Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction
Abstract
1. Introduction
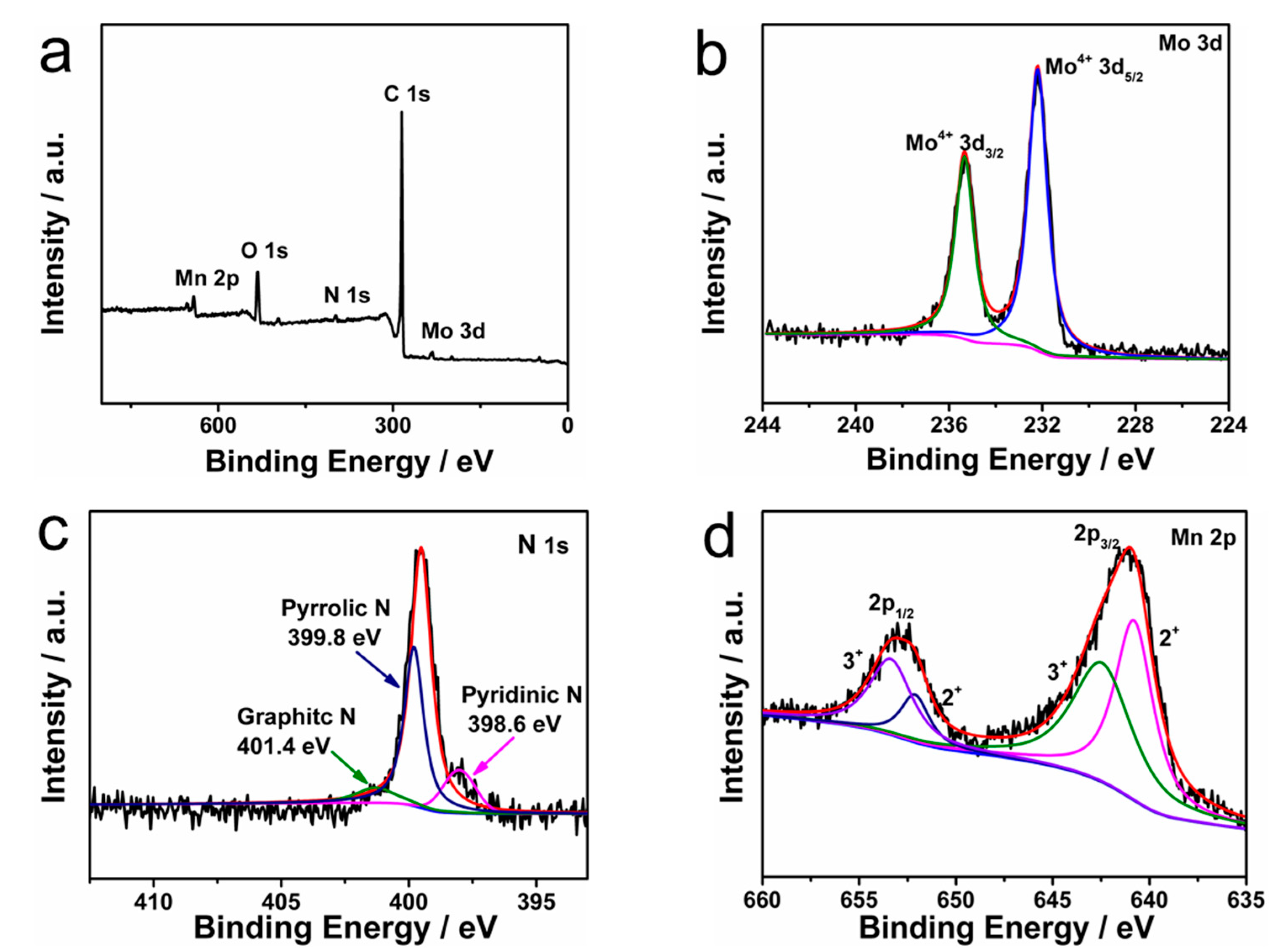
2. Results and Discussion
3. Experimental Section
3.1. Reagents and Chemicals
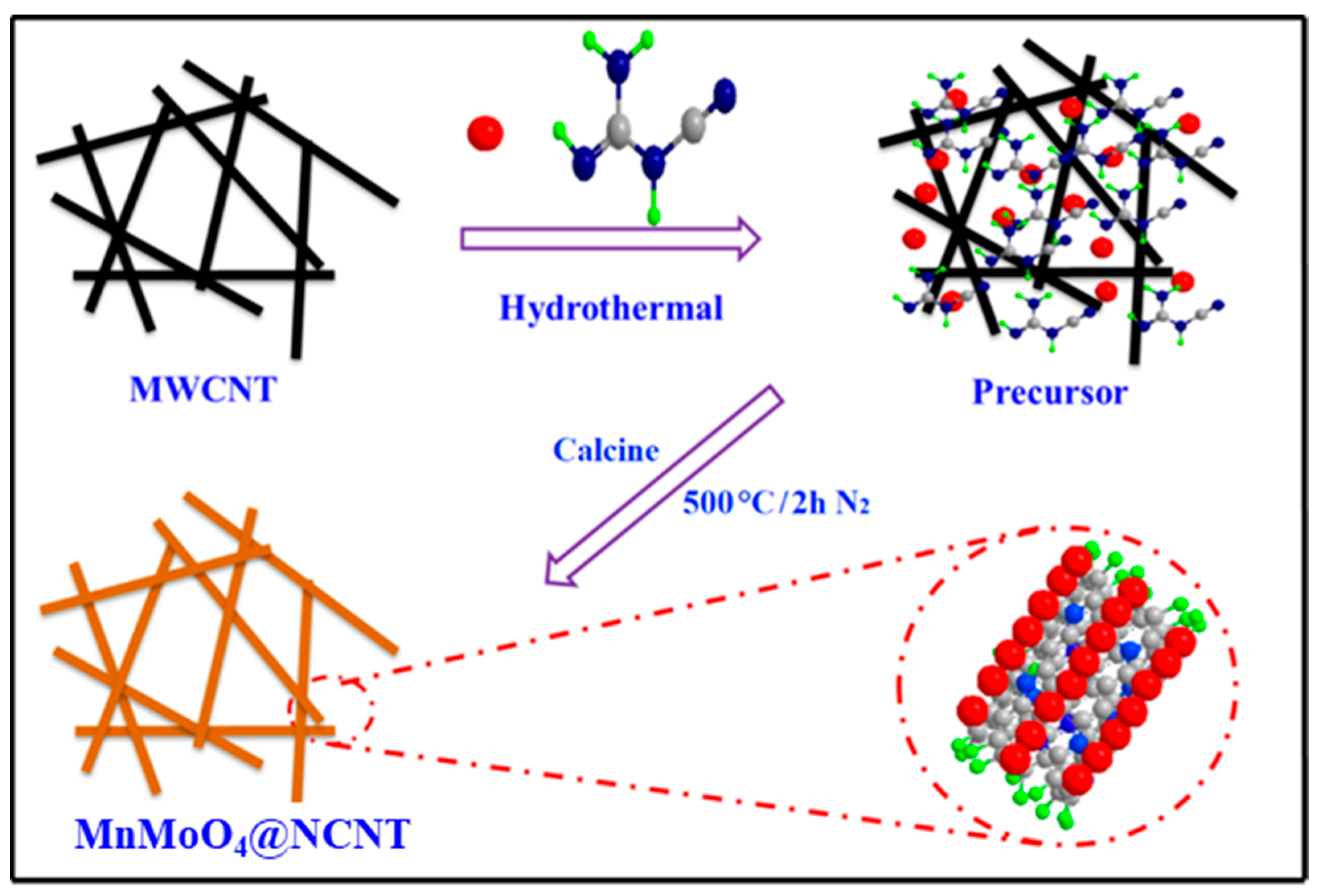
3.2. Preparation of MnMoO4@NCNT Composites
3.3. Materials Characterization
3.4. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akula, S.; Mooste, M.; Kozlova, J.; Käärik, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Paiste, P.; Tamm, A.; et al. Transition metal (Fe, Co, Mn, Cu) containing nitrogen-doped porous carbon as efficient oxygen reduction electrocatalysts for anion exchange membrane fuel cells. Chem. Eng. J. 2023, 458, 141468. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A review on anion exchange membranes for fuel cells: Anion-exchange polyelectrolytes and synthesis strategies. Int. J. Hydrog. Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Ni, Z.; Han, K.; Chen, X.; Wang, L.; Wang, B. Experimental and numerical efforts to improve oxygen mass transport in porous catalyst layer of proton exchange membrane fuel cells. Nano Res. Energy 2023, just accepted. [Google Scholar] [CrossRef]
- Naya, S.-i.; Suzuki, H.; Kobayashi, H.; Tada, H. Highly Active and Renewable Catalytic Electrodes for Two-Electron Oxygen Reduction Reaction. Langmuir 2022, 38, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhou, Y.; Hu, T.; Xu, Y.; Hou, M.; Zhu, F.; Liu, D.; Zhang, H.; Xu, K.; Liu, M.; et al. An Efficient High-Entropy Perovskite-Type Air Electrode for Reversible Oxygen Reduction and Water Splitting in Protonic Ceramic Cells. Adv. Mater. 2023, 35, 2209469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ricote, S.; Chen, M. Oxygen electrodes for protonic ceramic cells. Electrochim. Acta 2023, 446, 142101. [Google Scholar] [CrossRef]
- Dessalle, A.; Quílez-Bermejo, J.; Fierro, V.; Xu, F.; Celzard, A. Recent progress in the development of efficient biomass-based ORR electrocatalysts. Carbon 2023, 203, 237–260. [Google Scholar] [CrossRef]
- Zhu, E.; Wu, M.; Xu, H.; Peng, B.; Liu, Z.; Huang, Y.; Li, Y. Stability of Platinum-Group-Metal-Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2022, 32, 2203883. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Li, Y.; Wu, H.-R.; Lu, B.-A.; Zhang, J.-N. Highly Stable Pt-Based Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Materials 2023, 16, 2590. [Google Scholar] [CrossRef]
- Yan, J.; Ye, F.; Dai, Q.; Ma, X.; Fang, Z.; Dai, L.; Hu, C. Recent progress in carbon-based electrochemical catalysts: From structure design to potential applications. Nano Res. Energy 2023, 2, e9120047. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, M.; Yi, S.; Li, X.; Xin, R.; Yang, M.; Liu, B.; Chen, H.; Li, H.; Liu, Y. Metal-coordinated porous polydopamine nanospheres derived Fe3N-FeCo encapsulated N-doped carbon as a highly efficient electrocatalyst for oxygen reduction reaction. Nano Res. Energy 2022, 1, e9120027. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Xu, M.; Asset, T.; Yan, X.; Artyushkova, K.; Kodali, M.; Murphy, E.; Ly, A.; Pan, X.; et al. Catalysts by pyrolysis: Direct observation of transformations during re-pyrolysis of transition metal-nitrogen-carbon materials leading to state-of-the-art platinum group metal-free electrocatalyst. Mater. Today 2022, 53, 58–70. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Yang, F.; Li, B.; Stracensky, T.; Karakalos, S.; Mukerjee, S.; Jia, Q.; Su, D.; Wang, G.; et al. Atomically Dispersed MnN4 Catalysts via Environmentally Benign Aqueous Synthesis for Oxygen Reduction: Mechanistic Understanding of Activity and Stability Improvements. ACS Catal. 2020, 10, 10523–10534. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, J.; Yuan, P.; Hu, Y.; Qu, G.; Lu, B.-A.; Xue, X.; Yin, H.; Cheng, W.; Cheng, J.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Abidat, I.; Bouchenafa-Saib, N.; Habrioux, A.; Comminges, C.; Canaff, C.; Rousseau, J.; Napporn, T.W.; Dambournet, D.; Borkiewicz, O.; Kokoh, K.B. Electrochemically induced surface modifications of mesoporous spinels (Co3O4−δ, MnCo2O4−δ, NiCo2O4−δ) as the origin of the OER activity and stability in alkaline medium. J. Mater. Chem. A 2015, 3, 17433–17444. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Gorlin, Y.; Jaramillo, T.F. A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhao, T.; Chen, Y.; Li, Z.; Wu, W.; Wu, M. Firmly combination of CoMnOx nanocrystals supported on N-doped CNT for lithium-ion batteries. Chem. Eng. J. 2016, 306, 336–343. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko Jesse, S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Han, Y.; Shen, P.K.; Cui, Y. Porous MoO2 Nanosheets as Non-noble Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2016, 28, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Neburchilov, V.; Wang, H.; Martin, J.J.; Qu, W. A review on air cathodes for zinc–air fuel cells. J. Power Sources 2010, 195, 1271–1291. [Google Scholar] [CrossRef]
- Chen, S.; Qiao, S.-Z. Hierarchically Porous Nitrogen-Doped Graphene–NiCo2O4 Hybrid Paper as an Advanced Electrocatalytic Water-Splitting Material. ACS Nano 2013, 7, 10190–10196. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, H.; Wei, L.; Jiang, W.-J.; Wu, M.; Hu, J.-S. Lamellar Metal Organic Framework-Derived Fe–N–C Non-Noble Electrocatalysts with Bimodal Porosity for Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2017, 9, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xiao, T.-Y.; Qian, Y.-H.; Li, S.-S.; Yu, S.-H. A Nitrogen-Doped Graphene/Carbon Nanotube Nanocomposite with Synergistically Enhanced Electrochemical Activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, Conductive, and Processable Multifunctional Graphene Nanosheets Coated with High-Density Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Yurino, T.; Ueda, Y.; Shimizu, Y.; Tanaka, S.; Nishiyama, H.; Tsurugi, H.; Sato, K.; Mashima, K. Salt-Free Reduction of Nonprecious Transition-Metal Compounds: Generation of Amorphous Ni Nanoparticles for Catalytic C–C Bond Formation. Angew. Chem. Int. Ed. 2015, 54, 14437–14441. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-Metal Oxides/Graphene Nanocomposites as Multifunctional Electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Duan, J.; Zheng, Y.; Chen, S.; Tang, Y.; Jaroniec, M.; Qiao, S. Mesoporous hybrid material composed of Mn3O4 nanoparticles on nitrogen-doped graphene for highly efficient oxygen reduction reaction. Chem. Commun. 2013, 49, 7705–7707. [Google Scholar] [CrossRef]
- Holade, Y.; da Silva, R.G.; Servat, K.; Napporn, T.W.; Canaff, C.; de Andrade, A.R.; Kokoh, K.B. Facile synthesis of highly active and durable PdM/C (M = Fe, Mn) nanocatalysts for the oxygen reduction reaction in an alkaline medium. J. Mater. Chem. A 2016, 4, 8337–8349. [Google Scholar] [CrossRef]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy)hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Wang, Y.; Wang, Y.; Zaghib, K.; Zhou, Z. ZIF-derived Co–N–C ORR catalyst with high performance in proton exchange membrane fuel cells. Prog. Nat. Sci. 2020, 30, 855–860. [Google Scholar] [CrossRef]
- Wang, W.-T.; Batool, N.; Zhang, T.-H.; Liu, J.; Han, X.-F.; Tian, J.-H.; Yang, R. When MOFs meet MXenes: Superior ORR performance in both alkaline and acidic solutions. J. Mater. Chem. A 2021, 9, 3952–3960. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Y.; Jiang, Z.; Jiang, D.; Wei, W.; Hu, Z. Nitrogen-Doped Bimetallic Carbide-Graphite Composite as Highly Active and Extremely Stable Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2204031. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Z.; Zhang, J.; Xu, C.; Espiritu, R.; Cheng, J. High-Performance FeCo/NC-Mo2TiC2/Carbon Nanotube Hybrid Support Catalyst toward Oxygen Reduction for Alkaline Anion Exchange Membrane Fuel Cell. ACS Sustain. Chem. Eng. 2022, 10, 15735–15740. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Zhang, X.; Chen, B.; Lu, Z.; Xu, H.; Gao, R.; Shi, C. Engineering the Local Coordination Environment and Density of FeN4 Sites by Mn Cooperation for Electrocatalytic Oxygen Reduction. Small 2022, 18, 2200911. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]



| Electrocatalyst | Electrolyte | Onset Potential (V vs. RHE) | Half-Wave Potential (V vs. RHE) | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|
| MnMoO4@NCNT | 0.1 M KOH | 0.9 | 0.83 | 4 | This work |
| Mn-N-C | 0.5 M H2SO4 | 0.9 | 0.69 | 4 | [14] |
| Fe-Mn/N-C | 0.1M HClO4 | 0.95 | 0.80 | 2 | [15] |
| Mn-N-C | 0.5 M H2SO4 | 0.9 | 0.8 | 4 | [13] |
| Co-N-C | 0.1 M H2SO4 | 0.82 | 0.78 | 4 | [37] |
| Fe-N-C@MXene | 0.1 M KOH | 0.9 | 0.887 | 4 | [38] |
| N-Fe2MoC-GC | 0.1 M KOH | 0.95 | 0.887 | 4 | [39] |
| FeCo/NC-Mo2TiC2 | 0.1 M KOH | 1.017 | 0.887 | 2 | [40] |
| Fe-Mn/NC | 0.1 M KOH | 1.015 | 0.904 | 4 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, S.; Teng, J.; Zhao, S.; Li, Z.; Wu, M. Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction. Molecules 2023, 28, 5544. https://doi.org/10.3390/molecules28145544
Wang M, Zhang S, Teng J, Zhao S, Li Z, Wu M. Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction. Molecules. 2023; 28(14):5544. https://doi.org/10.3390/molecules28145544
Chicago/Turabian StyleWang, Min, Shilin Zhang, Juejin Teng, Shunsheng Zhao, Zhongtao Li, and Mingbo Wu. 2023. "Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction" Molecules 28, no. 14: 5544. https://doi.org/10.3390/molecules28145544
APA StyleWang, M., Zhang, S., Teng, J., Zhao, S., Li, Z., & Wu, M. (2023). Combination of Mn-Mo Oxide Nanoparticles on Carbon Nanotubes through Nitrogen Doping to Catalyze Oxygen Reduction. Molecules, 28(14), 5544. https://doi.org/10.3390/molecules28145544






