Ternary XBe4H5− (X = Si, Ge, Sn, Pb) Clusters: Planar Tetracoordinate Si/Ge/Sn/Pb Species with 18 Valence Electrons
Abstract
1. Introduction
2. Computational Details
3. Result and Discussion
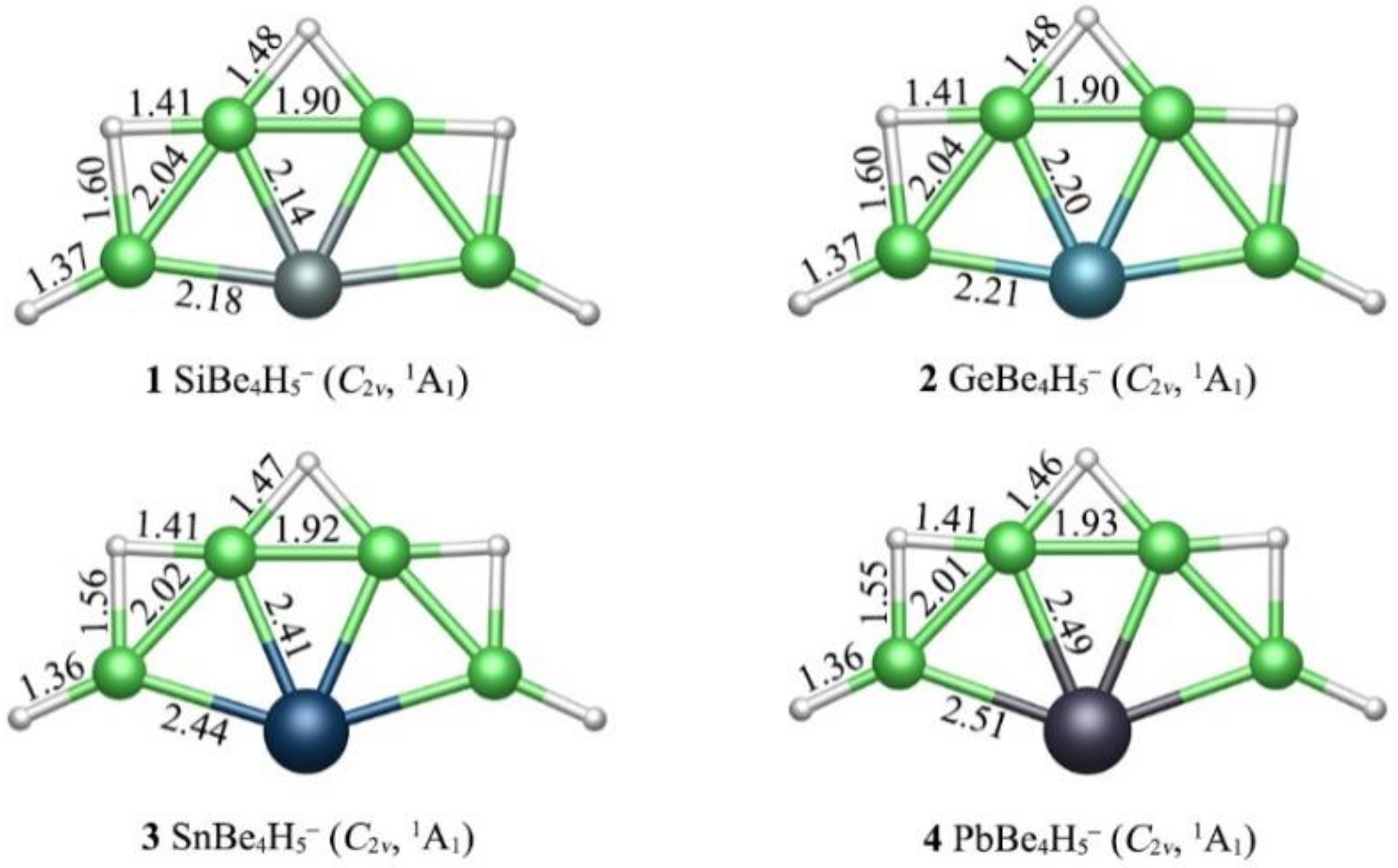
3.1. Geometies and Stability
3.2. Chemical Bonding
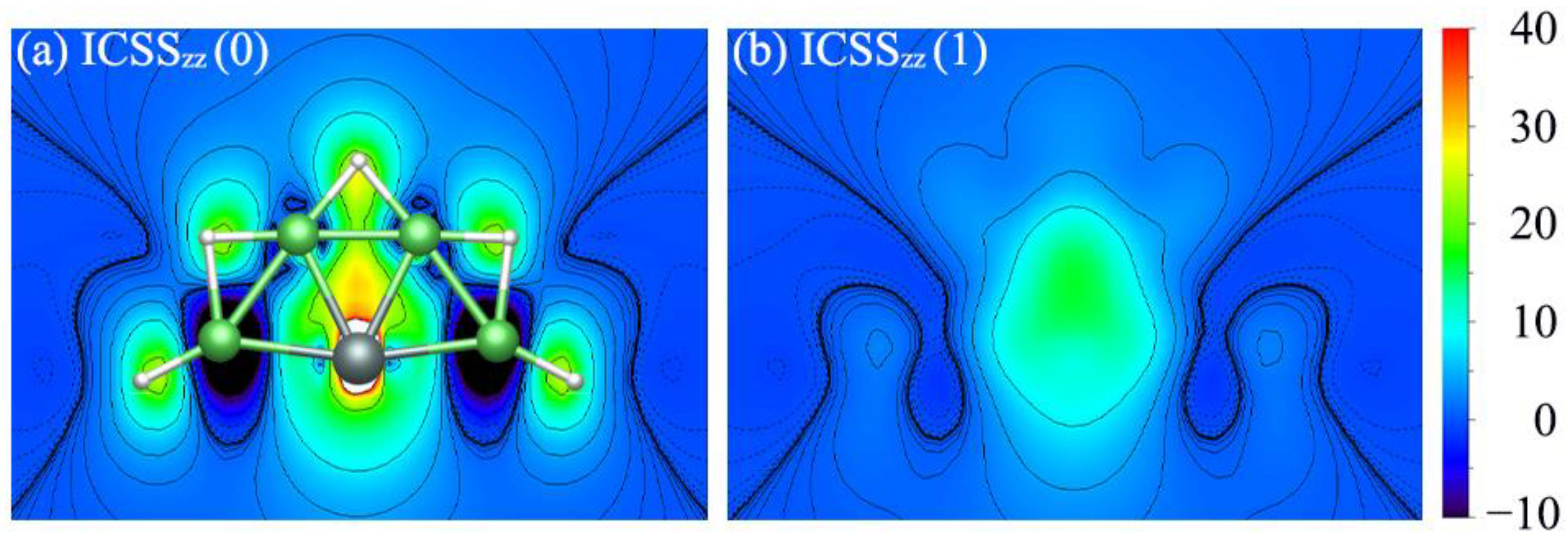
3.3. Double Aromaticity
3.4. Simulated Photoelectron Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Monkhorst, H.J. Activation energy for interconversion of enantiomers containing an asymmetric carbon atom without breaking bonds. Chem. Commun. 1968, 1111–1112. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alder, R.W.; Wilcox, C.F. Planar tetracoordinate carbon. J. Am. Chem. Soc. 1970, 92, 4992–4993. [Google Scholar] [CrossRef]
- Collins, J.B.; Dill, J.D.; Jemmis, E.D.; Apeloig, Y.; Schleyer, P.v.R.; Seeger, R.; Pople, J.A. Stabilization of planar tetracoordinate carbon. J. Am. Chem. Soc. 1976, 98, 5419–5427. [Google Scholar] [CrossRef]
- Cotton, F.A.; Millar, M. The probable existence of a triple bond between two vanadium atoms. J. Am. Chem. Soc. 1977, 99, 7886–7891. [Google Scholar] [CrossRef]
- Sorger, K.; Schleyer, P.v.R. Planar and inherently non-tetrahedral tetracoordinate carbon: A status report. J. Mol. Struct. (Theochem) 1995, 338, 317–346. [Google Scholar] [CrossRef]
- Erker, G. Using bent metallocenes for stabilizing unusual coordination geometries at carbon. Chem. Soc. Rev. 1999, 28, 307–314. [Google Scholar] [CrossRef]
- Siebert, W.; Gunale, A. Compounds containing a planar-tetracoordinate carbon atom as analogues of planar methane. Chem. Soc. Rev. 1999, 28, 367–371. [Google Scholar] [CrossRef]
- Keese, R. Carbon flatland: Planar tetracoordinate carbon and fenestranes. Chem. Rev. 2006, 106, 4787–4808. [Google Scholar] [CrossRef]
- Merino, G.; Méndez-Rojas, M.A.; Vela, A.; Heine, T. Recent advances in planar tetracoordinate carbon chemistry. J. Comput. Chem. 2007, 28, 362–372. [Google Scholar] [CrossRef]
- Yang, L.M.; Ganz, E.; Chen, Z.F.; Wang, Z.X.; Schleyer, P.v.R. Four decades of the chemistry of planar hypercoordinate compounds. Angew. Chem. Int. Ed. 2015, 54, 9468–9501. [Google Scholar] [CrossRef]
- Vassilev-Galindo, V.; Pan, S.; Donald, K.J.; Merino, G. Planar pentacoordinate carbons. Nat. Rev. Chem. 2018, 2, 0114. [Google Scholar] [CrossRef]
- Das, P.; Chattaraj, P.K. Structure and bonding in planar hypercoordinate carbon compounds. Chemistry 2022, 4, 1723–1756. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, Z. Planar hypercoordinate motifs in two-dimensional materials. Acc. Chem. Res. 2020, 53, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.S.; Boldyrev, A.I.; Simons, J. Tetracoordinated planar carbon in the Al4C− anion. A combined photoelectron spectroscopy and ab initio study. J. Am. Chem. Soc. 1999, 121, 6033–6038. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.F.; Wang, L.S.; Geske, G.D.; Boldyrev, A.I. Pentaatomic tetracoordinate planar carbon, [CAl4]2−: A new structural unit and its salt complexes. Angew. Chem. Int. Ed. 2000, 39, 3630–3632. [Google Scholar] [CrossRef]
- Wang, L.S.; Boldyrev, A.I.; Li, X.; Simon, J. Experimental observation of pentaatomic tetracoordinate planar carbon-containing molecules. J. Am. Chem. Soc. 2000, 122, 7681–7687. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Boldyrev, A.I. A new, general strategy for achieving planar tetracoordinate geometries for carbon and other second row periodic elements. J. Chem. Soc. Chem. Commun. 1991, 21, 1536–1538. [Google Scholar] [CrossRef]
- Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: A summary. J. Phys. Chem. A 2015, 119, 2326–2337. [Google Scholar] [CrossRef]
- Kolesnikov, A.S. Thermodynamic simulation of silicon and iron reduction and zinc and lead distillation in zincoligonite ore–carbon systems. Russ. J. Non-Ferr. Met. 2014, 55, 513–518. [Google Scholar] [CrossRef]
- Thermodynamic modeling of chemical and phase transformations in a waelz process-slag-carbon system. Refract. Ind. Ceram. 2020, 61, 289–292. [CrossRef]
- Boldyrev, A.I.; Li, X.; Wang, L.S. Experimental Observation of Pentaatomic Tetracoordinate Planar Si- and Ge-Containing Molecules: MAl4- and MAl4. Angew. Chem. Int. Ed. 2000, 39, 3307–3310. [Google Scholar] [CrossRef]
- Li, S.D.; Miao, C.Q.; Guo, J.C.; Ren, G.M. Planar tetra-, penta-, hexa-, hepta-, and octacoordinate silicons: A universal structural pattern. J. Am. Chem. Soc. 2004, 126, 16227–16231. [Google Scholar] [CrossRef]
- Tsipis, A.C.; Tsipis, C.A. Hydrometal analogues of aromatic hydrocarbons: A new class of cyclic hydrocoppers(I). J. Am. Chem. Soc. 2003, 125, 1136–1137. [Google Scholar] [CrossRef]
- Guo, J.C.; Li, S.D. Planar tetra-coordinate Si and Ge in perfectly squared Ni4Cl4X complexes. J. Mol. Struct. THEOCHEM 2007, 816, 59–65. [Google Scholar] [CrossRef]
- Li, S.D.; Miao, C.Q. M5H5X (M = Ag, Au, Pd, Pt; X = Si, Ge, P, S): Hydrometal pentagons with D5h planar pentacoordinate nonmetal centers. J. Phys. Chem. A 2005, 109, 7594–7597. [Google Scholar] [CrossRef]
- Li, S.D.; Ren, G.M.; Miao, C.Q. Hexacoordinate planar main group atoms centered in hexagonal hydrocopper complexes Cu6H6X (X = Si, P, As). Inorg. Chem. 2004, 43, 6331–6333. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Jalbout, A.F. Structural, electronic, and magnetic properties of heterofullerene C58Si with odd number of atoms and a near planar tetracoordinate Si atom. J. Mol. Graph. Model. 2008, 26, 1327–1332. [Google Scholar] [CrossRef]
- Belanzoni, P.; Giorgi, G.; Cerofolini, G.F.; Sgamellotti, A. Planar tetracoordinated silicon in silicon carbonyl complexes: A DFT approach. J. Phys. Chem. A 2006, 110, 4582–4591. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Nayhouse, M.J.; Huynh, M.T.; Kuo, J.L.; Melkonian, A.V.; Chavez, G.; Hernando, N.M.; Kowal, M.D.; Liu, C.-P. Selected AB42−/− (A = C, Si, Ge; B = Al, Ga, In) ions: A battle between covalency and aromaticity, and prediction of square planar Si in SiIn42−/−. Phys. Chem. Chem. Phys. 2012, 14, 14815–14821. [Google Scholar] [CrossRef]
- Xu, J.; Ding, Y.H. Pentaatomic planar tetracoordinate silicon with 14 valence electrons: A large-scale global search of SiXnYmq(n +m = 4; q = 0, ±1, −2; X, Y = Main Group Elements From H to Br). J. Comput. Chem. 2015, 36, 355–360. [Google Scholar] [CrossRef]
- Guo, J.C.; Wu, H.X.; Ren, G.M.; Miao, C.Q.; Li, Y.X. D3h X3Li3+ (X = C, Si and Ge): Superalkali cations containing three planar tetracoordinate X atoms. Comput. Theor. Chem. 2016, 1083, 1–6. [Google Scholar] [CrossRef]
- Guo, J.C.; Miao, C.Q.; Ren, G.M. Planar tetracoordinate Si and Ge in π-aromatic X3Cu3+ (X = Si, Ge) cations. Comput. Theor. Chem. 2014, 1032, 7–11. [Google Scholar] [CrossRef]
- Sui, J.J.; Xu, J.; Ding, Y.H. A template for a planar tetracoordinate heavier group 14 atom: A global study of C2Si2Xq.(X = C, Si, Ge, Sn, Pb; q = +1, 0, −1). Dalton Trans. 2016, 45, 56–60. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Guo, J.C.; Zhai, H.J. Ternary 14-electron XB2Be2 (X = Si, Ge, Sn, Pb) clusters: A planar tetracoordinate silicon (ptSi) system and its ptGe/Sn/Pb congeners. Phys. Chem. Chem. Phys. 2022, 24, 7068–7076. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Dong, X.; Cui, Z.H.; Orozco-Ic, M.; Ding, Y.H.; Barroso, J.; Merino, G. Planar pentacoordinate silicon and germanium atoms. Chem. Commun. 2020, 56, 13772–13775. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.H.; Feng, L.Y.; Zhao, L.Q.; Guo, J.C.; Zhai, H.J.; Cui, Z.H.; Pan, S.; Merino, G. Bare and ligand protected planar hexacoordinate silicon in SiSb3M3+ (M = Ca, Sr, Ba) clusters. Chem. Sci. 2022, 13, 8045–8051. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Zhou, Z.; Chen, Z. SiC2 silagraphene and its one-dimensional derivatives: Where planar tetracoordinate silicon happens. J. Am. Chem. Soc. 2011, 133, 900–908. [Google Scholar] [CrossRef]
- Ebner, F.; Greb, L. Calix[4]pyrrole hydridosilicate: The elusive planar tetracoordinate silicon imparts striking stability to its anionic silicon hydride. J. Am. Chem. Soc. 2018, 140, 17409–17412. [Google Scholar] [CrossRef]
- Nukazawa, T.; Iwamoto, T. An isolable tetrasilicon analogue of a planar bicyclo[1.1.0]butane with π-type single-bonding character. J. Am. Chem. Soc. 2020, 142, 9920–9924. [Google Scholar] [CrossRef]
- Ebner, F.; Greb, L. An isolable, crystalline complex of squareplanar silicon(IV). Chem 2021, 7, 2151–2159. [Google Scholar] [CrossRef]
- Ghana, P.; Rump, J.; Schnakenburg, G.; Arz, M.I.; Filippou, A.C. Planar tetracoordinated silicon (ptSi): Room-temperature stable compounds containing anti-van’t Hoff/Le Bel silicon. J. Am. Chem. Soc. 2021, 143, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Dong, S.; Yao, S.; Zhu, J.; Driess, M. Synthesis and reactivity of an anti-van’t Hoff/Le Bel compound with a planar tetracoordinate silicon(II) atom. J. Am. Chem. Soc. 2023, 145, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M. Stochastic search for isomers on a quantum mechanical surface. J. Comput. Chem. 2004, 25, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Averkiev, B.B.; Zhai, H.J.; Boldyrev, A.I.; Wang, L.S. All-boron analogues of aromatic hydrocarbons: B17− and B18−. J. Chem. Phys. 2011, 134, 224304. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.J.; Hommes, N.J.R.v.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zubarev, D.Y.; Boldyrev, A.I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Legault, C.Y. CYLview, 1.0b.; Universite. de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: http://www.cylview.org (accessed on 15 January 2019).
- Varetto, U. Molekel, Version 5.4.0.8; Swiss National Supercomputing Centre: Manno, Switzerland, 2009. [Google Scholar]
- Guo, J.C.; Ren, G.M.; Miao, C.Q.; Tian, W.J.; Wu, Y.B.; Wang, X. CBe5Hnn−4(n = 2–5): Hydrogen-stabilized CBe5 pentagons containing planar or quasi-planar pentacoordinate carbons. J. Phys. Chem. A 2015, 119, 13101–13106. [Google Scholar] [CrossRef] [PubMed]
- Kloda, S.; Kleinpeter, E. Ab initio calculation of the anisotropy effect of multiple bonds and the ring current effect of arenes– application in conformational and configurational analysis. J. Chem. Soc. Perkin Trans. 2001, 2, 1893–1898. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Bai, L.-X.; Guo, J.-C. Ternary XBe4H5− (X = Si, Ge, Sn, Pb) Clusters: Planar Tetracoordinate Si/Ge/Sn/Pb Species with 18 Valence Electrons. Molecules 2023, 28, 5583. https://doi.org/10.3390/molecules28145583
Li Y-X, Bai L-X, Guo J-C. Ternary XBe4H5− (X = Si, Ge, Sn, Pb) Clusters: Planar Tetracoordinate Si/Ge/Sn/Pb Species with 18 Valence Electrons. Molecules. 2023; 28(14):5583. https://doi.org/10.3390/molecules28145583
Chicago/Turabian StyleLi, Yong-Xia, Li-Xia Bai, and Jin-Chang Guo. 2023. "Ternary XBe4H5− (X = Si, Ge, Sn, Pb) Clusters: Planar Tetracoordinate Si/Ge/Sn/Pb Species with 18 Valence Electrons" Molecules 28, no. 14: 5583. https://doi.org/10.3390/molecules28145583
APA StyleLi, Y.-X., Bai, L.-X., & Guo, J.-C. (2023). Ternary XBe4H5− (X = Si, Ge, Sn, Pb) Clusters: Planar Tetracoordinate Si/Ge/Sn/Pb Species with 18 Valence Electrons. Molecules, 28(14), 5583. https://doi.org/10.3390/molecules28145583






