Shape-Persistent Dendrimers
Abstract
1. Introduction
2. 2D Shape-Persistent Dendrimers with Co-Planar Rigid Linkers and Flexible Peripheral Groups
3. 2D Shape-Persistent Dendrimers with Co-Planar Rigid Linkers and Bulky Peripheral Groups
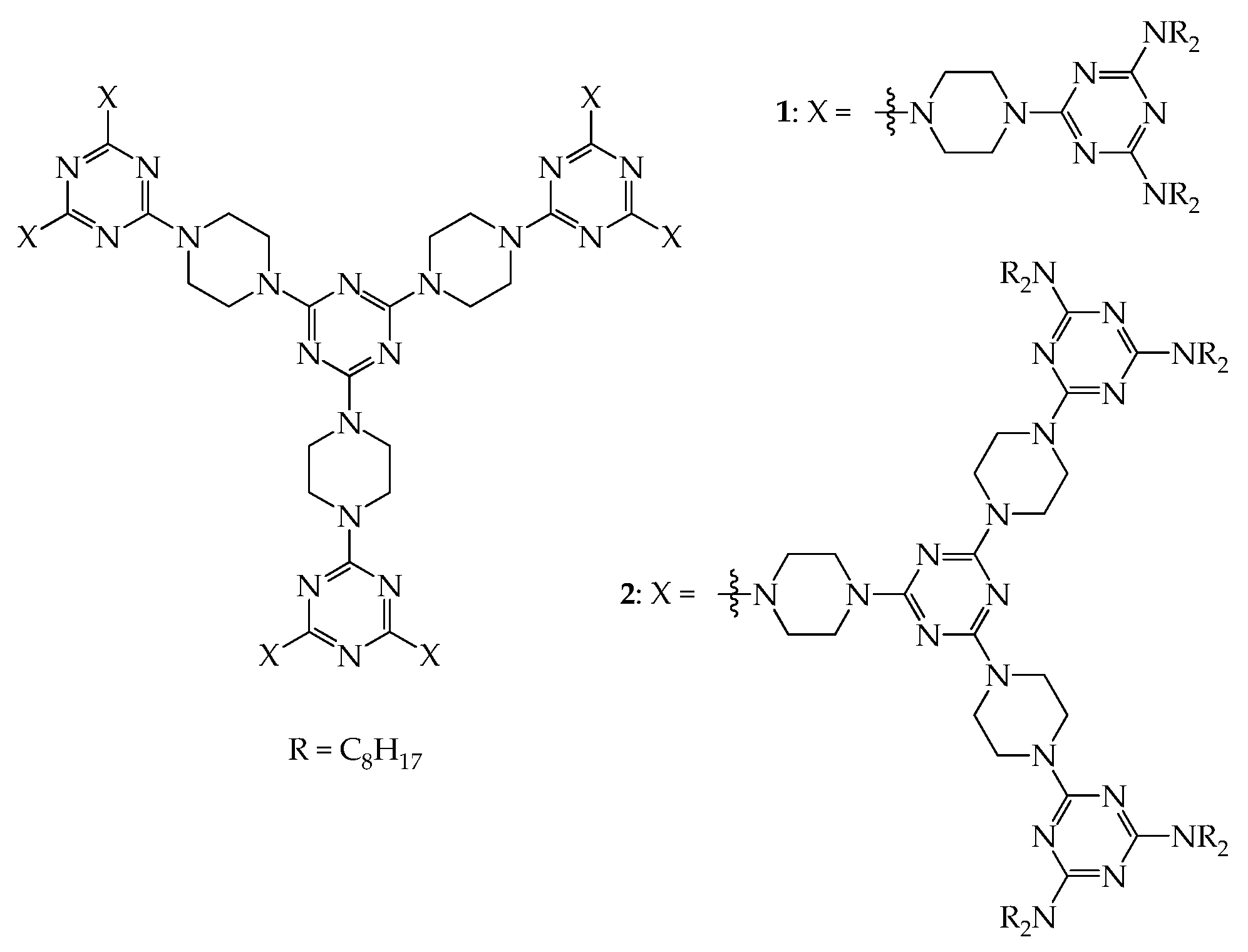
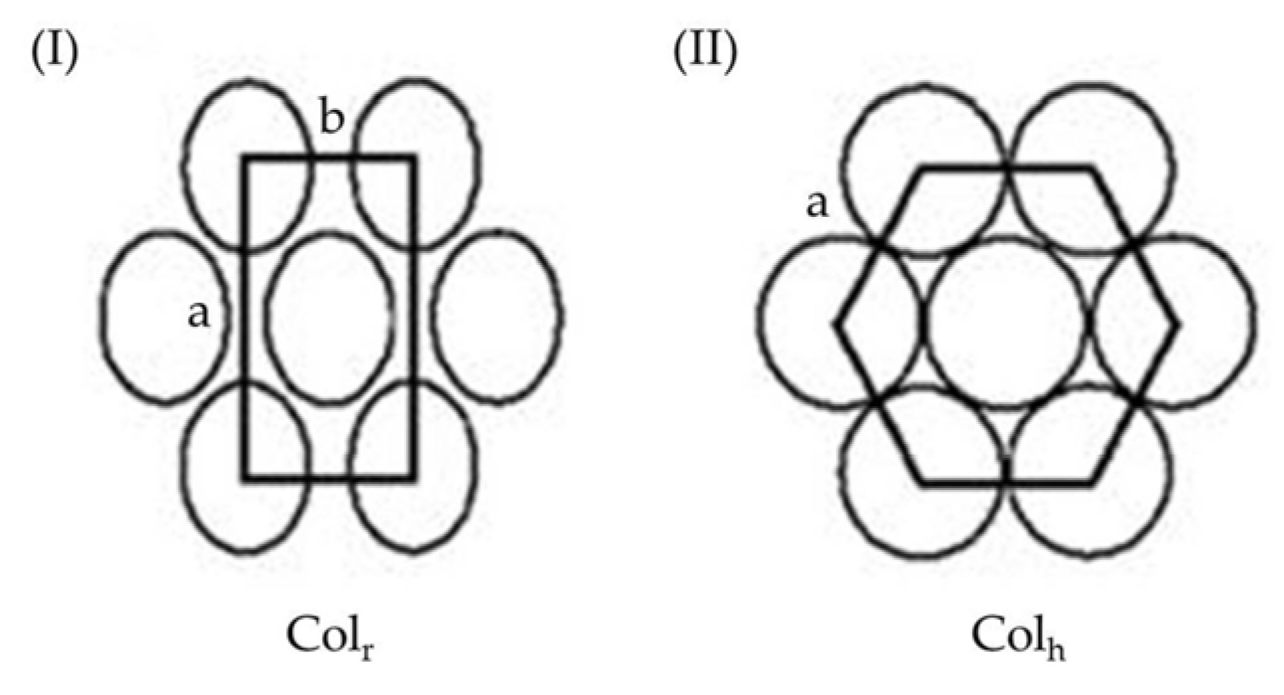
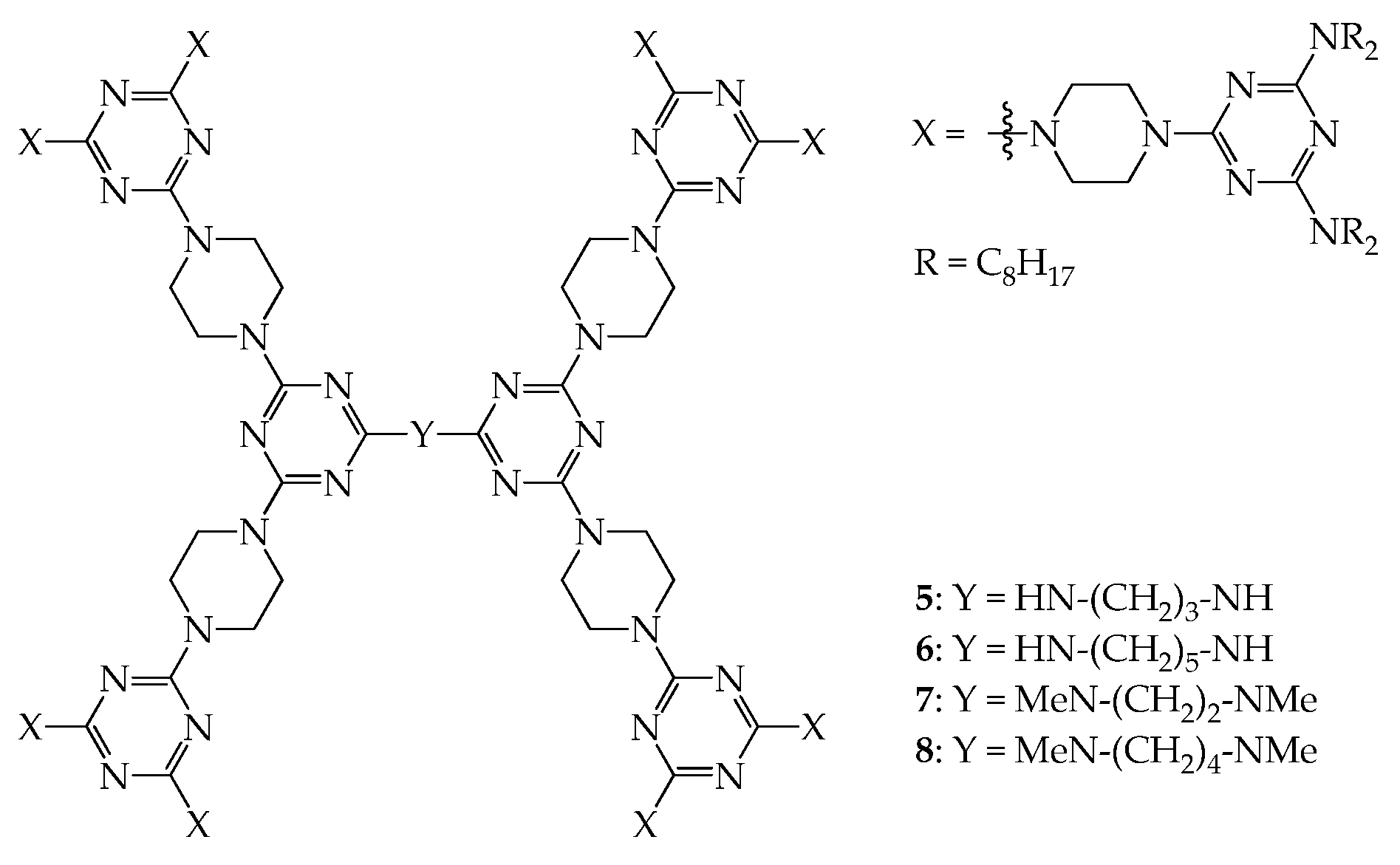
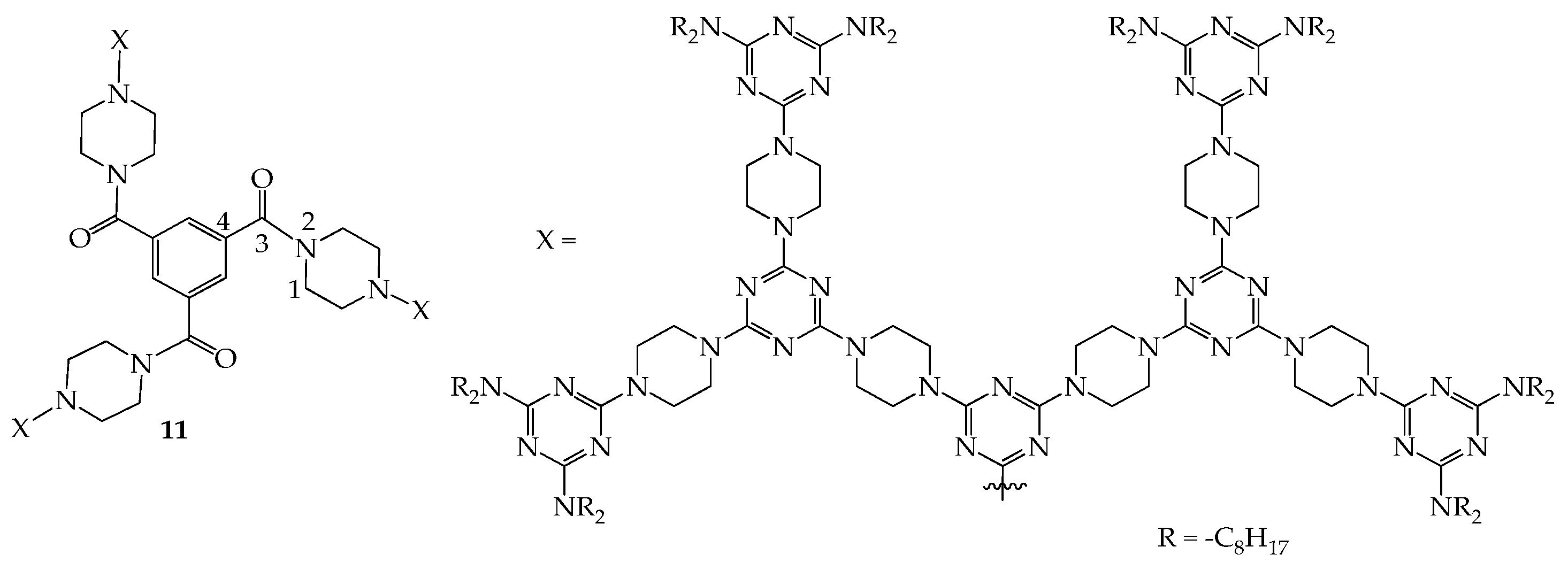
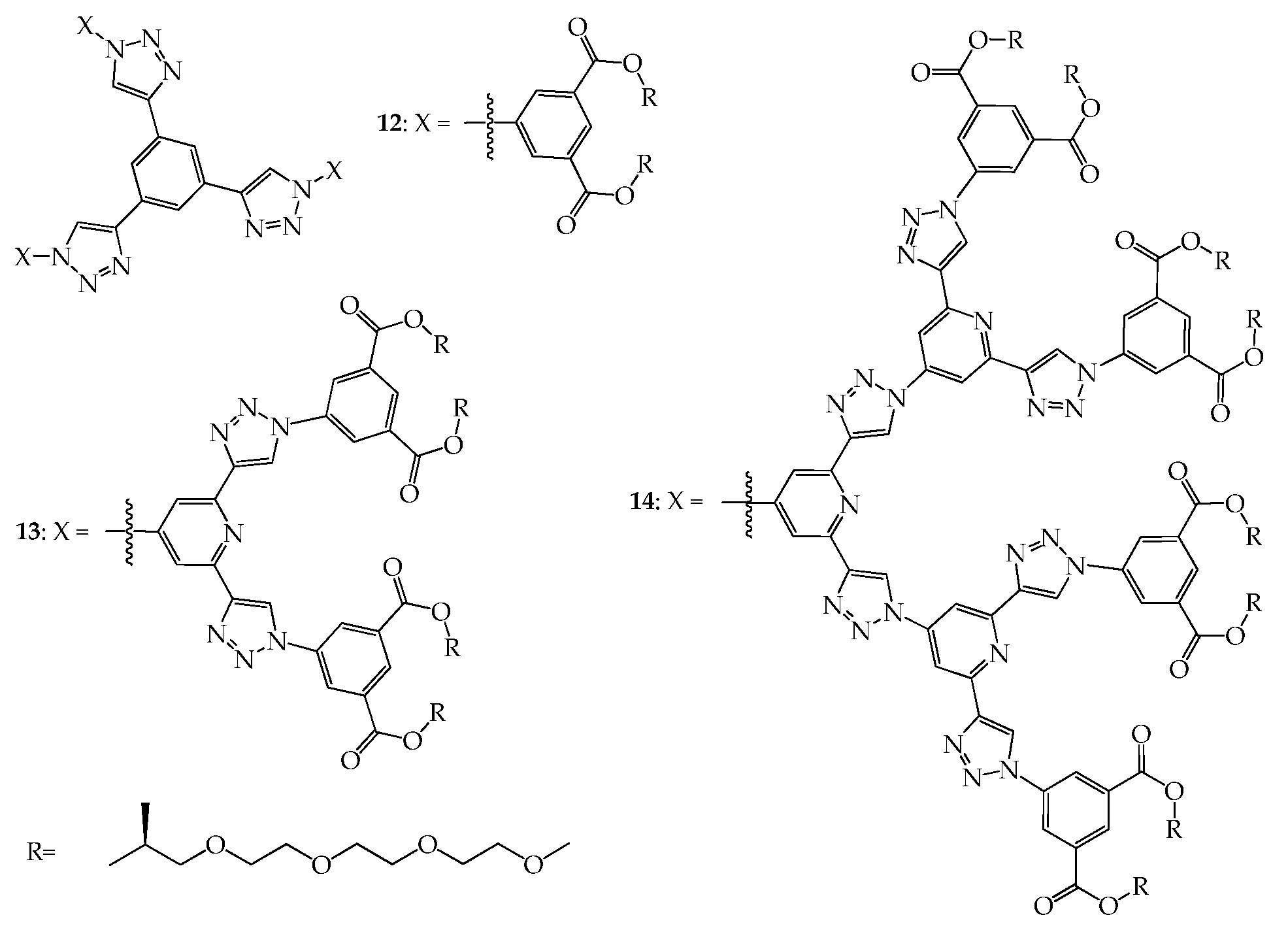
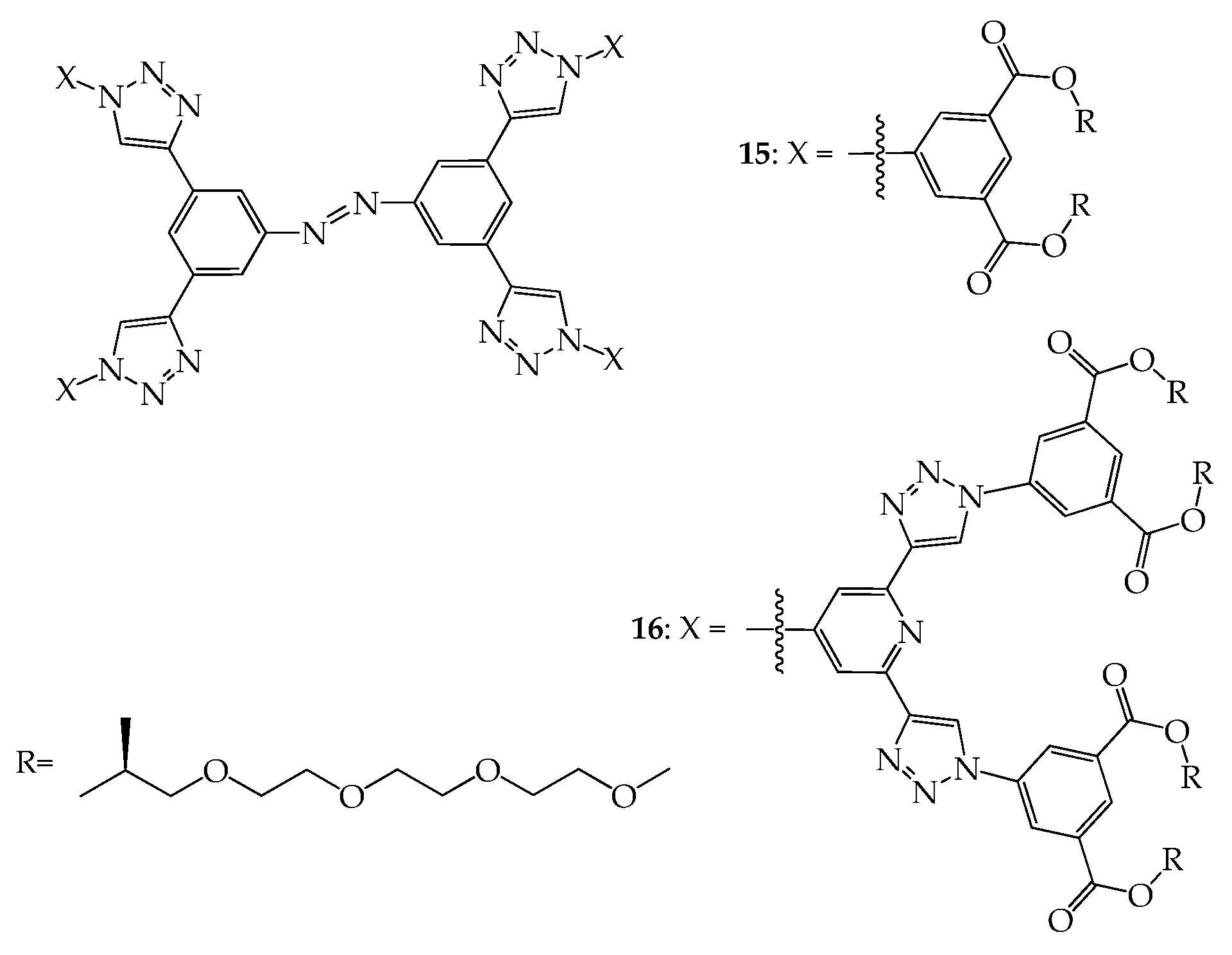
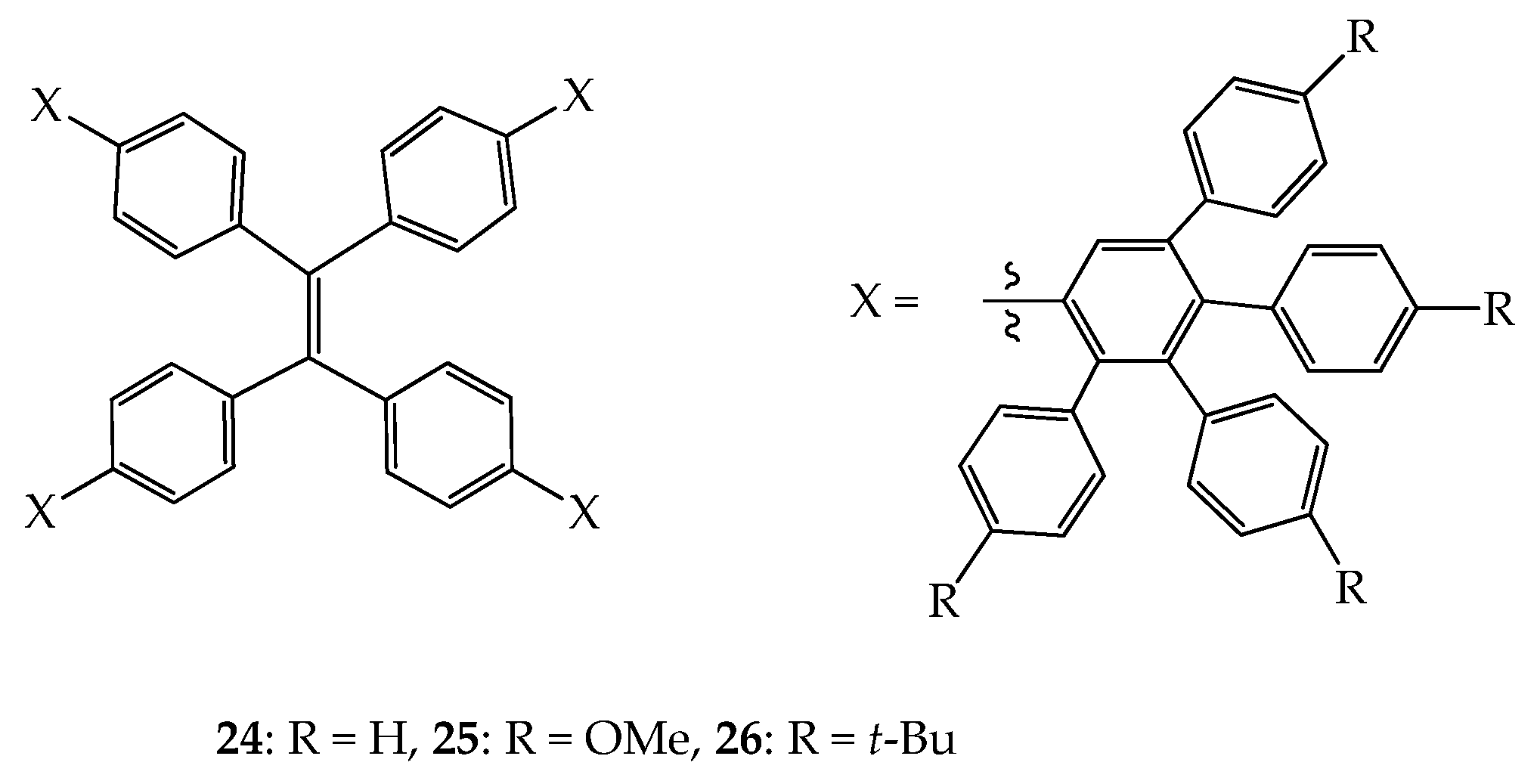
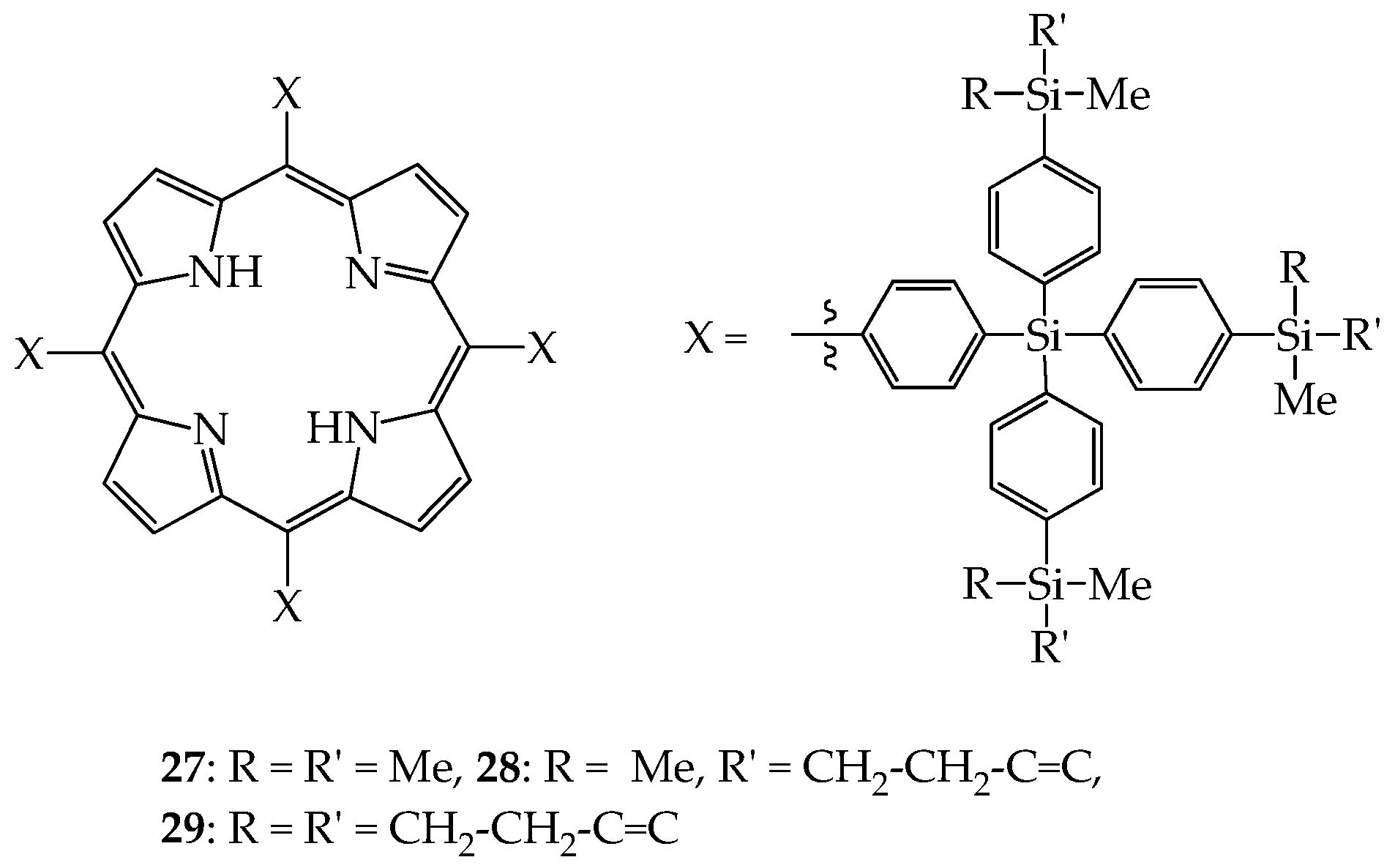
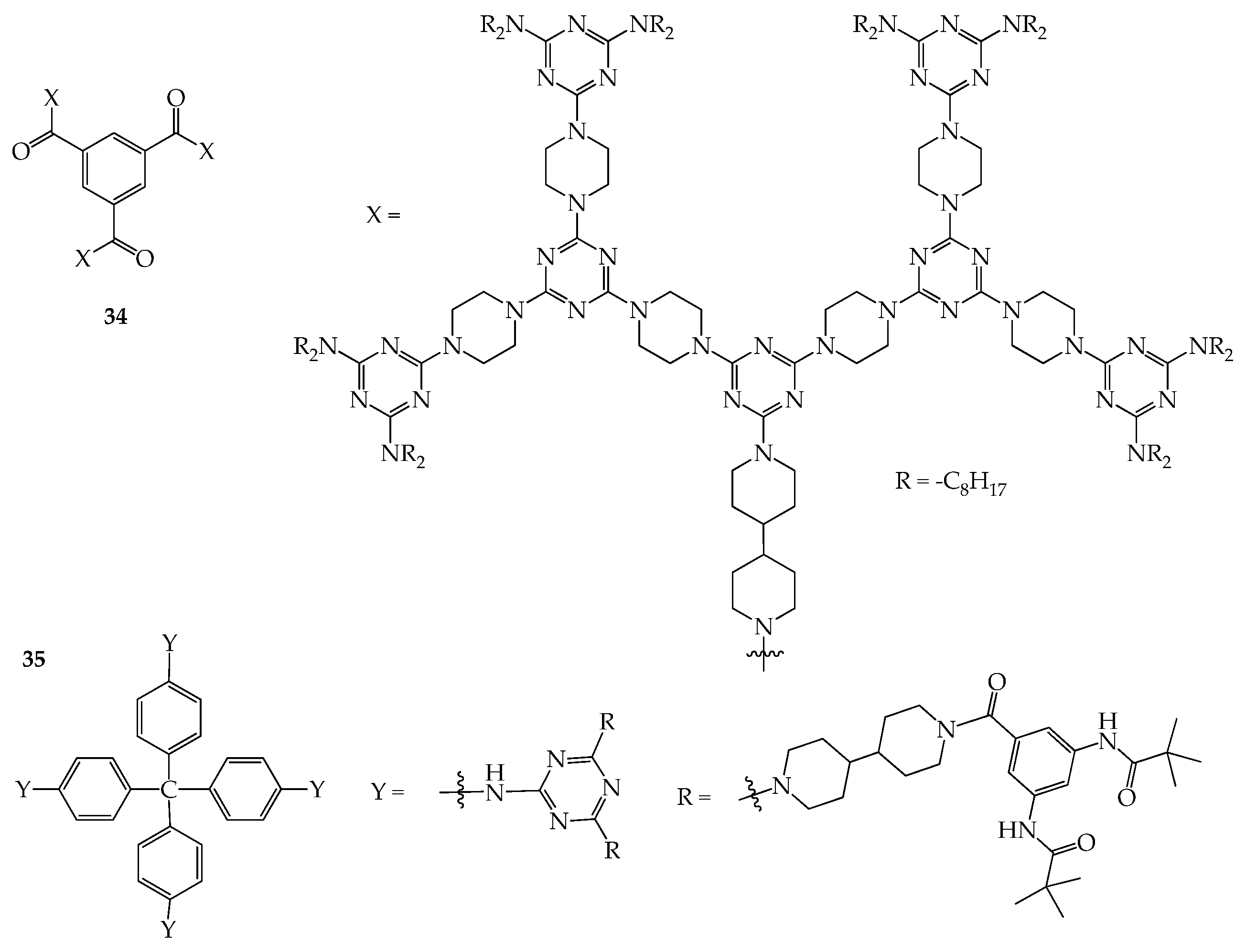
4. 2D or 3D Shape-Persistent Dendrimers with Highly Twisted Rigid Linkers
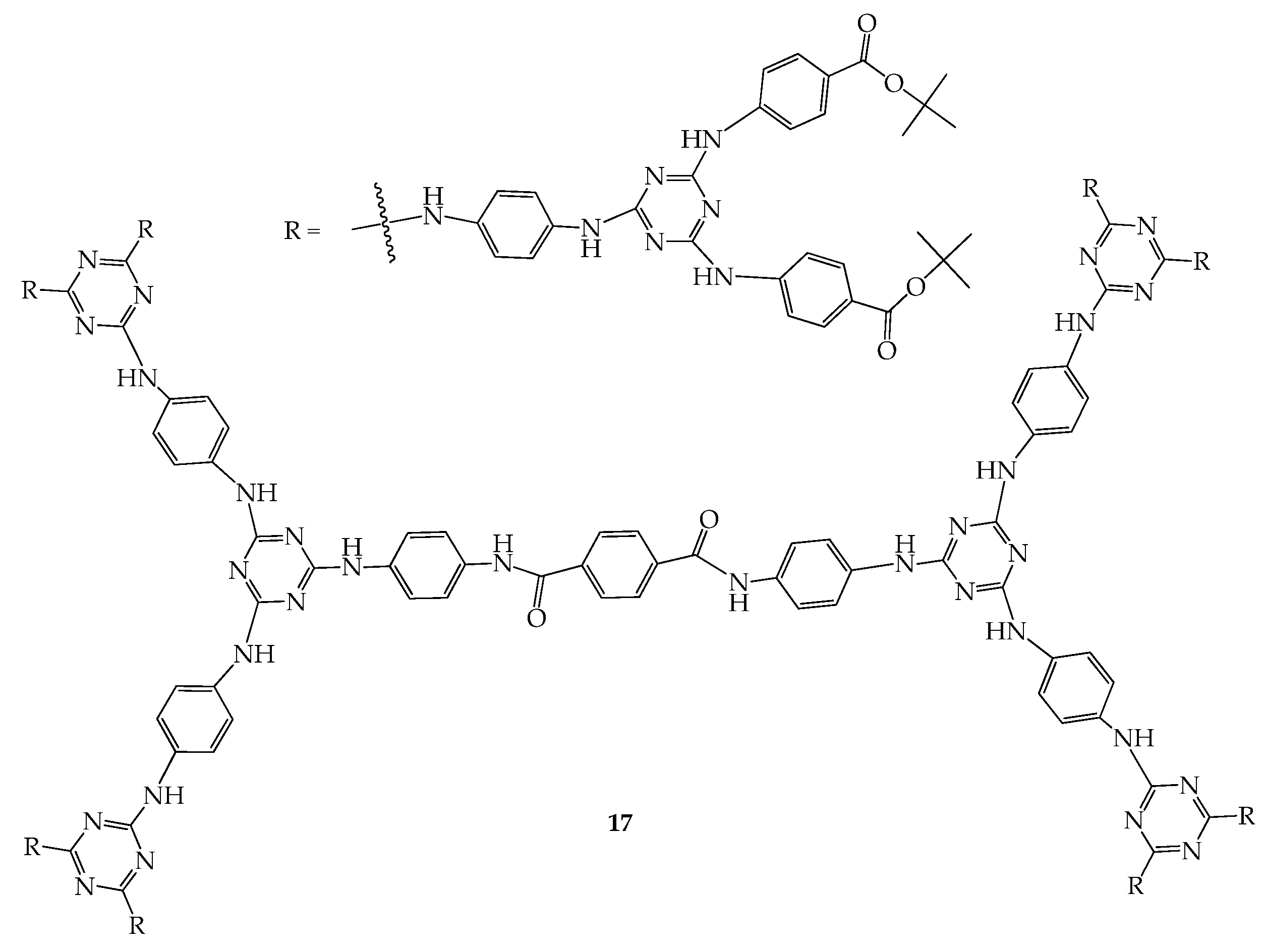
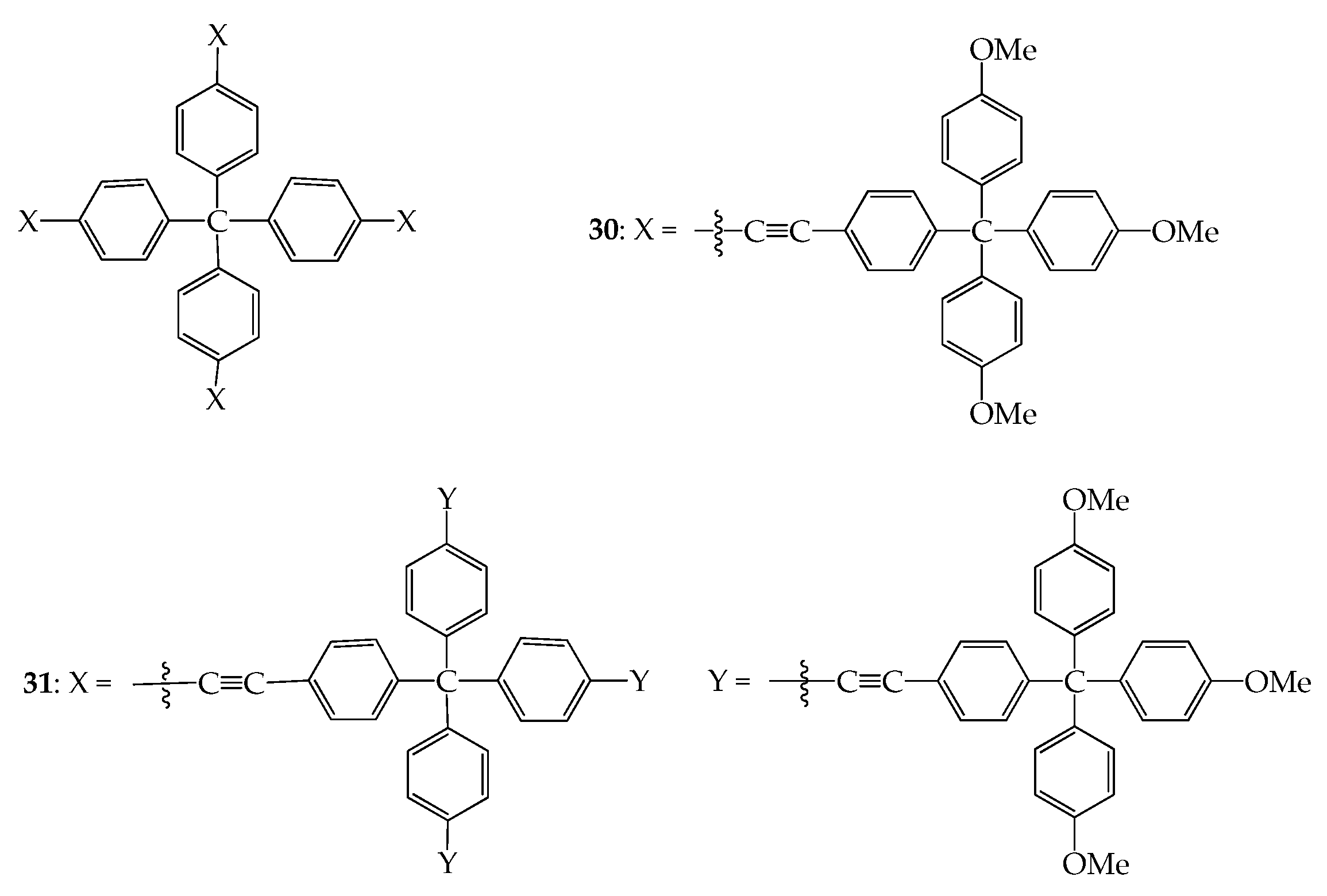
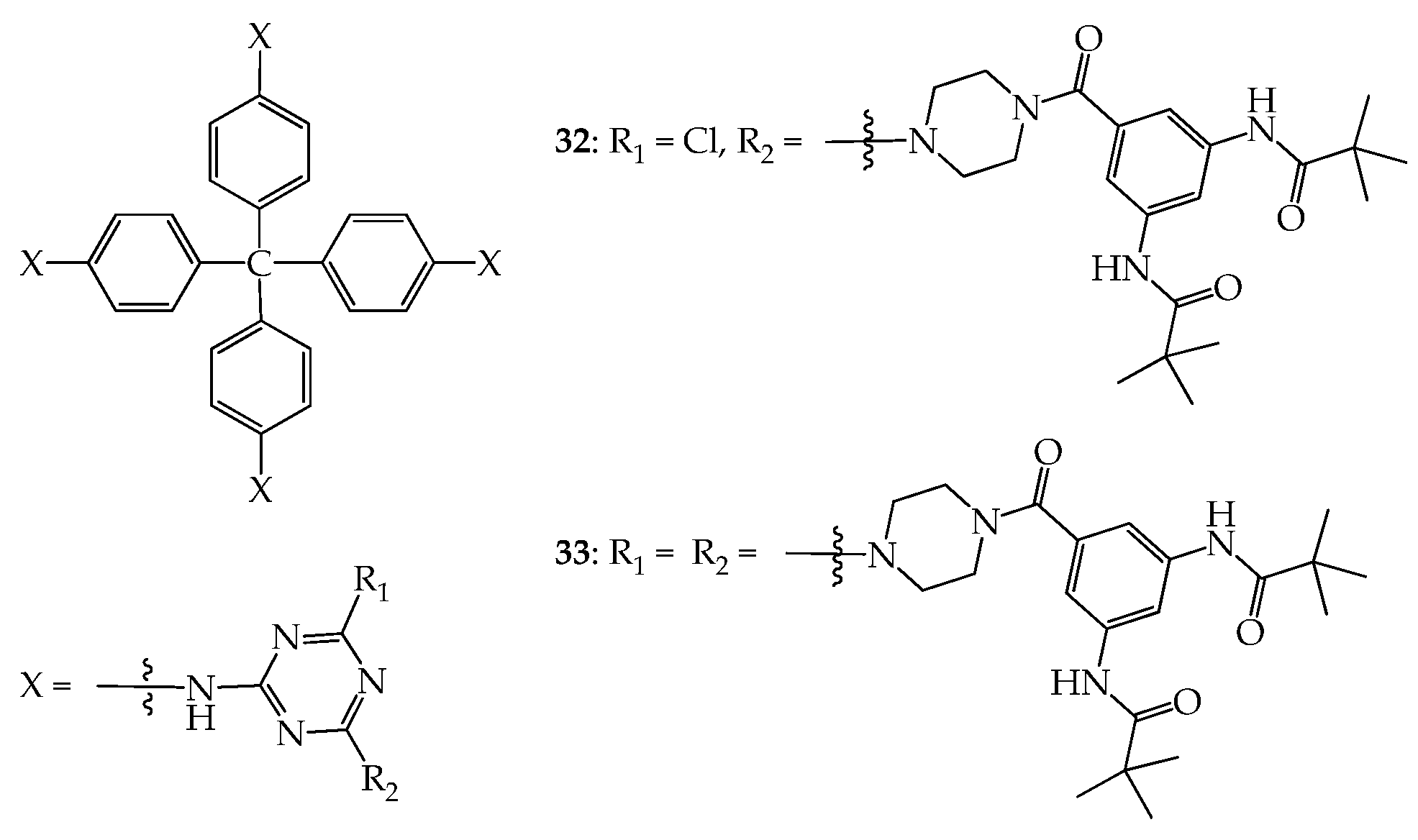
5. 3D Molecules with Three-Dimensional Rigid Linkers or Cores
6. Possible Applications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A review on comparative study of PPI and PAMAM dendrimers. J. Nanoparticle Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent advances in dendrimer-based nanoplatform for cancer treatment: A review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- Liu, Y.; Lopes, R.P.; Lüdtke, T.; Di Silvio, D.; Moya, S.; Hamon, J.-R.; Astruc, D. “Click” dendrimer-Pd nanoparticle assemblies as enzyme mimics: Catalytic o-phenylenediamine oxidation and application in colorimetric H2O2 detection. Inorg. Chem. Front. 2021, 8, 3301–3307. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Cangiotti, M.; Kao, C.-L.; Ottaviani, M.F. EPR Characterization of Copper(II) Complexes of PAMAM-Py Dendrimers for Biocatalysis in the Absence and Presence of Reducing Agents and a Spin Trap. J. Phys. Chem. B 2017, 121, 10498–10507. [Google Scholar] [CrossRef]
- Neumann, P.; Dib, H.; Caminade, A.-M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314. [Google Scholar] [CrossRef]
- Twyman, L.J.; King, A.S.H.; Martin, I.K. Catalysis inside dendrimers. Chem. Soc. Rev. 2002, 31, 69–82. [Google Scholar] [CrossRef]
- Astruc, D.; Chardac, F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3024. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Imahori, H. Giant Multiporphyrin Arrays as Artificial Light-Harvesting Antennas. J. Phys. Chem. B 2004, 108, 6130–6143. [Google Scholar] [CrossRef]
- Nantalaksakul, A.; Reddy, D.R.; Bardeen, C.J.; Thayumanavan, S. Light Harvesting Dendrimers. Photosyn. Res. 2006, 87, 133–150. [Google Scholar] [CrossRef]
- Bonardd, S.; Díaz Díaz, D.; Leiva, A.; Saldías, C. Chromophoric Dendrimer-Based Materials: An Overview of Holistic-Integrated Molecular Systems for Fluorescence Resonance Energy Transfer (FRET) Phenomenon. Polymers 2021, 13, 4404. [Google Scholar] [CrossRef]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Serrano, J.L. Highly congested liquid crystal structures: Dendrimers, dendrons, dendronized and hyperbranched polymers. Chem. Soc. Rev. 2007, 36, 1889–1901. [Google Scholar] [CrossRef]
- Saez, I.M.; Goodby, J.W. Supermolecular liquid crystals. J. Mater. Chem. 2005, 15, 26–40. [Google Scholar] [CrossRef]
- Deschenaux, R.; Donnio, B.; Guillon, D. Liquid-crystalline fullerodendrimers. New J. Chem. 2007, 31, 1064–1073. [Google Scholar] [CrossRef]
- Pirzadeh, K.; Ghoreyshi, A.A.; Rohani, S.; Rahimnejad, M. Strong Influence of Amine Grafting on MIL-101 (Cr) Metal–Organic Framework with Exceptional CO2/N2 Selectivity. Ind. Eng. Chem. Res. 2020, 59, 366–378. [Google Scholar] [CrossRef]
- Sudan, S.; Gładysiak, A.; Valizadeh, B.; Lee, J.-H.; Stylianou, K.C. Sustainable Capture of Aromatic Volatile Organic Compounds by a Pyrene-Based Metal–Organic Framework under Humid Conditions. Inorg. Chem. 2020, 59, 9029–9036. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.M.H.; Jhung, S.H. Oxidative modification of metal-organic framework-derived carbon: An effective strategy for adsorptive elimination of carbazole and benzonitrile. Fuel 2022, 307, 121764. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Pan, Q.; Wu, C.; Hao, W.; Xu, J.; Chen, R.; Liu, J.; Li, Z.; Zhao, Y. Construction of Fully Conjugated Covalent Organic Frameworks via Facile Linkage Conversion for Efficient Photoenzymatic Catalysis. J. Am. Chem. Soc. 2020, 142, 5958–5963. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Diwakara, S.D.; Thompson, C.M.; Smaldone, R.A. Supramolecular design in 2D covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1344–1356. [Google Scholar] [CrossRef]
- Ghahari, A.; Raissi, H.; Farzad, F. Design of a new drug delivery platform based on surface functionalization 2D covalent organic frameworks. J. Taiwan Inst. Chem. Eng. 2021, 125, 15–22. [Google Scholar] [CrossRef]
- Huang, L.; Miao, J.; Shuai, Q. Carboxyl-functionalized magnetic porous organic polymers as efficient adsorbent for wastewater remediation. J. Taiwan Inst. Chem. Eng. 2020, 109, 97–102. [Google Scholar] [CrossRef]
- Subodh; Prakash, K.; Masram, D.T. A reversible chromogenic covalent organic polymer for gas sensing applications. Dalton Trans. 2020, 49, 1007–1010. [Google Scholar] [CrossRef]
- Roy Chowdhury, A.; Maiti, S.; Mondal, A.; Das, A.K. Picolinohydrazide-Based Covalent Organic Polymer for Metal-Free Catalysis and Removal of Heavy Metals from Wastewater. J. Phys. Chem. C 2020, 124, 7835–7843. [Google Scholar] [CrossRef]
- Takagi, K.; Hattori, T.; Kunisada, H.; Yuki, Y. Triazine dendrimers by divergent and convergent methods. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4385–4395. [Google Scholar] [CrossRef]
- Steffensen, M.B.; Hollink, E.; Kuschel, F.; Bauer, M.; Simanek, E.E. Dendrimers based on [1,3,5]-triazines. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3411–3433. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.A.G.; Müllen, K. Expanding the limits of synthetic macromolecular chemistry through Polyphenylene Dendrimers. J. Nanoparticle Res. 2018, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.A.G.; Müllen, K. Dimensional Evolution of Polyphenylenes: Expanding in All Directions. Chem. Rev. 2016, 116, 2103–2140. [Google Scholar] [CrossRef]
- Lai, L.-L.; Hsu, S.-J.; Hsu, H.-C.; Wang, S.-W.; Cheng, K.-L.; Chen, C.-J.; Wang, T.-H.; Hsu, H.-F. Formation of Columnar Liquid Crystals on the Basis of Unconventional Triazine-Based Dendrimers by the C3-Symmetric Approach. Chem. Eur. J. 2012, 18, 6542–6547. [Google Scholar] [CrossRef]
- Lai, L.-L.; Lee, C.-H.; Wang, L.-Y.; Cheng, K.-L.; Hsu, H.-F. Star-Shaped Mesogens of Triazine-Based Dendrons and Dendrimers as Unconventional Columnar Liquid Crystals. J. Org. Chem. 2008, 73, 485–490. [Google Scholar] [CrossRef]
- Lai, L.-L.; Wang, S.-W.; Cheng, K.-L.; Lee, J.-J.; Wang, T.-H.; Hsu, H.-F. Induction of the Columnar Phase of Unconventional Dendrimers by Breaking the C2 Symmetry of Molecules. Chem. Eur. J. 2012, 18, 15361–15367. [Google Scholar] [CrossRef]
- Lai, L.-L.; Hsieh, J.-W.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. A Small Change in Central Linker Has a Profound Effect in Inducing Columnar Phases of Triazine-Based Unconventional Dendrimers. Chem. Eur. J. 2014, 20, 5160–5166. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Hsieh, J.-W.; Lai, L.-L.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. Converting Nonliquid Crystals into Liquid Crystals by N-Methylation in the Central Linker of Triazine-Based Dendrimers. J. Org. Chem. 2016, 81, 5007–5013. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, C.-C.; Li, C.-Y.; Lai, L.-L.; Lee, J.-J.; Hsu, H.-F. Both increasing the Iso-to-Col transition and lowering the solidifying temperatures of a triazine-based dendrimer by introducing CN polar groups in the dendritic core. J. Mater. Chem. C 2019, 7, 14232–14238. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Anedda, R.; Chen, H.-H.; Hsu, H.-C.; Hsu, S.-J.; Ratcliffe, C.; Lai, L.-L.; Ripmeester, J.; Hsu, H.-F. Direct 129Xe-NMR spectroscopy evidence of a mesogenic dendrimer with free void space. J. Mater. Chem. C 2023, 11, 3710–3714. [Google Scholar] [CrossRef]
- El Malah, T.; Rolf, S.; Weidner, S.M.; Thünemann, A.F.; Hecht, S. Amphiphilic Folded Dendrimer Discs and Their Thermosensitive Self-Assembly in Water. Chem. Eur. J. 2012, 18, 5837–5842. [Google Scholar] [CrossRef] [PubMed]
- Hourani, R.; Sharma, A.; Kakkar, A. Designing dendritic frameworks using versatile building blocks suitable for CuI-catalyzed. alkyne azide ‘click’ chemistry. Tetrahedron Lett. 2010, 51, 3792–3795. [Google Scholar] [CrossRef]
- Stangenberg, R.; Türp, D.; Müllen, K. Shape persistent hybrid dendrimers from benzene and triazole via ‘click chemistry’. Tetrahedron 2014, 70, 3178–3184. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F. Click Synthesis of Shape-Persistent Azodendrimers and their Orthogonal Self-Assembly to Nanofibres. Aust. J. Chem. 2018, 71, 463–472. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tsai, M.-R.; Chang, Y.-T.; Lai, L.-L.; Lu, K.-L.; Cheng, K.-L. Preparation of Unconventional Dendrimers that Contain Rigid NH—Triazine Linkages and Peripheral tert-Butyl Moieties for CO2-Selective Adsorption. Chem. Eur. J. 2013, 19, 10573–10579. [Google Scholar] [CrossRef]
- Lee, C.-H.; Soldatov, D.V.; Tzeng, C.-H.; Lai, L.-L.; Lu, K.-L. Design of a Peripheral Building Block for H-Bonded Dendritic Frameworks and Analysis of the Void Space in the Bulk Dendrimers. Sci. Rep. 2017, 7, 3649. [Google Scholar] [CrossRef]
- Patel, H.A.; Karadas, F.; Canlier, A.; Park, J.; Deniz, E.; Jung, Y.; Atilhan, M.; Yavuz, C.T. High capacity carbon dioxide adsorption by inexpensive covalent organic polymers. J. Mater. Chem. 2012, 22, 8431–8437. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chien, C.-Y.; Hsu, H.-F.; Lai, L.-L. Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g. Molecules 2021, 26, 4862. [Google Scholar] [CrossRef]
- Xia, M.; Jin, C.; Kong, X.; Jiang, M.; Lei, D.; Lei, X. Green removal of pyridine from water via adsolubilization with lignosulfonate intercalated layered double hydroxide. Adsorpt. Sci. Technol. 2018, 36, 982–998. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, X.; Liu, S.; Li, Y.; Wang, L.; Liu, Y. Dendritic polyphenylene AIEgens: Fluorescence detection of explosives and stimulus-responsive luminescence. Polym. Chem. 2022, 13, 6197–6204. [Google Scholar] [CrossRef]
- Ishtaiwi, Z.; Rüffer, T.; Klaib, S.; Buschbeck, R.; Walfort, B.; Lang, H. Porphyrins with a carbosilane dendrimer periphery as synthetic components for supramolecular self-assembly. Dalton Trans. 2014, 43, 7868–7888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Yan, J.; Tang, Z.-M.; Xiao, Q.; Ma, Y.; Pei, J. Gradient Shape-Persistent π-Conjugated Dendrimers for Light-Harvesting: Synthesis, Photophysical Properties, and Energy Funneling. J. Am. Chem. Soc. 2008, 130, 9952–9962. [Google Scholar] [CrossRef]
- Urzúa, J.I.; Regueira, M.A.; Lazzari, M.; Torneiro, M. Shape-persistent fluorescent tetraphenylmethane dendrimers. Polym. Chem. 2016, 7, 5641–5645. [Google Scholar] [CrossRef]
- Urzúa, J.I.; Torneiro, M. Divergent Synthesis of Porous Tetraphenylmethane Dendrimers. J. Org. Chem. 2017, 82, 13231–13238. [Google Scholar] [CrossRef]
- Gu, Z.-T.; Tzeng, C.-H.; Chien, H.-J.; Chen, C.-C.; Lai, L.-L. Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds. Int. J. Mol. Sci. 2022, 23, 11155. [Google Scholar] [CrossRef]
- Kumar, S.; Bisoyi, H.K. Aligned Carbon Nanotubes in the Supramolecular Order of Discotic Liquid Crystals. Angew. Chem. Int. Ed. 2007, 46, 1501–1503. [Google Scholar] [CrossRef]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. A dendrimer emitter doped in a dendrimer host: Efficient thermally activated delayed fluorescence OLEDs with fully-solution processed organic-layers. Mater. Chem. Front. 2018, 2, 1097–1103. [Google Scholar] [CrossRef]
- Liu, C.-X.; Wang, H.; Du, J.-Q.; Zhao, K.-Q.; Hu, P.; Wang, B.-Q.; Monobe, H.; Heinrich, B.; Donnio, B. Molecular design of benzothienobenzothiophene-cored columnar mesogens: Facile synthesis, mesomorphism, and charge carrier mobility. J. Mater. Chem. C 2018, 6, 4471–4478. [Google Scholar] [CrossRef]
- Termine, R.; Golemme, A. Charge Mobility in Discotic Liquid Crystals. Int. J. Mol. Sci. 2021, 22, 877. [Google Scholar] [CrossRef]
- Baskoro, F.; Chiang, P.-C.; Lu, Y.-C.; Patricio, J.N.; Arco, S.D.; Chen, H.-C.; Kuo, W.-S.; Lai, L.-L.; Yen, H.-J. Columnar liquid-crystalline triazine-based dendrimer with carbon nanotube filler for efficient organic lithium-ion batteries. Electrochim. Acta 2022, 434, 141306. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The Evaporation, Condensation and Reflection of Molecules and the Mechanism of Adsorption. Phys. Rev. 1916, 8, 149–176. [Google Scholar] [CrossRef]
- Osterrieth, J.W.M.; Rampersad, J.; Madden, D.; Rampal, N.; Skoric, L.; Connolly, B.; Allendorf, M.D.; Stavila, V.; Snider, J.L.; Ameloot, R.; et al. How Reproducible are Surface Areas Calculated from the BET Equation? Adv. Mater. 2022, 34, 2201502. [Google Scholar] [CrossRef] [PubMed]


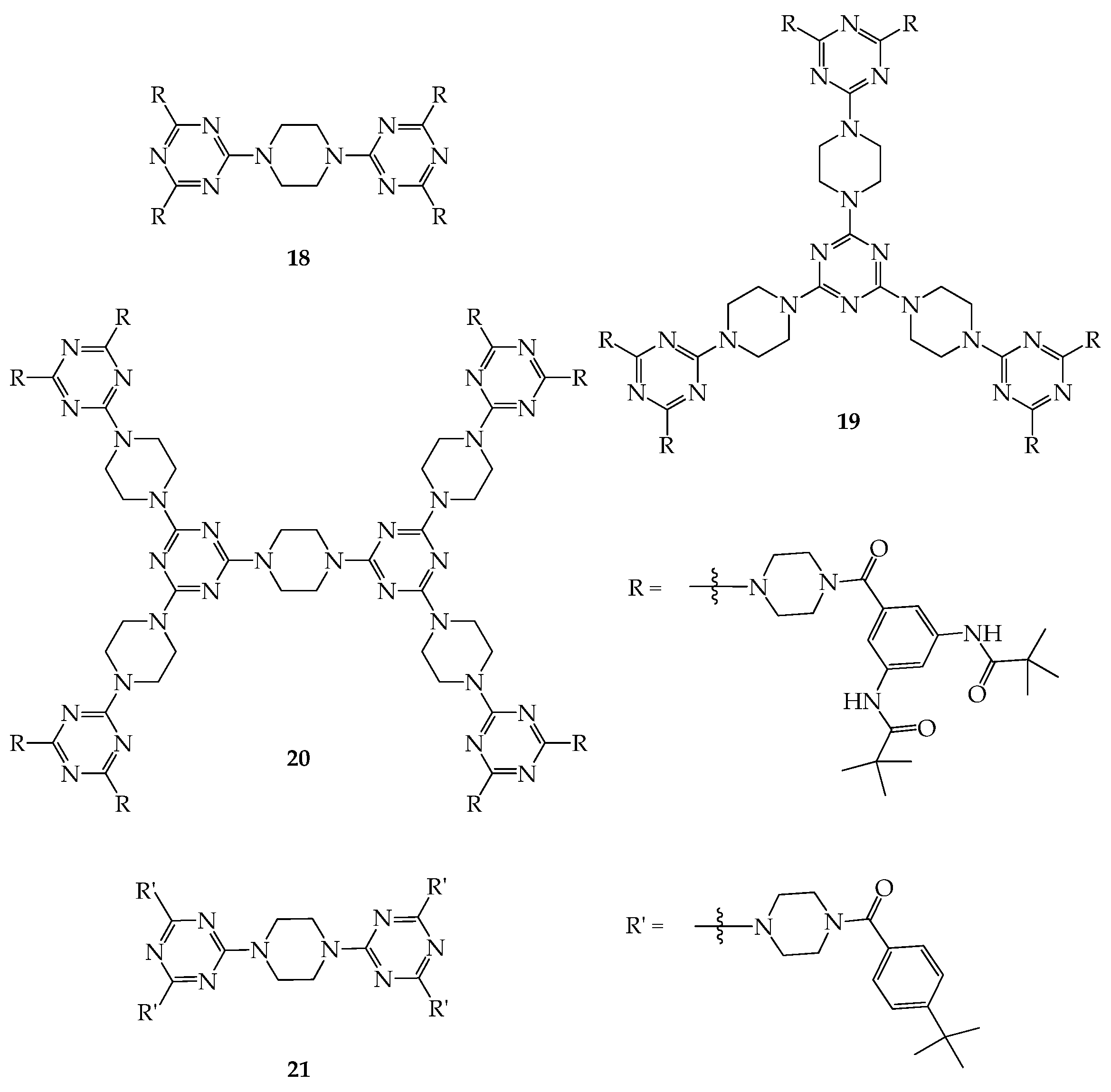

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Anedda, R.; Lai, L.-L. Shape-Persistent Dendrimers. Molecules 2023, 28, 5546. https://doi.org/10.3390/molecules28145546
Lu Y-C, Anedda R, Lai L-L. Shape-Persistent Dendrimers. Molecules. 2023; 28(14):5546. https://doi.org/10.3390/molecules28145546
Chicago/Turabian StyleLu, Yao-Chih, Roberto Anedda, and Long-Li Lai. 2023. "Shape-Persistent Dendrimers" Molecules 28, no. 14: 5546. https://doi.org/10.3390/molecules28145546
APA StyleLu, Y.-C., Anedda, R., & Lai, L.-L. (2023). Shape-Persistent Dendrimers. Molecules, 28(14), 5546. https://doi.org/10.3390/molecules28145546







