Atomistic Insights into the Effect of Functional Groups on the Adsorption of Water by Activated Carbon for Heat Energy Storage
Abstract
:1. Introduction
2. Results and Discussion
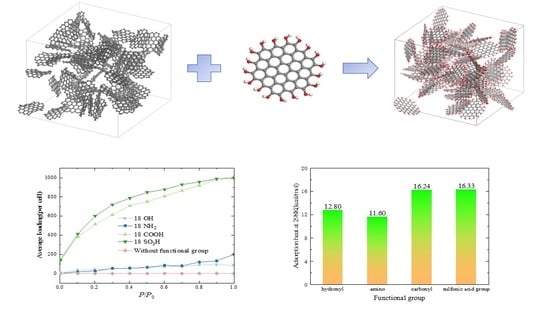
2.1. The Influence of Functional Group Types
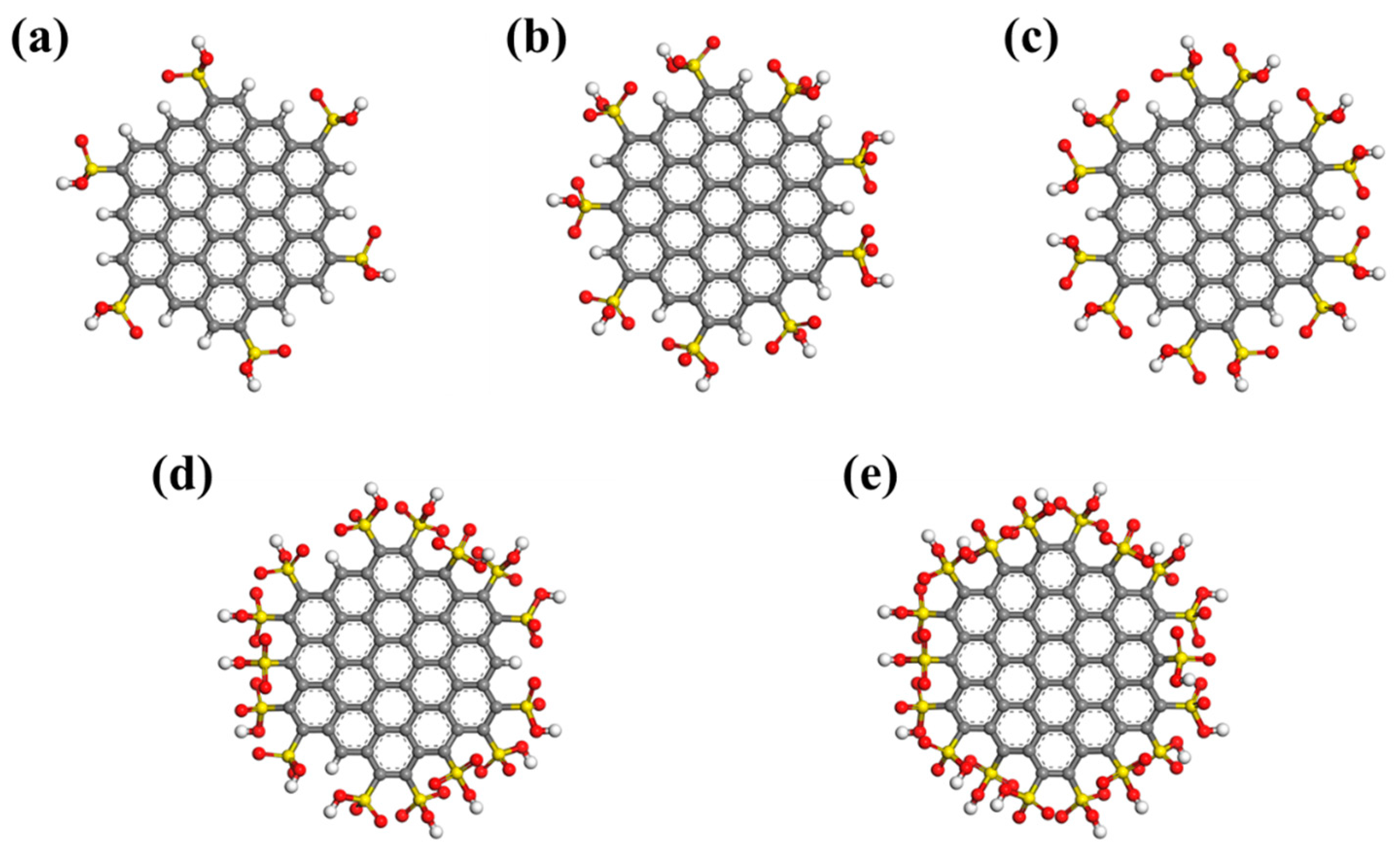
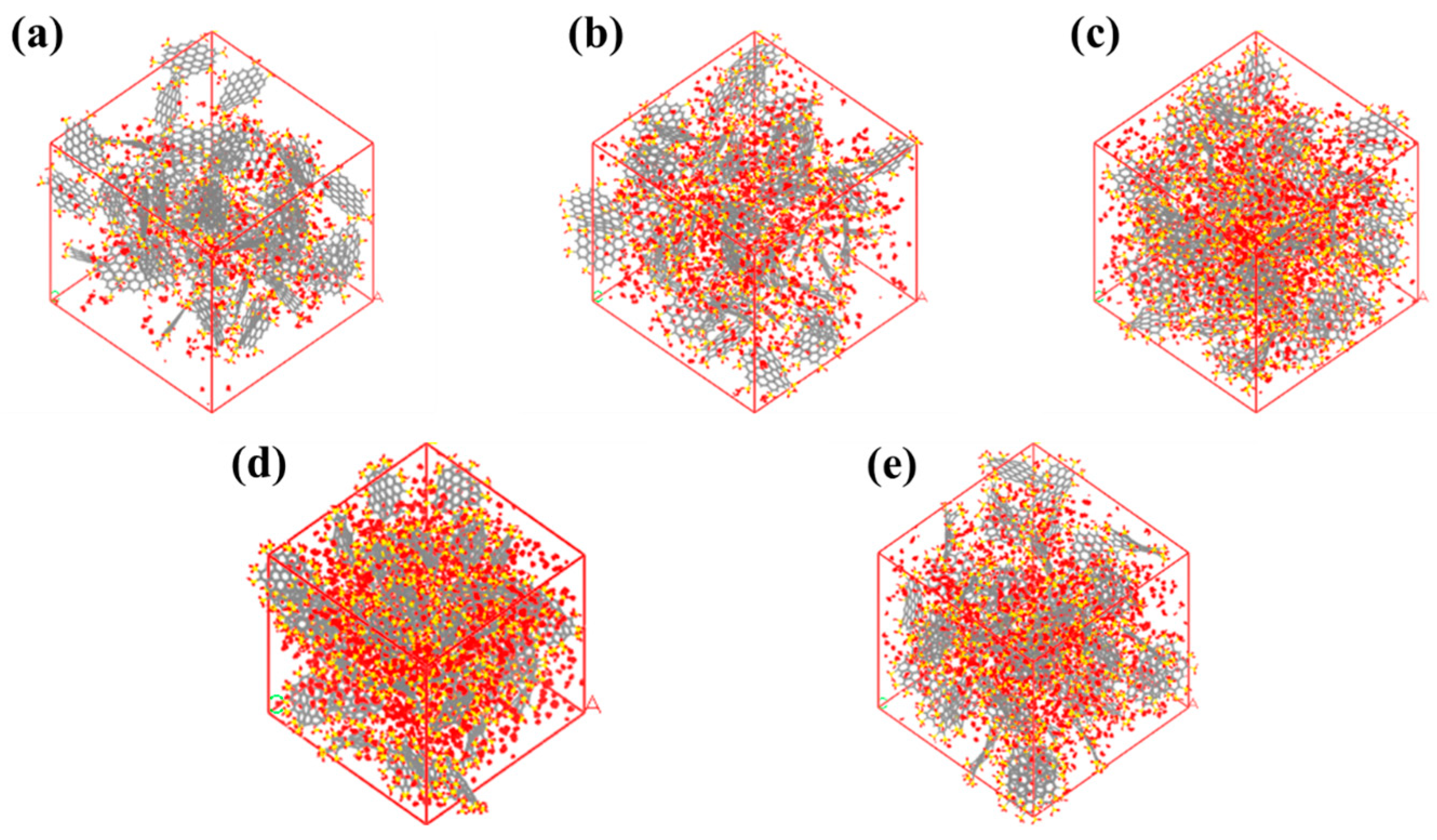
2.2. The Influence of Functional Group Numbers
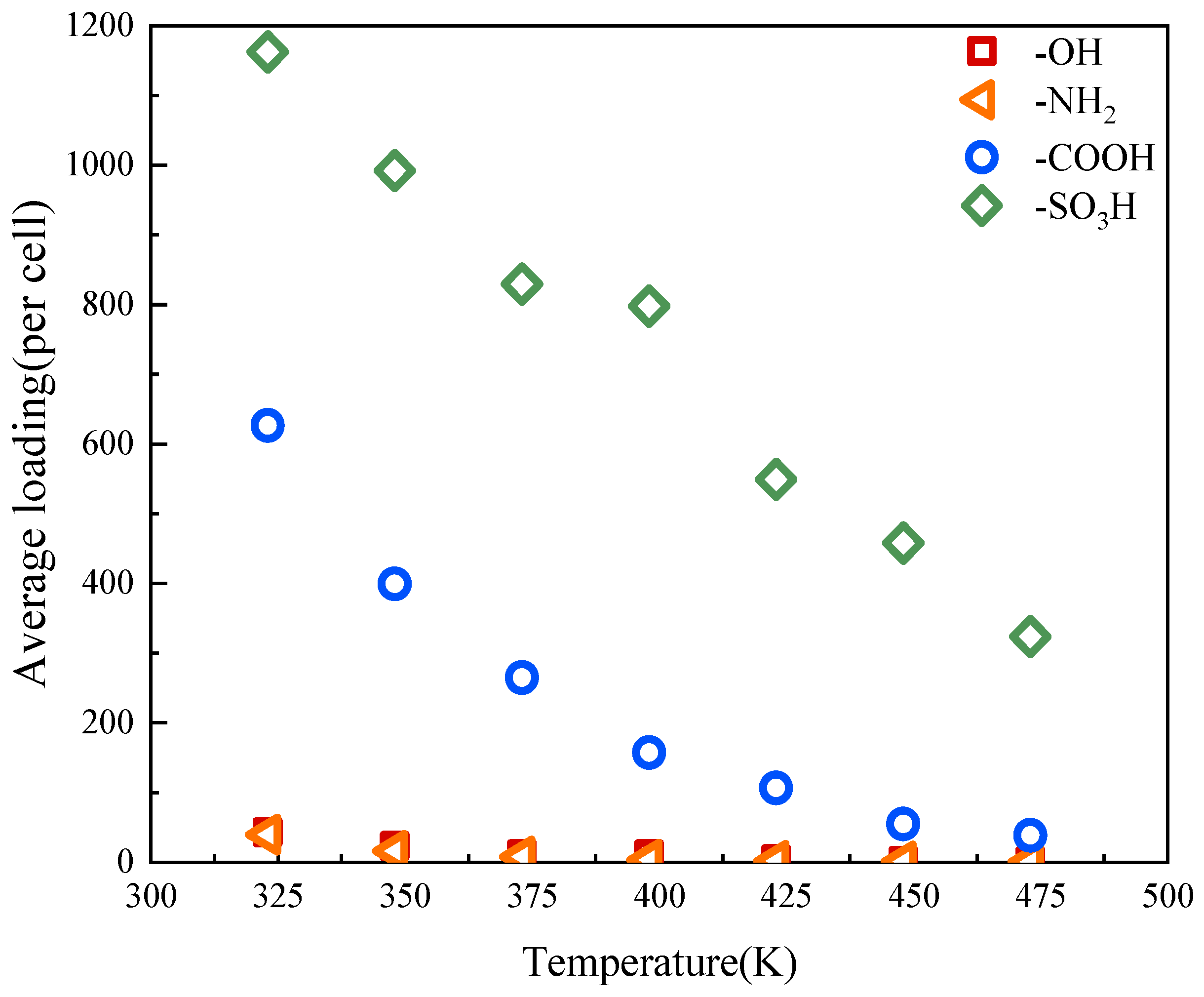
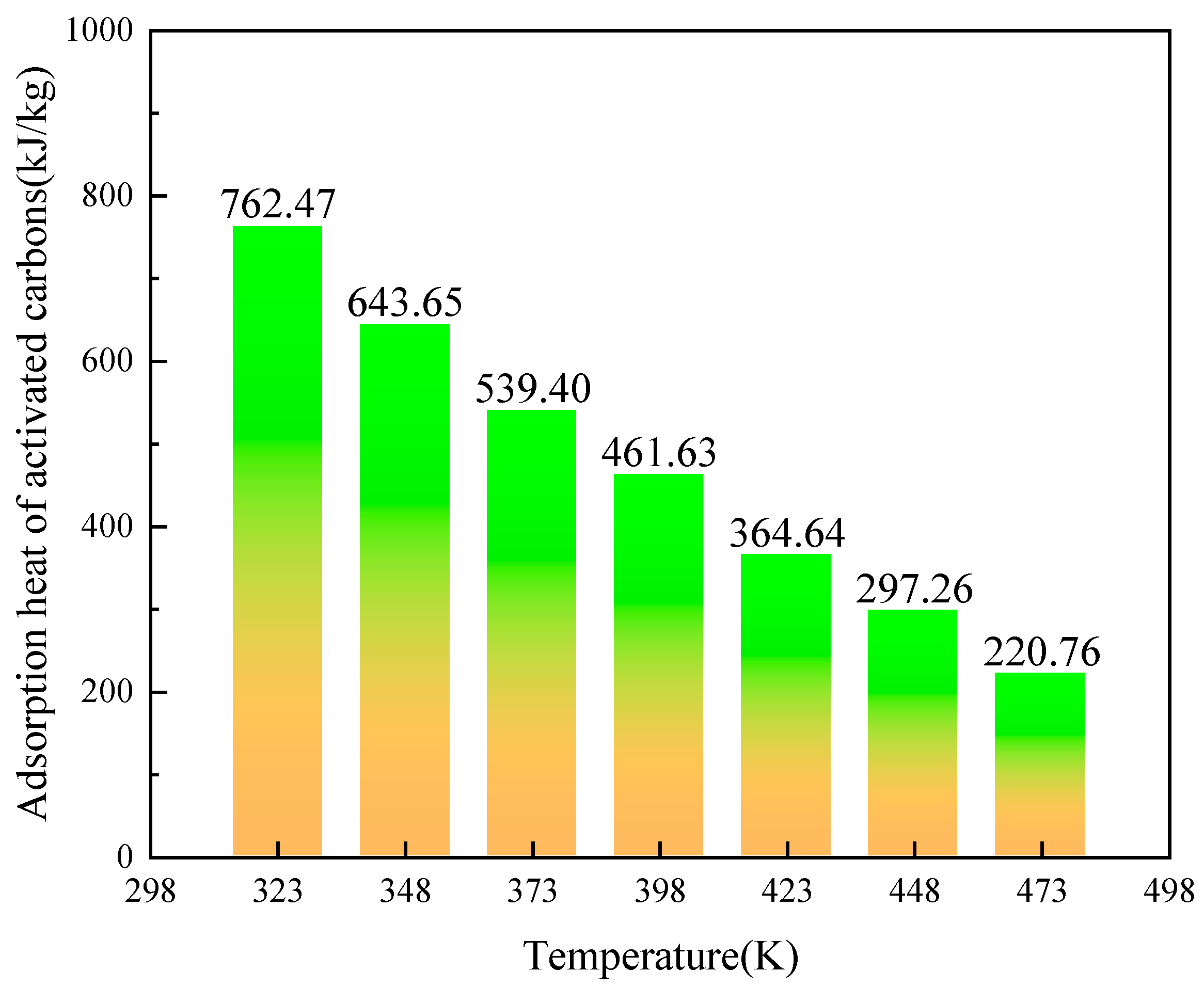
2.3. The Influence of Temperature
3. Physical Model and Numerical Details
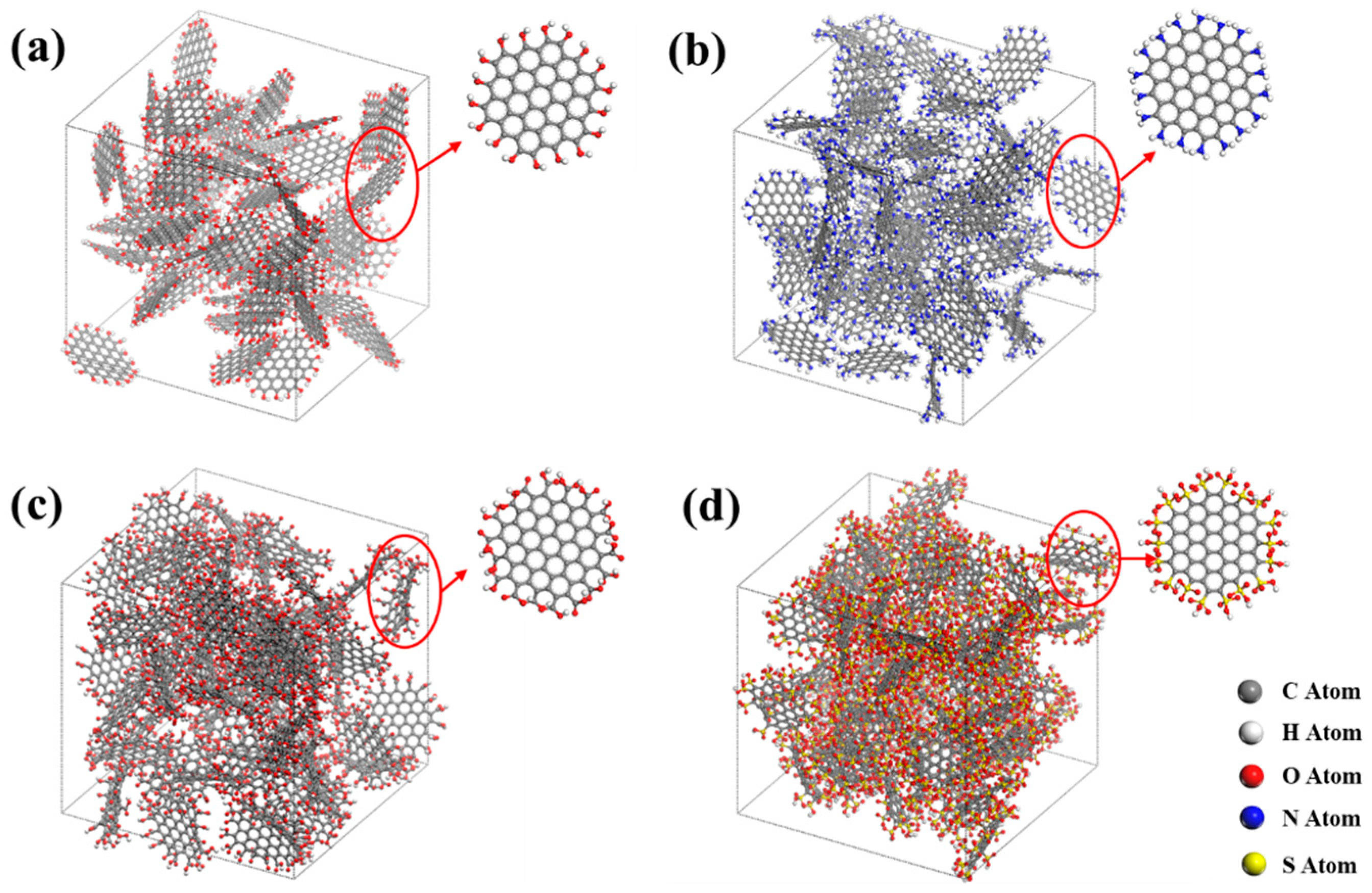
3.1. Activated Carbon Model
3.2. Theory and Calculation Details
4. Conclusions
- The activated carbon without functional groups is superhydrophobic and hardly absorbs water under low and medium pressure. The functional groups can significantly improve the water adsorption capacity of activated carbon. Under low and medium pressure, the activated carbon with -SO3H shows the best adsorption capacity, followed by the activated carbon with -COOH. The functional groups of -OH and -NH2 can also improve the adsorption capacity of the activated carbon but are not as effective as -SO3H and -COOH.
- Under low and medium pressure, increasing the number of -SO3H functional groups can increase the water adsorption capacity. However, increasing the functional group numbers may decrease the water adsorption capacity due to porosity reduction when the pressure is high. The length and number of hydrogen bonds between functional groups and water molecules affect the adsorption capacity significantly.
- In the temperature range of 323~473 K, the adsorption capacity of activated carbons with -OH, -NH2, -COOH, and -SO3H decreases with temperature increase. The activated carbon with -SO3H exhibits excellent adsorption performance and the prospect of application for heat storage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BP. Statistical Review of World Energy/Energy in 2020: The Year of COVID, (n.d.) 22; BP: London, UK, 2020. [Google Scholar]
- Zhang, S.; Li, Z.; Wang, H.; Tian, L.; Jin, Y.; Alston, M.; Yan, Y. Component-dependent thermal properties of molten salt eutectics for solar thermal energy storage: Experiments, molecular simulation and applications. Appl. Therm. Eng. 2022, 209, 118333. [Google Scholar] [CrossRef]
- Costa, S.C.; Kenisarin, M. A review of metallic materials for latent heat thermal energy storage: Thermophysical properties, applications, and challenges. Renew. Sustain. Energy Rev. 2022, 154, 111812. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2021, 33, 101913. [Google Scholar] [CrossRef]
- Lefebvre, D.; Tezel, F.H. A review of energy storage technologies with a focus on adsorption thermal energy storage processes for heating applications. Renew. Sustain. Energy Rev. 2017, 67, 116–125. [Google Scholar] [CrossRef]
- Di Palo, M.; Sabatelli, V.; Buzzi, F.; Gabbrielli, R. Experimental and Numerical Assessment of a Novel All-In-One Adsorption Thermal Storage with Zeolite for Thermal Solar Applications. Appl. Sci. 2020, 10, 8517. [Google Scholar] [CrossRef]
- Gautam, A.; Saini, R.P. A review on technical, applications and economic aspect of packed bed solar thermal energy storage system. J. Energy Storage 2020, 27, 101046. [Google Scholar] [CrossRef]
- Nasruddin; Arsyad, A.P.; Djubaedah; Wulandari, D.A.; Hidayat, H.F. Experimental analysis of Indonesian natural zeolites-water pair for closed system adsorption thermal energy storage. AIP Conf. Proc. 2020, 2255, 040036. [Google Scholar]
- Miller, M.A.; Wang, C.-Y.; Merrill, G.N. Experimental and Theoretical Investigation Into Hydrogen Storage via Spillover in IRMOF-8. J. Phys. Chem. C 2009, 113, 3222–3231. [Google Scholar] [CrossRef]
- Kohler, T.; Müller, K. Storage of low grade solar thermal energy by adsorption of organics. AIP Conf. Proc. 2017, 1850, 080014. [Google Scholar]
- Hu, Q.; Lu, Y.; Meisner, G.P. Preparation of Nanoporous Carbon Particles and Their Cryogenic Hydrogen Storage Capacities. J. Phys. Chem. C 2008, 112, 1516–1523. [Google Scholar] [CrossRef]
- Deshmukh, H.; Maiya, M.P.; Srinivasa Murthy, S. Study of sorption based energy storage system with silica gel for heating application. Appl. Therm. Eng. 2017, 111, 1640–1646. [Google Scholar] [CrossRef]
- Ayisi, E.N.; Fraňa, K. The Design and Test for Degradation of Energy Density of a Silica Gel-Based Energy Storage System Using Low Grade Heat for Desorption Phase. Energies 2020, 13, 4513. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, Y.; Shen, C.; Shi, J.; Xu, G.; Xiao, X.; Cao, R. Water sorption properties of functionalized MIL-101(Cr)-X (X = –NH2, –SO3H, H, –CH3, –F) based composites as thermochemical heat storage materials. Microporous Mesoporous Mater. 2019, 285, 129–136. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Tuning the Metal Binding Energy and Hydrogen Storage in Alkali Metal Decorated MOF-5 through Boron Doping: A Theoretical Investigation. J. Phys. Chem. C 2011, 115, 16984–16991. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and municipal sludge. Mater. Chem. Phys. 2020, 241, 122327. [Google Scholar] [CrossRef]
- Sultan, M.; El-Sharkaw, I.I.; Miyazaki, T.; Saha, B.B.; Koyama, S. Experimental Study on Carbon Based Adsorbents for Greenhouse Dehumidification. Evergreen 2014, 1, 5–11. [Google Scholar] [CrossRef]
- Chairunnisa; Mikšík, F.; Miyazaki, T.; Thu, K.; Miyawaki, J.; Nakabayashi, K.; Wijayanta, A.T.; Rahmawati, F. Enhancing water adsorption capacity of acorn nutshell based activated carbon for adsorption thermal energy storage application. Energy Rep. 2020, 6, 255–263. [Google Scholar] [CrossRef]
- Wiig, E.O.; Juhola, A.J. The Adsorption of Water Vapor on Activated Charcoal. J. Am. Chem. Soc. 1949, 71, 561–568. [Google Scholar] [CrossRef]
- Qian, Q.; Sunohara, S.; Kato, Y.; Zaini, M.A.A.; Machida, M.; Tatsumoto, H. Water vapor adsorption onto activated carbons prepared from cattle manure compost (CMC). Appl. Surf. Sci. 2008, 254, 4868–4874. [Google Scholar] [CrossRef]
- Horikawa, T.; Sakao, N.; Do, D.D. Effects of temperature on water adsorption on controlled microporous and mesoporous carbonaceous solids. Carbon 2013, 56, 183–192. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Uygur, Y.; Thomas, K.M. Role of Surface Functional Groups in the Adsorption Kinetics of Water Vapor on Microporous Activated Carbons. J. Phys. Chem. C 2007, 111, 8349–8359. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Ngernyen, Y.; Tangsathitkulchai, M. Surface modification and adsorption of eucalyptus wood-based activated carbons: Effects of oxidation treatment, carbon porous structure and activation method. Korean J. Chem. Eng 2009, 26, 1341–1352. [Google Scholar] [CrossRef]
- Koyanagi, J.; Itano, N.; Yamamoto, M.; Mori, K.; Ishida, Y.; Bazhirov, T. Evaluation of the mechanical properties of carbon fiber/polymer resin interfaces by molecular simulation. Adv. Compos. Mater. 2019, 28, 639–652. [Google Scholar] [CrossRef]
- Xie, J.; Xiao, C.; Zhang, L.; Lü, F.; Xie, Q.; Cheng, L. Molecular simulation of nano polyhedral oligomeric silsesquioxane doping effect on the properties of two-component crosslinked epoxy resin. J. Mol. Graph. Model. 2021, 107, 107961. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.P.; Jiang, T.; Sun, C.; Lihan, M.; Pant, S.; Mahinthichaichan, P.; Trifan, A.; Tajkhorshid, E. Characterization of Lipid–Protein Interactions and Lipid-Mediated Modulation of Membrane Protein Function through Molecular Simulation. Chem. Rev 2019, 119, 6086–6161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, H.; Bian, X.; Li, J.; Li, J.; Zhang, H. Insight into the binding of ACE-inhibitory peptides to angiotensin-converting enzyme: A molecular simulation. Mol. Simul. 2019, 45, 215–222. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Alaabed, S.; AL-Akhras, M.-A.; Obaidat, I.M. Molecular simulation of adsorption of methylene blue and rhodamine B on graphene and graphene oxide for water purification. Mater. Today Proc. 2020, 28, 1078–1083. [Google Scholar] [CrossRef]
- Chu, X.; Liu, S.; Zhou, S.; Zhao, Y.; Xing, W.; Lee, C.-H. Adsorption behaviors of CO2 and CH4 on zeolites JSR and NanJSR using the GCMC simulations. Adsorption 2016, 22, 1065–1073. [Google Scholar] [CrossRef]
- Smykowski, D.; Szyja, B.; Szczygieł, J. GCMC simulations of CO2 adsorption on zeolite-supported Ir4 clusters. J. Mol. Graph. Model. 2014, 50, 35–43. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Zhang, T.; Yang, C.; Shan, H. Adsorption and Separation Mechanism of Thiophene/Benzene in MFI Zeolite: A GCMC Study. J. Phys. Chem. C 2017, 121, 25818–25826. [Google Scholar] [CrossRef]
- Chen, L.; Morrison, C.A.; Düren, T. Improving Predictions of Gas Adsorption in Metal–Organic Frameworks with Coordinatively Unsaturated Metal Sites: Model Potentials, ab initio Parameterization, and GCMC Simulations. J. Phys. Chem. C 2012, 116, 18899–18909. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, X.; Cao, D. Computer simulations for the adsorption and separation of CO2/CH4/H2/N2 gases by UMCM-1 and UMCM-2 metal organic frameworks. J. Mater. Chem 2011, 21, 11259. [Google Scholar] [CrossRef]
- Camp, J.; Stavila, V.; Allendorf, M.D.; Prendergast, D.; Haranczyk, M. Critical Factors in Computational Characterization of Hydrogen Storage in Metal–Organic Frameworks. J. Phys. Chem. C 2018, 122, 18957–18967. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; He, D.; Zhu, L.; Du, Y.; Xing, W.; Zhang, T. Sulfur–Nitrogen Codoped Graphite Slit-Pore for Enhancing Selective Carbon Dioxide Adsorption: Insights from Molecular Simulations. ACS Sustain. Chem. Eng. 2017, 5, 8815–8823. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Wu, D.; Lu, X.; Cao, W.; Yang, T.; Zhu, Y. Molecular Simulation Study of the Adsorption and Diffusion of a Mixture of CO2/CH4 in Activated Carbon: Effect of Textural Properties and Surface Chemistry. J. Chem. Eng. Data 2016, 61, 4139–4147. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.; Shao, Q.; Lu, L.; Lu, X.; Jiang, S.; Shen, W. Simulations of Binary Mixture Adsorption of Carbon Dioxide and Methane in Carbon Nanotubes: Temperature, Pressure, and Pore Size Effects. J. Phys. Chem. C 2007, 111, 11912–11920. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Sutjahja, I.M.; Rahayu, A.U.S.; Kurniati, N.; Pallitine, I.D.; Kurnia, D. The role of chemical additives to the phase change process of CaCl2 6H2O to optimize its performance as latent heat energy storage system. J. Phys. Conf. Ser. 2016, 739, 012064. [Google Scholar]
- Striolo, A.; Chialvo, A.A.; Cummings, P.T.; Gubbins, K.E. Water Adsorption in Carbon-Slit Nanopores. Langmuir 2003, 19, 8583–8591. [Google Scholar] [CrossRef]
- Kumar, K.V.; Salih, A.; Lu, L.; Müller, E.A.; Rodríguez-Reinoso, F. Molecular Simulation of Hydrogen Physisorption and Chemisorption in Nanoporous Carbon Structures. Adsorpt. Sci. Technol. 2011, 29, 799–817. [Google Scholar] [CrossRef]
- Sihn, S.; Roy, A.K. Modeling and prediction of bulk properties of open-cell carbon foam. J. Mech. Phys. Solids 2004, 52, 167–191. [Google Scholar] [CrossRef]
- Gu, Z.-K.; Zhu, C.-Y.; Huang, Z.-Q.; Xu, M.-H.; Gong, L. Numerical simulations on the adsorption characteristics of aromatics on activated carbon by the GCMC method. Case Stud. Therm. Eng. 2021, 26, 101116. [Google Scholar] [CrossRef]
- Li, S.; Song, K.; Zhao, D.; Rugarabamu, J.R.; Diao, R.; Gu, Y. Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mater. 2020, 302, 110220. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Huang, L.; Wang, S.; Zhu, Y.; Li, L.; Lu, X. Acetone adsorption on activated carbons: Roles of functional groups and humidity. Fluid Phase Equilibria 2020, 521, 112645. [Google Scholar] [CrossRef]
- Ao, Z.M.; Li, S.; Jiang, Q. Correlation of the applied electrical field and CO adsorption/desorption behavior on Al-doped graphene. Solid State Commun. 2010, 150, 680–683. [Google Scholar] [CrossRef]





| Functional Group | The Solid Volume, Å3 | The Pore Volume, Å3 | Porosity, % |
|---|---|---|---|
| -- | 33,305 | 91,695 | 73.36 |
| -OH | 42,230 | 82,770 | 66.22 |
| -NH2 | 47,797 | 77,203 | 61.76 |
| -COOH | 68,992 | 56,008 | 44.81 |
| -SO3H | 84,344 | 40,656 | 32.52 |
| C | H | O | N | S | |
|---|---|---|---|---|---|
| -- | −0.1268 | 0.1268 | / | / | / |
| -OH | 0.042 | 0.41 | −0.452 | / | / |
| -NH2 | 0.01 | 0.353 | / | −0.716 | / |
| -COOH | 0.53/−0.035 | 0.41 | −0.45/−0.455 | / | / |
| -SO3H | 0.16 | 0.41 | −0.543/−0.535 | / | 1.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.-Y.; Qian, Z.-H.; Tuo, Y.-X.; Gong, L.; Zhu, C.-Y. Atomistic Insights into the Effect of Functional Groups on the Adsorption of Water by Activated Carbon for Heat Energy Storage. Molecules 2024, 29, 11. https://doi.org/10.3390/molecules29010011
Duan X-Y, Qian Z-H, Tuo Y-X, Gong L, Zhu C-Y. Atomistic Insights into the Effect of Functional Groups on the Adsorption of Water by Activated Carbon for Heat Energy Storage. Molecules. 2024; 29(1):11. https://doi.org/10.3390/molecules29010011
Chicago/Turabian StyleDuan, Xin-Yue, Zeng-Hui Qian, Yong-Xiao Tuo, Liang Gong, and Chuan-Yong Zhu. 2024. "Atomistic Insights into the Effect of Functional Groups on the Adsorption of Water by Activated Carbon for Heat Energy Storage" Molecules 29, no. 1: 11. https://doi.org/10.3390/molecules29010011
APA StyleDuan, X.-Y., Qian, Z.-H., Tuo, Y.-X., Gong, L., & Zhu, C.-Y. (2024). Atomistic Insights into the Effect of Functional Groups on the Adsorption of Water by Activated Carbon for Heat Energy Storage. Molecules, 29(1), 11. https://doi.org/10.3390/molecules29010011