Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells
Abstract
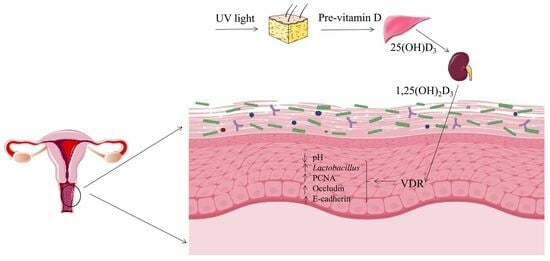
:1. Introduction
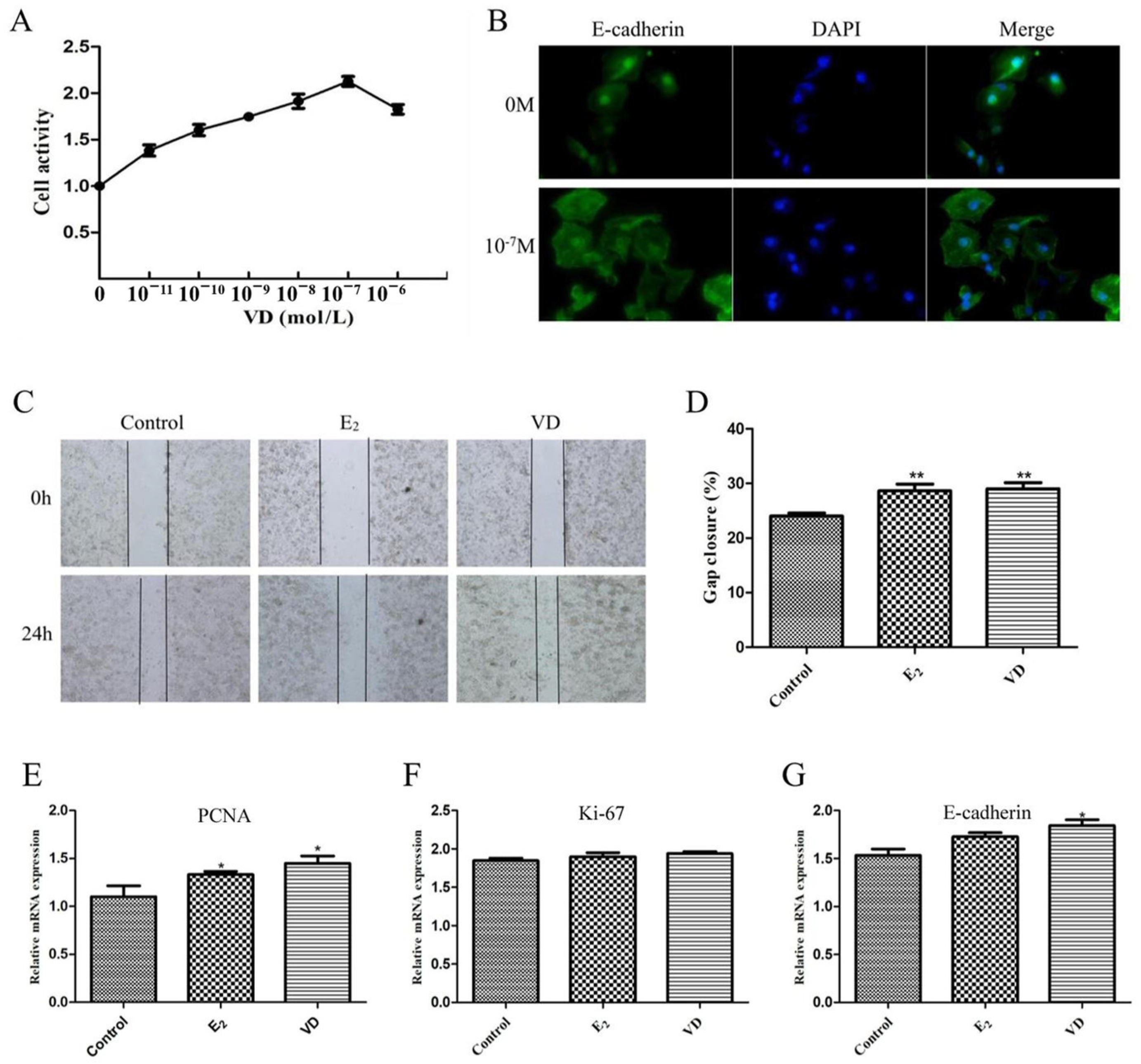
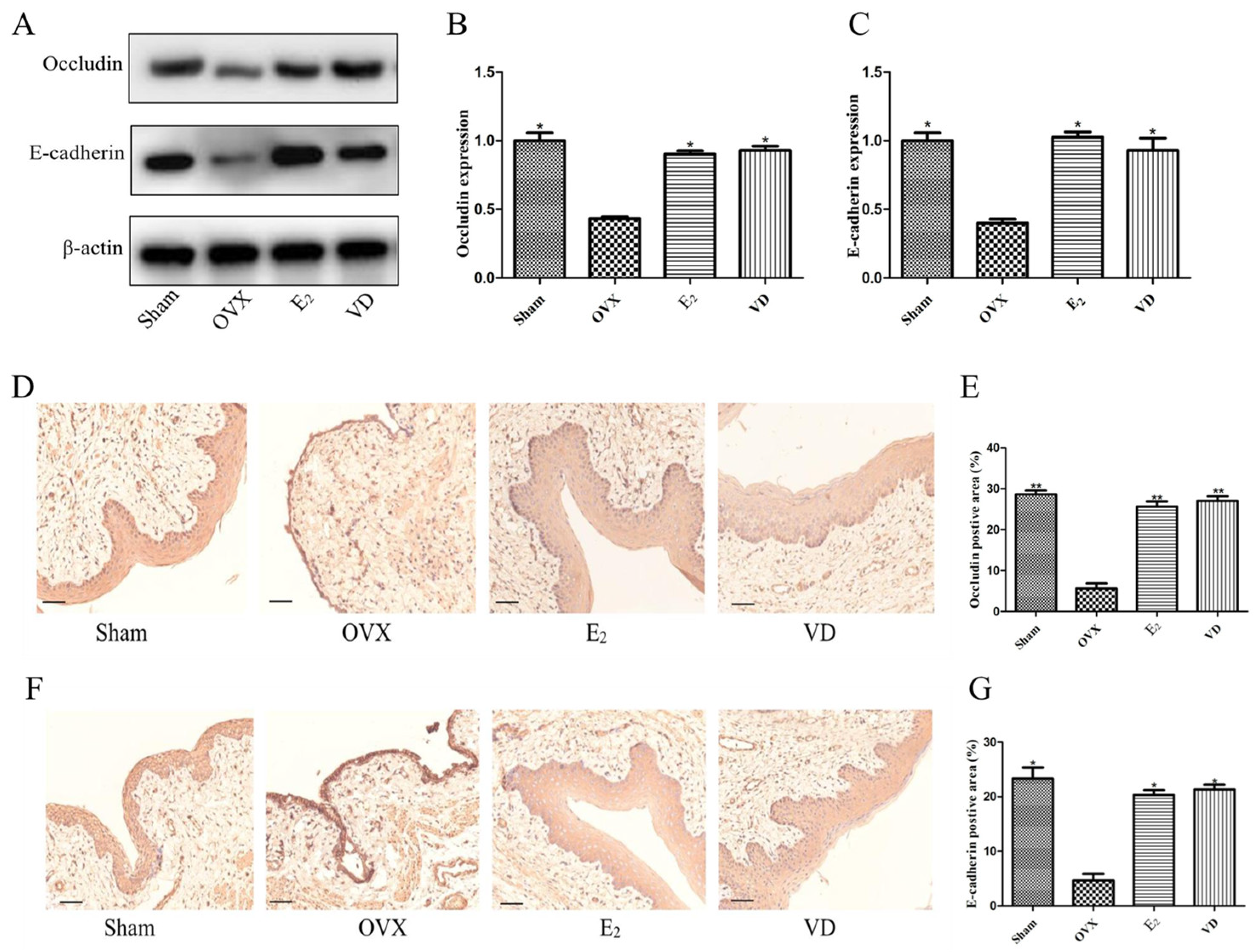
2. Results
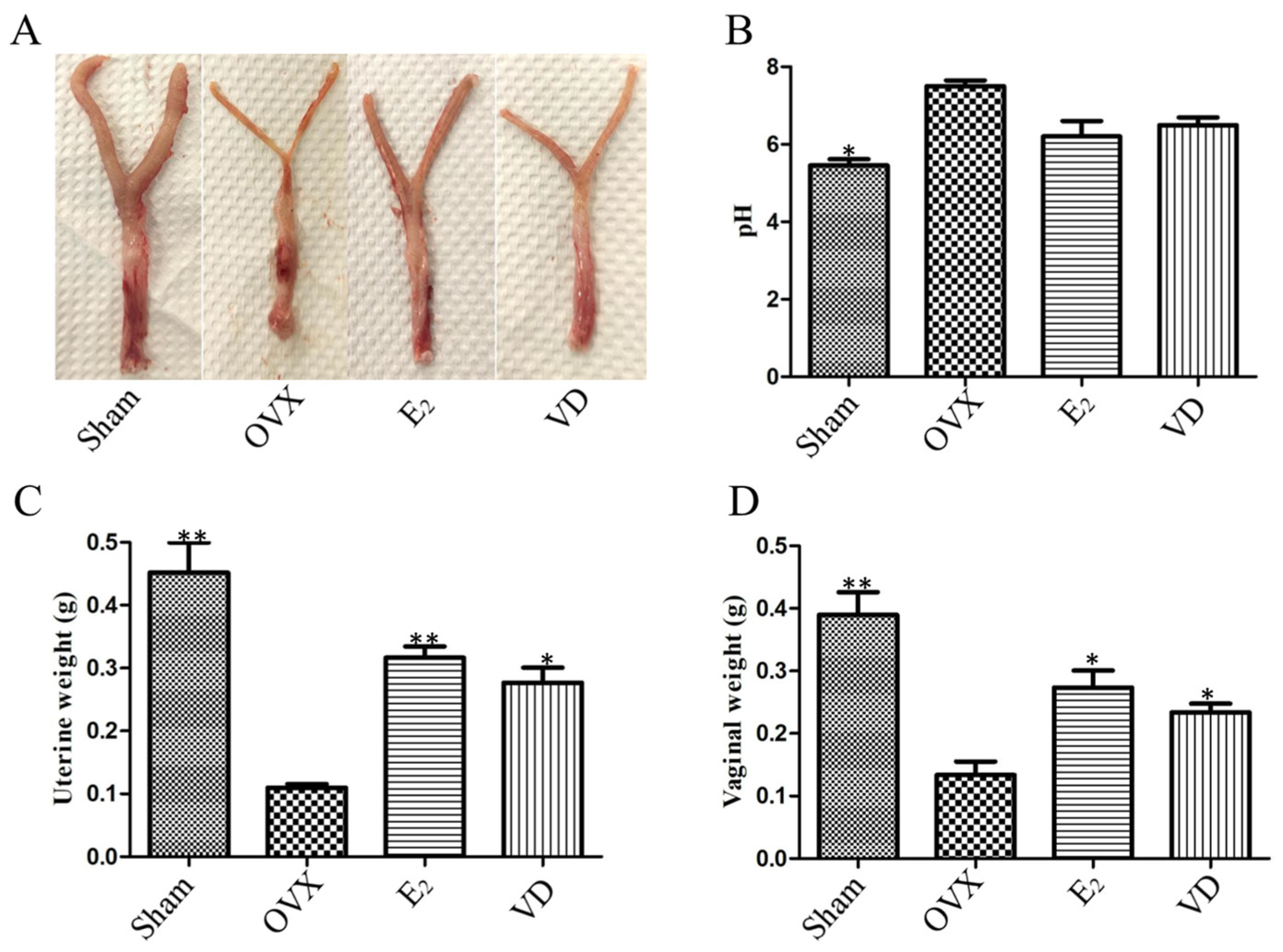
2.1. Effect of Vitamin D on Uterine and Vaginal Tissues of Ovariectomised Rats
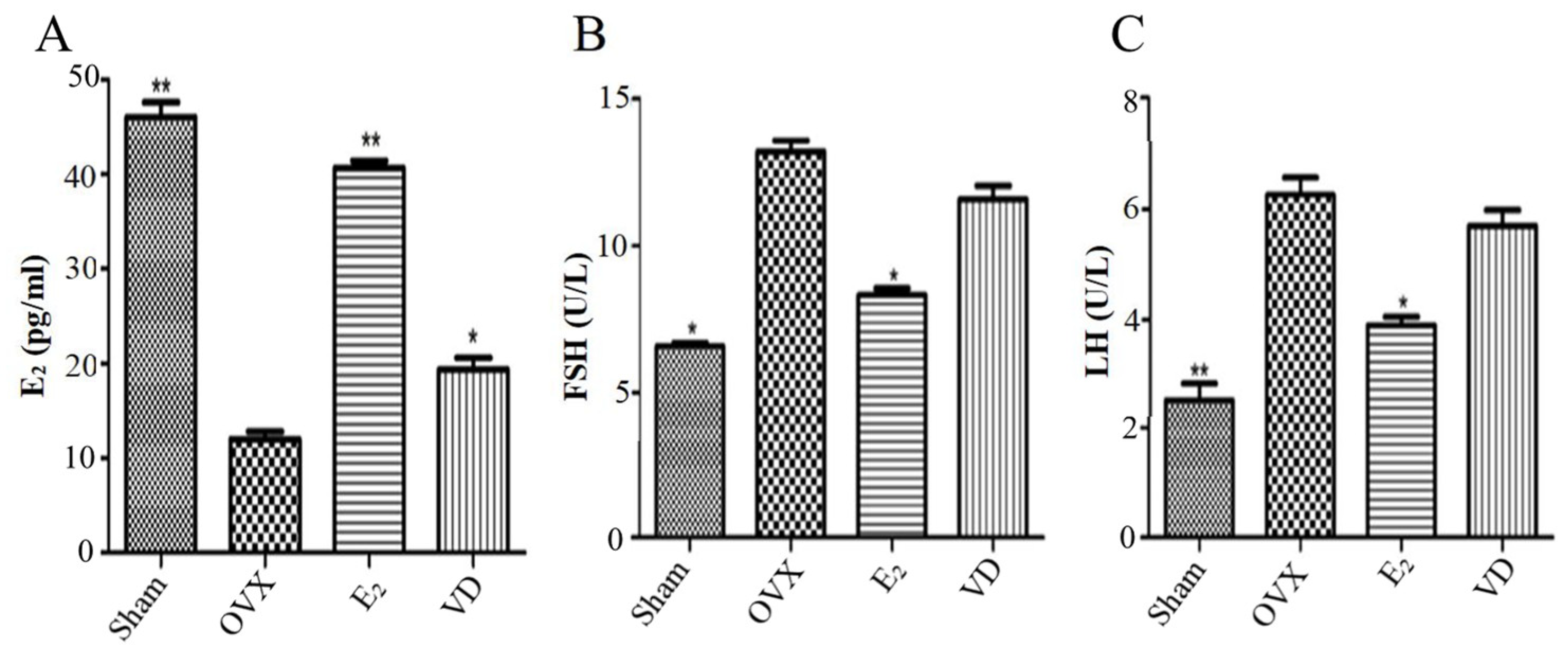
2.2. Effect of Vitamin D on Hormone Levels in Ovariectomised Rats
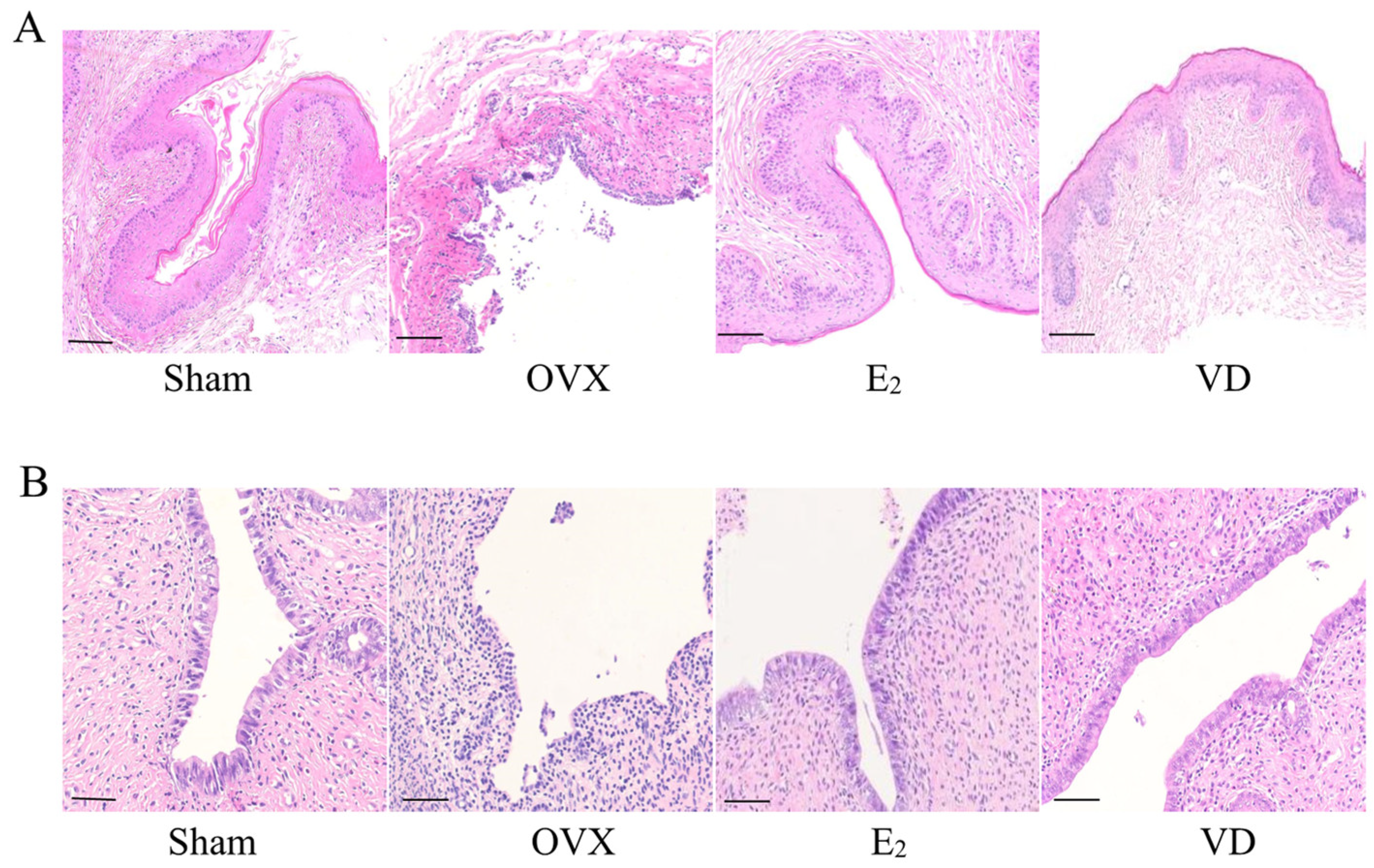
2.3. Effect of Vitamin D on Vaginal and Uterine Morphology in Rats
2.4. Effect of Vitamin D on Gene Expression in Vaginal Tissues
2.5. Effect of Vitamin D on Microorganisms in the Rat Vagina
2.6. Effect of Vitamin D on the VK2/E6E7 Cell Line
2.7. Effect of Vitamin D on Protein Expression in Rat Vaginal Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Serum Sex Hormone Level Testing
4.3. Histological Analysis
4.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.5. Intravaginal Microbiological Testing
4.6. Cell Culture
4.7. Cell Counting Kit 8 Assay
4.8. Cell Migration Assay
4.9. Immunofluorescence Staining
4.10. Western Blot Analysis
4.11. Immunohistochemical Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Sample Availability
References
- Zhu, Y.; Wei, J.; Yang, X.; Zhu, W.; Zhang, W. Investigation on prevalence and risk factors associated with genitourinary syndrome of menopause in middle-aged and older women in Beijing community: A cross sectional study. BMC Women’s Health 2022, 22, 558. [Google Scholar] [CrossRef]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Zaid, S.S.; Sulaiman, S.A.; Sirajudeen, K.N.; Othman, N.H. The effects of Tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats—Animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Agius, J.; Brincat, M.P. The urogenital system and the menopause. Climacteric J. Int. Menopause Soc. 2015, 18 (Suppl. 1), 18–22. [Google Scholar] [CrossRef]
- Yodplob, T.; Sirisopana, K.; Jongwannasiri, M.; Sirisreetreerux, P.; Viseshsindh, W.; Kochakarn, W. Local treatment with a polycarbophil-based cream in postmenopausal women with genitourinary syndrome of menopause. Int. Urogynecol. J. 2021, 32, 317–322. [Google Scholar] [CrossRef]
- Cirpan, T.; Guliyeva, A.; Onder, G.; Terek, M.C.; Ozsaran, A.; Kabasakal, Y.; Zekioglu, O.; Yucebilgin, S. Comparison of human papillomavirus testing and cervical cytology with colposcopic examination and biopsy in cervical cancer screening in a cohort of patients with Sjogren’s syndrome. Eur. J. Gynaecol. Oncol. 2007, 28, 302–306. [Google Scholar]
- Bergman, A.; Karram, M.M.; Bhatia, N.N. Changes in urethral cytology following estrogen administration. Gynecol. Obstet. Investig. 1990, 29, 211–213. [Google Scholar] [CrossRef]
- Fleet, J.C. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients 2022, 14, 3351. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.H.; Park, J. Immunohistochemical detection of the 1,25-dihydroxy vitamin D receptor in the human vagina. Iran. J. Reprod. Med. 2014, 12, 805–810. [Google Scholar]
- Kamronrithisorn, T.; Manonai, J.; Vallibhakara, S.A.; Sophonsritsuk, A.; Vallibhakara, O. Effect of Vitamin D Supplement on Vulvovaginal Atrophy of the Menopause. Nutrients 2020, 12, 2876. [Google Scholar] [CrossRef] [PubMed]
- Rad, P.; Tadayon, M.; Abbaspour, M.; Latifi, S.M.; Rashidi, I.; Delaviz, H. The effect of vitamin D on vaginal atrophy in postmenopausal women. Iran. J. Nurs. Midwifery Res. 2015, 20, 211–215. [Google Scholar]
- Abban, G.; Yildirim, N.B.; Jetten, A.M. Regulation of the vitamin D receptor and cornifin beta expression in vaginal epithelium of the rats through vitamin D3. Eur. J. Histochem. 2008, 52, 107–114. [Google Scholar] [CrossRef]
- Björkman, E.V.; Edebo, A.; Oltean, M.; Casselbrant, A. Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: Functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand. J. Gastroenterol. 2013, 48, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhang, Z.; Yang, L.; Li, R.; Ma, Y. Combined effect of vitamin C and vitamin D(3) on intestinal epithelial barrier by regulating Notch signaling pathway. Nutr. Metab. 2021, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022, 12, 963868. [Google Scholar] [CrossRef] [PubMed]
- Serré, J.; Mathyssen, C.; Ajime, T.T.; Heigl, T.; Verlinden, L.; Maes, K.; Verstuyf, A.; Cataldo, D.; Vanoirbeek, J.; Vanaudenaerde, B.; et al. Local nebulization of 1α,25(OH)(2)D(3) attenuates LPS-induced acute lung inflammation. Respir. Res. 2022, 23, 76. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Geng, C.; Song, S.; Xie, X.; Wang, C. Vitamin D receptor involves in the protection of intestinal epithelial barrier function via up-regulating SLC26A3. J. Steroid Biochem. Mol. Biol. 2023, 227, 106231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Luo, B.; Xu, S.; Zhang, Z.C.; Xing, W.Y.; Chen, Y.H.; Zhang, C.; Wang, H.; Xie, D.D.; Xu, D.X. Long-term vitamin D deficiency promotes renal fibrosis and functional impairment in middle-aged male mice. Br. J. Nutr. 2021, 125, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Agrawal, D.K. Vitamin D regulating TGF-β induced epithelial-mesenchymal transition. Respir. Res. 2014, 15, 146. [Google Scholar] [CrossRef]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef]
- Mohanty, S.; Kamolvit, W.; Hertting, O.; Brauner, A. Vitamin D strengthens the bladder epithelial barrier by inducing tight junction proteins during E. coli urinary tract infection. Cell Tissue Res. 2020, 380, 669–673. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Xu, G.; Zhang, R.; Zhou, C.; Qian, Y.; Liu, Y.; Chen, L.; Zhu, B.; Ye, X.; et al. Follicle-stimulating hormone promotes age-related endometrial atrophy through cross-talk with transforming growth factor beta signal transduction pathway. Aging Cell 2015, 14, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Hait, H.; Reape, K.Z.; Shu, H. Measuring symptom relief in studies of vaginal and vulvar atrophy: The most bothersome symptom approach. Menopause 2008, 15, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef]
- Allemailem, K.S. Prophylactic and Therapeutic Role of Vitamin D3 in Combination with Fluconazole Against Vaginal Candidiasis in a Murine Model. Curr. Pharm. Biotechnol. 2021, 22, 1812–1820. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Janghorban, R.; Alipour, S.; Tahmasebi, S.; Jokar, A. The effect of vitamin D and E vaginal suppositories on tamoxifen-induced vaginal atrophy in women with breast cancer. Support. Care Cancer 2019, 27, 1325–1334. [Google Scholar] [CrossRef]
- Lee, A.; Lee, M.R.; Lee, H.H.; Kim, Y.S.; Kim, J.M.; Enkhbold, T.; Kim, T.H. Vitamin D Proliferates Vaginal Epithelium through RhoA Expression in Postmenopausal Atrophic Vagina tissue. Mol. Cells 2017, 40, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995, 7, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, M.; Jamora, C.; Fuchs, E. Sticky business: Orchestrating cellular signals at adherens junctions. Cell 2003, 112, 535–548. [Google Scholar] [CrossRef]
- Hussein, H.M.; Elyamany, M.F.; Rashed, L.A.; Sallam, N.A. Vitamin D mitigates diabetes-associated metabolic and cognitive dysfunction by modulating gut microbiota and colonic cannabinoid receptor 1. Eur. J. Pharm. Sci. 2022, 170, 106105. [Google Scholar] [CrossRef]
- Lee, C.; Lau, E.; Chusilp, S.; Filler, R.; Li, B.; Zhu, H.; Yamoto, M.; Pierro, A. Protective effects of vitamin D against injury in intestinal epithelium. Pediatr. Surg. Int. 2019, 35, 1395–1401. [Google Scholar] [CrossRef]
- Schröder-Heurich, B.; von Hardenberg, S.; Brodowski, L.; Kipke, B.; Meyer, N.; Borns, K.; von Kaisenberg, C.S.; Brinkmann, H.; Claus, P.; von Versen-Höynck, F. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. FASEB J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C. Vaginal estrogen preparations: A review of safety and efficacy for vaginal atrophy. J. Women’s Health 2002, 11, 857–877. [Google Scholar] [CrossRef]
- Caillouette, J.C.; Sharp, C.F., Jr.; Zimmerman, G.J.; Roy, S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am. J. Obstet. Gynecol. 1997, 176, 1270–1275; discussion 1275–1277. [Google Scholar] [CrossRef]
- Gniadecki, R.; Gajkowska, B.; Hansen, M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: Involvement of protein kinase C. Endocrinology 1997, 138, 2241–2248. [Google Scholar] [CrossRef]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Parikh, H.I.; Garcia, E.M.; Edwards, D.J.; Serrano, M.G.; Hewison, M.; Shary, J.R.; Powell, A.M.; Hollis, B.W.; Fettweis, J.M.; et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836. [Google Scholar] [CrossRef]
- Hasan, S.F.; Al-Hashemi, I.H.M.; Al-Rammahi, T.M.M.; Abbas, Z.F. Interleukin-6 and C-Reactive Protein: Their association with vitamin D in women with recurrent infections of reproductive system. Egypt. J. Immunol. 2022, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Hussien, N.I.; Elwia, S.K. Vitamin D Replacement Mitigates Menopause-Associated Dyslipidaemia and Atherogenic Indices in Ovariectomized Rats; A Biochemical Study. Exp. Clin. Endocrinol. Diabetes 2020, 128, 144–151. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, T.; Yang, H.; Yang, W.; Zhang, C.; Gao, G. Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells. Molecules 2023, 28, 6605. https://doi.org/10.3390/molecules28186605
Li D, Zhang T, Yang H, Yang W, Zhang C, Gao G. Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells. Molecules. 2023; 28(18):6605. https://doi.org/10.3390/molecules28186605
Chicago/Turabian StyleLi, Dandan, Tao Zhang, He Yang, Wenlan Yang, Chi Zhang, and Guolan Gao. 2023. "Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells" Molecules 28, no. 18: 6605. https://doi.org/10.3390/molecules28186605
APA StyleLi, D., Zhang, T., Yang, H., Yang, W., Zhang, C., & Gao, G. (2023). Effect of Vitamin D on the Proliferation and Barrier of Atrophic Vaginal Epithelial Cells. Molecules, 28(18), 6605. https://doi.org/10.3390/molecules28186605