.136 | |
| Degrees of freedom | 18 | |
| Method repeatability | 0.095 | |
| r/r(M) | 1.435 | |
| Method reproducibility | 0.249 | |
| Uncertainty | 0.352 | |
| T experimental | 2.684 | |
| P | 0.000 | |
| T critical | 2.10092204 | |
| MODERATELY SWEET WINE | ||
| Automated method Abs | Manual method Abs | |
| 7.08 | 7.187 | |
| 7.051 | 7.256 | |
| 6.986 | 7.235 | |
| 6.95 | 7.137 | |
| 6.919 | 7.29 | |
| 6.955 | 7.101 | |
| 6.959 | 7.317 | |
| 7.053 | 7.243 | |
| 6.995 | 7.378 | |
| 7.012 | 7.346 | |
| Automated method | Manual method | |
| Average (xm) | 6.996 | 7.249 |
| Standard deviation | 0.052 | 0.089 |
| Repeatability | 0.251 | |
| Degrees of freedom | 18 | |
| Method repeatability | 0.406 | |
| r/r(M) | 0.619 | |
| Method reproducibility | 0.671 | |
| Uncertainty | 0.949 | |
| T experimental | 7.360 | |
| P | 0.000 | |
| T critical | 2.100922 | |
| SWEET WINE | ||
| Automated method Abs | Manual method Abs | |
| 31.767 | 32.541 | |
| 31.09 | 33.125 | |
| 30.262 | 32.823 | |
| 31.793 | 32.979 | |
| 31.325 | 32.751 | |
| 31.583 | 31.077 | |
| 31.124 | 34.02 | |
| 31.197 | 31.683 | |
| 31.582 | 31.463 | |
| 31.912 | 32.483 | |
| Automated method | Manual method | |
| Average (xm) | 31.364 | 32.495 |
| Standard deviation (S) | 0.485 | 0.874 |
| Repeatability | 2.471 | |
| Degrees of freedom | 18 | |
| Method repeatability | 1.820 | |
| r/r(M) | 1.358 | |
| Method reproducibility | 2.590 | |
| Uncertainty | 3.662 | |
| T experimental | 3.396 | |
| P | 0.003 | |
| T critical | 2.100922 | |
| Sample | Concentration g/L | T-Test Result | Uncertainty | ||
|---|---|---|---|---|---|
| Automated Method | Manual Method | ||||
| 1 | red wine | <5 g/L | Significant difference | 0.052 | 0.074 |
| 2 | white wine | <5 g/L | Significant difference | 0.026 | 0.096 |
| 3 | moderately sweet wine | 5–12 g/L | Significant difference | 0.104 | 0.356 |
| 4 | sweet wine | >5 g/L | Significant difference | 0.97 | 1.748 |
| Event | Possible Causes | Decision To Be Taken |
|---|---|---|
| A point is out of control limits. | The inexperience of the operator’s ex-pired check or incorrect conservation of the same. | Repeat the analysis; if the point is within the limit of control continues, otherwise stop, locate, and resolve the cause. |
| Seven consecutive points are above or below the central line | Defective kit control or incorrect con-servation of the same | If the eighth point falls on the side opposite to the line central continue, otherwise stop, locate, and resolve the cause. |
| Seven consecutive points are in ascending order (derive positive) | Obsolescence of reagents, progressive evaporation of solvent from the standard solution | If the eighth point changes, the order continues; otherwise, stop, locate, and resolve the cause. |
| Seven consecutive points are in descending order (derives negative) | Solution obsolescence, standards or reagents | If the eighth point changes, the order continues; otherwise, stop, locate, and resolve the cause. |
| METHOD | |
| Sample volume (µL) | 3 |
| Reactive 1 | 250 |
| Reactive2 | 50 |
| Wash | 1.2 |
| Abs (nm) | 340 |
| Reading 1 | 72 s |
| Reading 2 | 600 s |
| Reactive 2 | 96 s |
| Temperature (°C) | 37 |
| CALIBRATION | |
| Calibration | Multiple calibrations |
| Calibrate replicates | 3 |
| Blank replicates | 3 |
| OPTIONS | |
| Blank limit (Abs; nm) | 0.300 |
| Linearity limit (g/L) | 8 |
| Concentration Range (g/L) | Dilution Factor |
|---|---|
| 0–8.00 | 0 |
| 8.00–16.00 | 2 |
| 16.00–32.00 | 4 |
| 32.00–88.00 | 11 |
| 88.00–160.00 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Tuccillo, D.; Coppola, D.; De Biasi, M.-G.; Morelli, E.; Mancusi, A. Validation of an Eco-Friendly Automated Method for the Determination of Glucose and Fructose in Wines. Molecules 2023, 28, 5585. https://doi.org/10.3390/molecules28145585
Dini I, Tuccillo D, Coppola D, De Biasi M-G, Morelli E, Mancusi A. Validation of an Eco-Friendly Automated Method for the Determination of Glucose and Fructose in Wines. Molecules. 2023; 28(14):5585. https://doi.org/10.3390/molecules28145585
Chicago/Turabian StyleDini, Irene, Dario Tuccillo, Daniele Coppola, Margherita-Gabriella De Biasi, Elena Morelli, and Andrea Mancusi. 2023. "Validation of an Eco-Friendly Automated Method for the Determination of Glucose and Fructose in Wines" Molecules 28, no. 14: 5585. https://doi.org/10.3390/molecules28145585
APA StyleDini, I., Tuccillo, D., Coppola, D., De Biasi, M.-G., Morelli, E., & Mancusi, A. (2023). Validation of an Eco-Friendly Automated Method for the Determination of Glucose and Fructose in Wines. Molecules, 28(14), 5585. https://doi.org/10.3390/molecules28145585







