The Antiproliferative Activity of Adiantum pedatum Extract and/or Piceatannol in Phenylhydrazine-Induced Colon Cancer in Male Albino Rats: The miR-145 Expression of the PI-3K/Akt/p53 and Oct4/Sox2/Nanog Pathways
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening of AP Extract
2.2. Oxidative Stress Markers
2.3. Antioxidant Markers
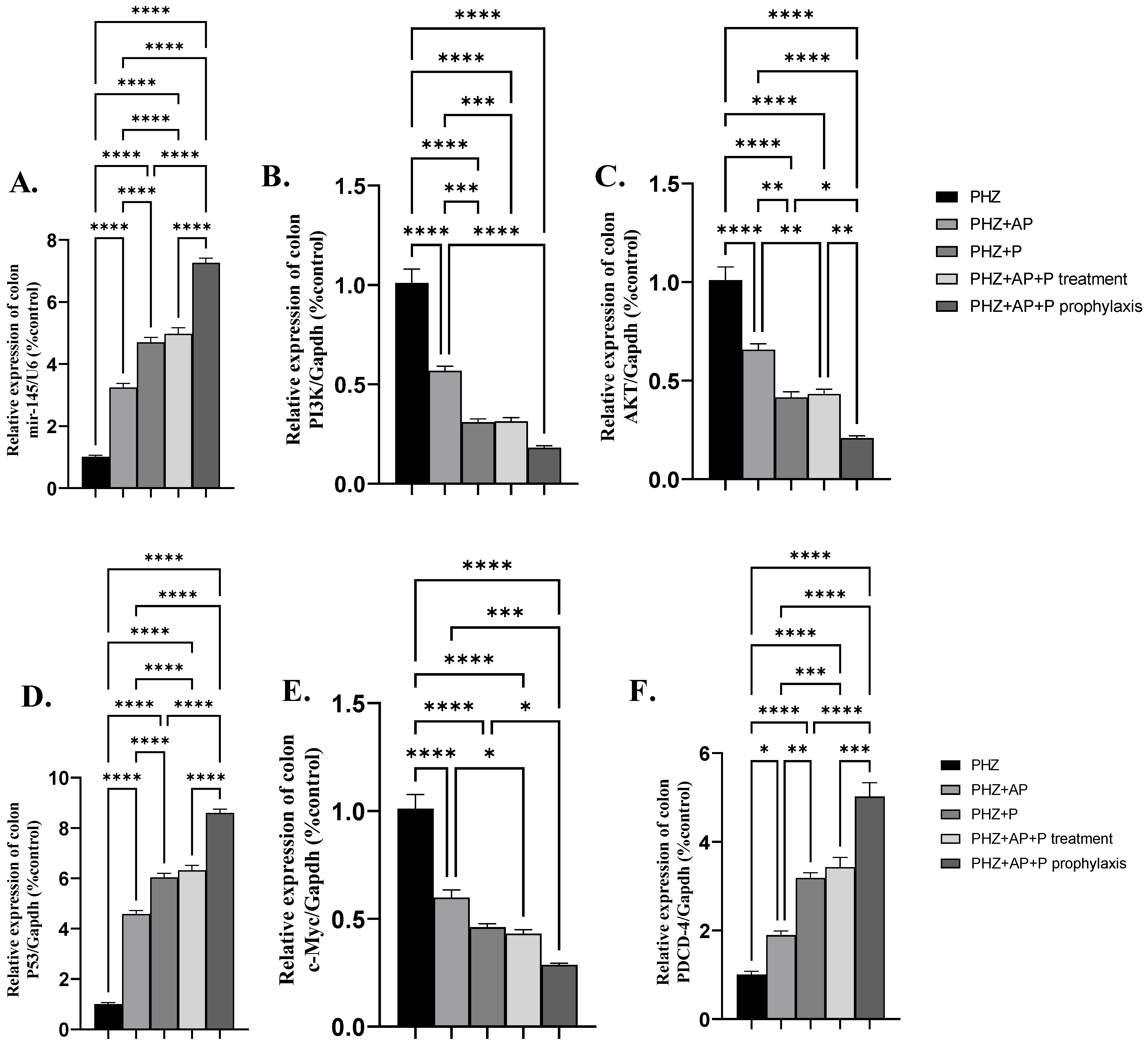
2.4. mir-145 and mRNA Expression of PI3K, AKT, P53, c-Myc, and PDCD-4
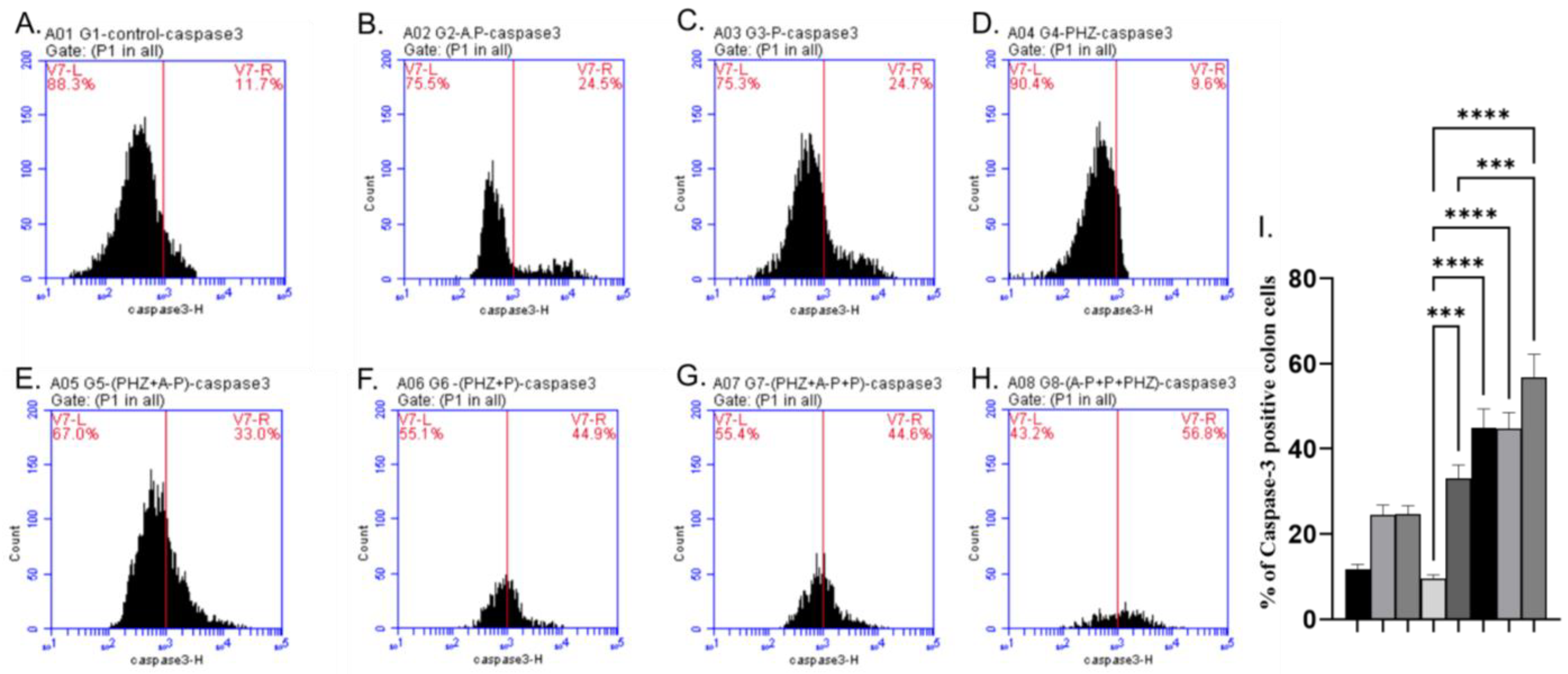
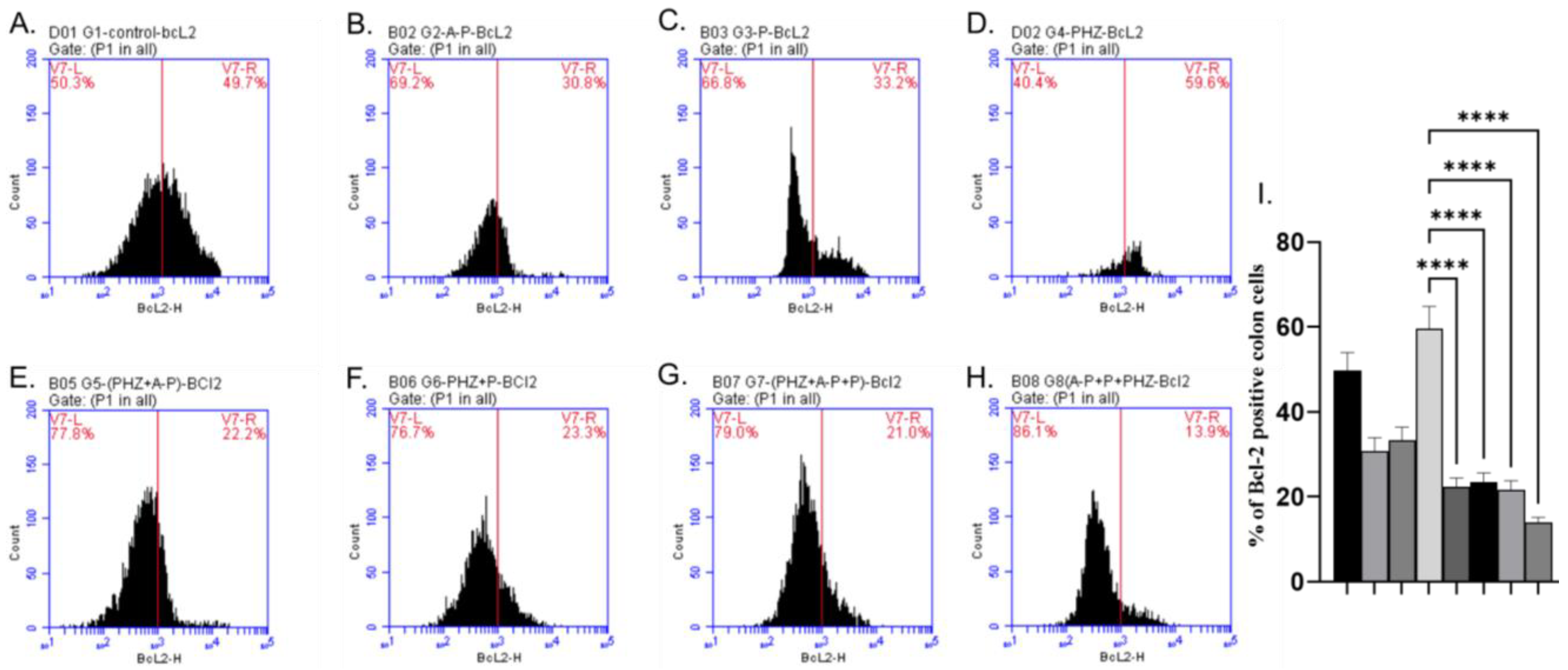
2.5. Apoptotic and Antiapoptotic Proteins
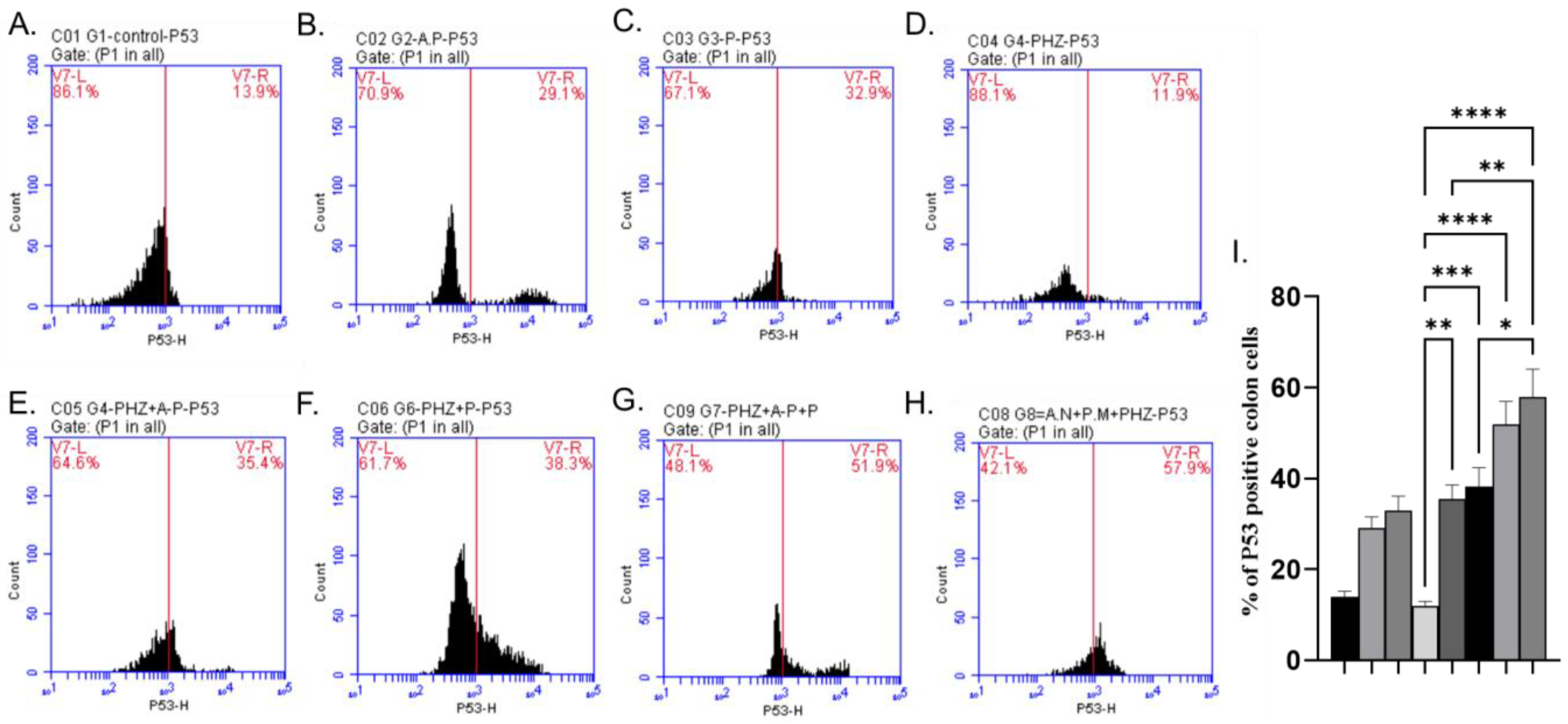
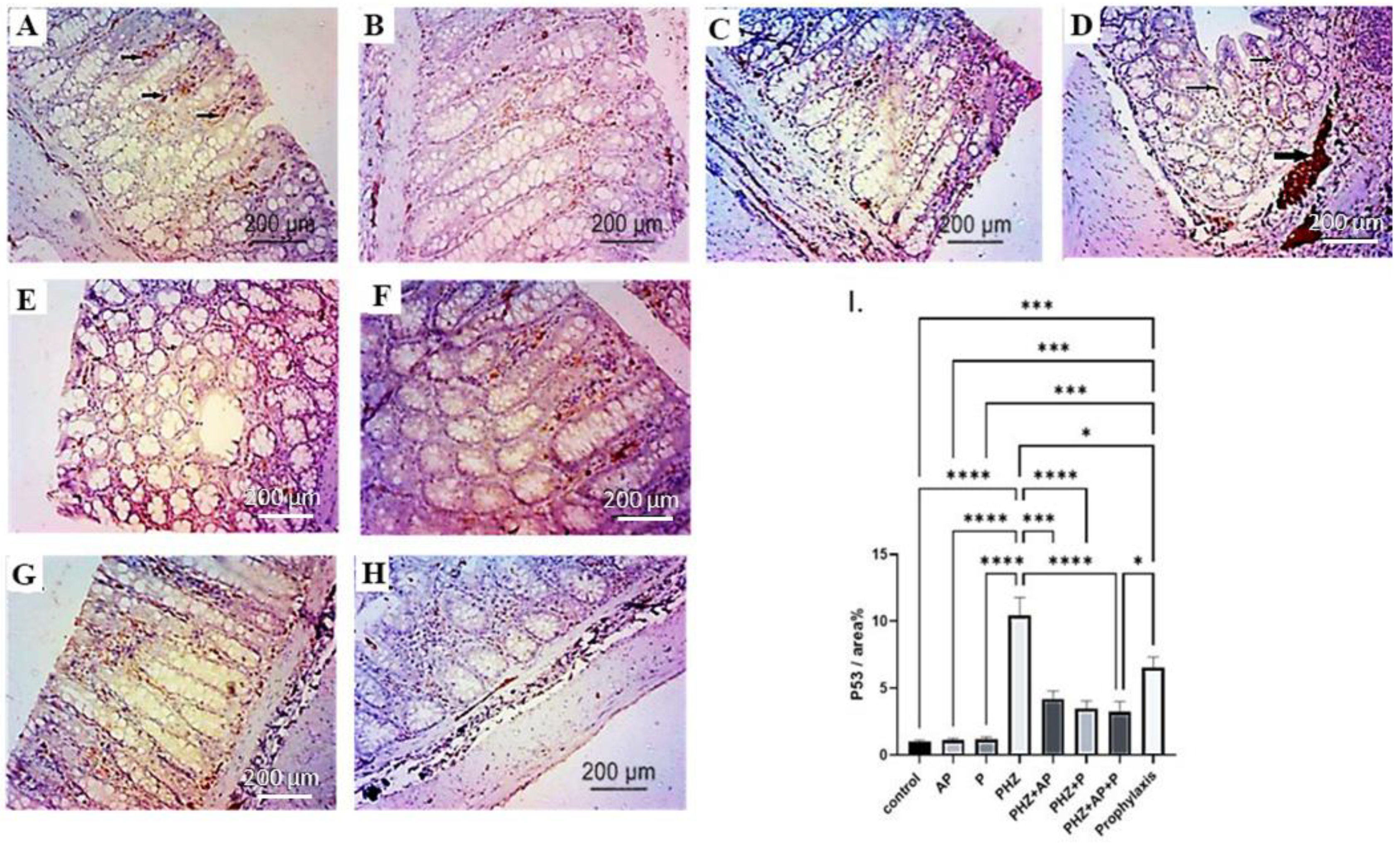
2.6. Immunohistochemical Analysis of P53
2.7. mRNA Expression of CK-20, SOX-2, OCT-4 and NanoG
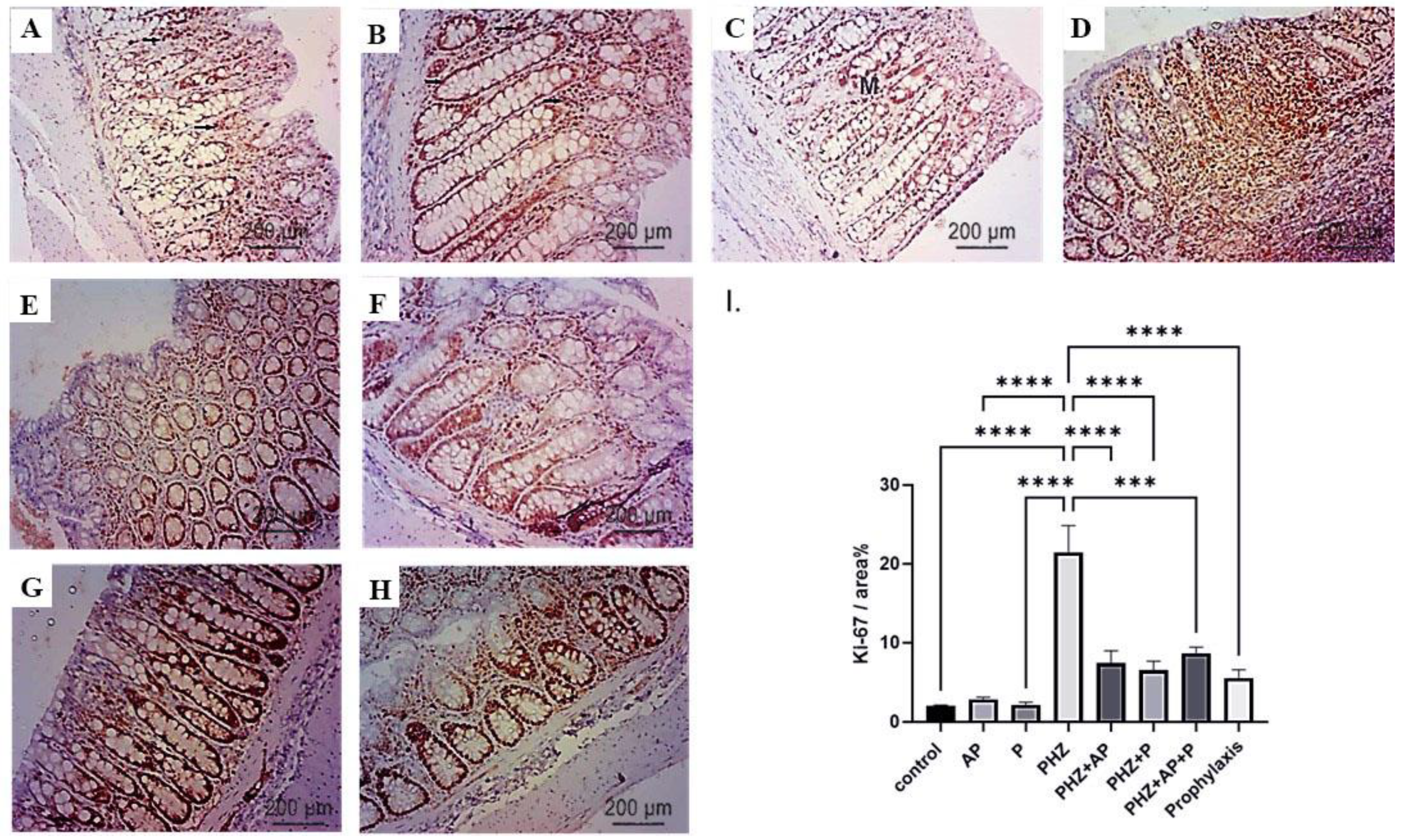
2.8. Immunohistochemical Analysis of Ki-67
2.9. Histopathological Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation, Phytochemical Screening, and Physicochemical Standardization of the Adiantum pedatum (AP) Extract
4.3. Animals Care
4.4. Induction of Colon Cancer
4.5. Experimental Animals Design
4.6. Preparation of Colon Tissue Homogenate for Biochemistry Analysis
4.7. Determination of Oxidative Stress and Antioxidant Markers
4.8. Preparation of Colon Tissue Suspension for Flow Cytometry
4.9. Flow Cytometry Determination of Apoptotic Markers
4.10. Histopathological Investigation and Immunohistochemical Detection of P53 and Ki67
4.11. RT-qPCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Hämälistö, S.; Jäättelä, M. Lysosomes in cancer—Living on the edge (of the cell). Curr. Opin. Cell Biol. 2016, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, A. Cancer cell lines involving cancer stem cell populations respond to oxidative stress. Biotechnol. Rep. 2018, 17, 24–30. [Google Scholar] [CrossRef]
- Alcaraz, R.; Muñiz, P.; Cavia, M.; Palacios, Ó.; Samper, K.G.; Gil-García, R.; Jiménez-Pérez, A.; García-Tojal, J.; García-Girón, C. Thiosemicarbazone-metal complexes exhibiting cytotoxicity in colon cancer cell lines through oxidative stress. J. Inorg. Biochem. 2020, 206, 110993. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Obtułowicz, T.; Winczura, A.; Speina, E.; Swoboda, M.; Janik, J.; Janowska, B.; Cieśla, J.M.; Kowalczyk, P.; Jawien, A.; Gackowski, D. Aberrant repair of etheno–DNA adducts in leukocytes and colon tissue of colon cancer patients. Free Radic. Biol. Med. 2010, 49, 1064–1071. [Google Scholar] [CrossRef]
- Erekat, N.S. Programmed cell death in cerebellar Purkinje neurons. J. Integr. Neurosci. 2022, 21, 30. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Hassan, M.; Feyen, O.; Grinstein, E. Fas-Induced Apoptosis of Renal Cell Carcinoma is Mediated by Apoptosis Signal-Regulating Kinase 1 via Mitochondrial Damage-Dependent Caspase-8 Activation. Anal. Cell. Pathol. 2009, 31, 437–456. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, V.; Torriglia, A.; Scovassi, A.I. Conversation between apoptosis and autophagy: “Is it your turn or mine?”. Apoptosis 2011, 16, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bukholm, I.K.; Nesland, J.M. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000, 436, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.; Goulet, E.J.; Livingston, E.H. Colon cancer and apoptosis. Am. J. Surg. 2006, 191, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Emerging Roles of C-Myc in Cancer Stem Cell-Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target Against Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2340. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.C.; Liu, X.Y.; Teng, L.; Ji, Q.; Wu, Y.; Li, J.M.; Gao, W.; Zhang, Y.Y.; La, T.; Tabatabaee, H.; et al. c-Myc inactivation of p53 through the pan-cancer lncRNA MILIP drives cancer pathogenesis. Nat. Commun. 2020, 11, 4980. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.S. The role of Pdcd4 in tumour suppression and protein translation. Biol. Cell 2018, 110, 169–177. [Google Scholar] [CrossRef]
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2305. [Google Scholar] [CrossRef]
- Ahadi, A. The significance of microRNA deregulation in colorectal cancer development and the clinical uses as a diagnostic and prognostic biomarker and therapeutic agent. Non-Coding RNA Res. 2020, 5, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dusenbery, B.N.; Hata, A. MicroRNA in Cancer: The Involvement of Aberrant MicroRNA Biogenesis Regulatory Pathways. Genes Cancer 2010, 1, 1100–1114. [Google Scholar] [CrossRef]
- Imedio, L.; Cristóbal, I.; Rubio, J.; Santos, A.; Rojo, F.; García-Foncillas, J. MicroRNAs in Rectal Cancer: Functional Significance and Promising Therapeutic Value. Cancers 2020, 12, 2040. [Google Scholar] [CrossRef]
- Ge, N.; Lin, H.X.; Xiao, X.S.; Guo, L.; Xu, H.M.; Wang, X.; Jin, T.; Cai, X.Y.; Liang, Y.; Hu, W.H.; et al. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J. Transl. Med. 2010, 8, 94. [Google Scholar] [CrossRef]
- Wang, Z.X.; Teh, C.H.; Kueh, J.L.; Lufkin, T.; Robson, P.; Stanton, L.W. Oct4 and Sox2 directly regulate expression of another pluripotency transcription factor, Zfp206, in embryonic stem cells. J. Biol. Chem. 2007, 282, 12822–12830. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- Shirendeb, U.; Hishikawa, Y.; Moriyama, S.; Win, N.; Minn Myint Thu, M.; Swe Mar, K.; Khatanbaatar, G.; Masuzaki, H.; Koji, T. Human Papillomavirus Infection and Its Possible Correlation with p63 Expression in Cervical Cancer in Japan, Mongolia, and Myanmar. Acta Histochem. Cytochem. 2009, 42, 181–190. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- KlÖPpel, G.; Perren, A.; Heitz, P.U. The Gastroenteropancreatic Neuroendocrine Cell System and Its Tumors: The WHO Classification. Ann. N. Y. Acad. Sci. 2004, 1014, 13–27. [Google Scholar] [CrossRef]
- Sueishi, Y.; Nii, R.; Kakizaki, N. Resveratrol analogues like piceatannol are potent antioxidants as quantitatively demonstrated through the high scavenging ability against reactive oxygen species and methyl radical. Bioorg. Med. Chem. Lett. 2017, 27, 5203–5206. [Google Scholar] [CrossRef]
- Wittgen, H.G.M.; van Kempen, L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res./Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; Alghaith, A.F.; et al. Piceatannol-Loaded Emulsomes Exhibit Enhanced Cytostatic and Apoptotic Activities in Colon Cancer Cells. Antioxidants 2020, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Lan, Y.M.; Lee, P.S.; Lin, Q.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Piceatannol Prevents Colon Cancer Progression via Dual-Targeting to M2-Polarized Tumor-Associated Macrophages and the TGF-β1 Positive Feedback Signaling Pathway. Mol. Nutr. Food Res. 2022, 66, e2200248. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.B.; Li, C.; Taotafa, U.; Ding, B.; Guan, Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Chandrappa, C.P.; Shilpashree, C.B.; Karthik, M.R.; Govindappa, M.; Sadananda, T.S. Antibacterial and Antioxidant Activities of Adiantum pedatum L. J. Phytol. 2011, 3. [Google Scholar]
- Fan, P.; Zhao, L.; Hostettmann, K.; Lou, H. Chemical constituents of Asplenium rutamuraria L. Nat. Prod. Res. 2012, 26, 1413–1418. [Google Scholar] [CrossRef]
- Stein, S.E.; Ausloos, P.; Lias, S.G. Comparative evaluations of mass spectral databases. J. Am. Soc. Mass Spectrom. 1991, 2, 441–443. [Google Scholar] [CrossRef]
- Stein, S. Mass spectral reference libraries: An ever-expanding resource for chemical identification. Anal. Chem. 2012, 84, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hosen, M.A.; Ahmad, S.; ul Qamar, M.T.; Dey, S.; Hasan, I.; Fujii, Y.; Ozeki, Y.; Kawsar, S.M.A. Synthesis, antimicrobial, anticancer activities, PASS prediction, molecular docking, molecular dynamics and pharmacokinetic studies of designed methyl α-D-glucopyranoside esters. J. Mol. Struct. 2022, 1260, 132761. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Zain, W.Z.W.M.; Ramli, N.W.; Hamzah, F.; Hamid, N.A. Antioxidant and GC-MS Analysis of Cyperus iria, Fimbristyis miliacea, and Fimbristylis globulosa. IOP Conf. Ser. Earth Environ. Sci. 2022, 1114, 012047. [Google Scholar] [CrossRef]
- Shahin, A.; Nabil-Adam, A.; Elnagar, K.; Osman, H.; Shreadah, M.A. Bioactivity and metabolomics fingerprinting characterization of different organic solvents extracts of Padina pavonica collected from Abu Qir Bay, Egypt. Egypt. J. Chem. 2022, 65, 207–225. [Google Scholar] [CrossRef]
- Nasr, M.; Naeem, S.A.; El-Shenbaby, I.; Mohamed, F.M.A.; Mahmoud, S.M.; Abuamara, T.M.M.; Abd-Elhay, W.M.; Elbayoumy, F.; Elkot, A.; Shikhon, T.; et al. Pomegranate Seeds and Peel Ethanolic Extracts Anticancer Potentials and Related Genetic, Histological, Immunohistochemical, Apoptotic and Oxidative Stress Profiles: In vitro Study. J. Exp. Pharmacol. 2023, 15, 191–205. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Rodziewicz, J.; Butarewicz, A. Possible Protective Effects of TA on the Cancerous Effect of Mesotrione. Nutrients 2020, 12, 1343. [Google Scholar] [CrossRef]
- Kim, H.S.; Lim, J.M.; Kim, J.Y.; Kim, Y.; Park, S.; Sohn, J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int. J. Cancer 2016, 138, 1432–1441. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Kałużna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant Properties of Green Coffee Extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules 2022, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Lyantagaye, S.L. Methyl-α-D-glucopyranoside from Tulbaghia violacea extract induces apoptosis in vitro in cancer cells. Bangladesh J. Pharmacol. 2013, 8, 93–101. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.; Baothman, O.; Shash, L.; Abuaraki, H.; Zeyadi, M.; Hosawi, S.; Altayb, H.; Abo-Golayel, M. Hepatoprotective effect of date palm fruit extract against doxorubicin intoxication in Wistar rats: In vivo and in silico studies. Asian Pac. J. Trop. Biomed. 2022, 12, 357–366. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Ali, N.A.; El Gendy, A.N.G.; Mohamed, S.H.; Shalby, A.B.; Farrag, A.H.; Shalaby, A.S.G. Renoprotective and therapeutic effects of newly water, ethanol, and butanol ginseng fractions in hypertensive and chronic kidney disease with L-NAME. Biomed. Pharmacother. 2021, 142, 111978. [Google Scholar] [CrossRef]
- Spanou, C.; Manta, S.; Komiotis, D.; Dervishi, A.; Kouretas, D. Antioxidant Activity of a Series of Fluorinated Pyrano-nucleoside Analogues of N(4)-benzoyl Cytosine and N(6)-benzoyl Adenine. Int. J. Mol. Sci. 2007, 8, 695–704. [Google Scholar] [CrossRef]
- Chen, M.; Meng, H.; Zhao, Y.; Chen, F.; Yu, S. Antioxidant and in vitro anticancer activities of phenolics isolated from sugar beet molasses. BMC Complement. Altern. Med. 2015, 15, 313. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae. Molecules 2023, 28, 3939. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Sruthi, V.; Padmaja, B.; Asha, V.V. In vitro anti-inflammatory and anti-cancer activities of Cuscuta reflexa Roxb. J. Ethnopharmacol. 2011, 134, 872–877. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Taie, H. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and sorbitol. Aust. J. Basic Appl. Sci. 2009, 3, 3609–3616. [Google Scholar]
- Bekhouche, K.; Özen, T.; Boussaha, S.; Koldaş, S.; Yenigün, S.; Lassed, S.; Demirtas, I.; Benayache, F.; Samir, B.; Zama, D. Anti-oxidant, DNA-damage protection and anti-cancer properties of n-butanol extract of the endemic Perralderia coronopifolia. Bangladesh J. Pharmacol. 2018, 13, 82–88. [Google Scholar] [CrossRef]
- Pal, L.C.; Gautam, A.; Pande, V.; Rao, C.V. Anticancer property of Selaginella bryopteris (L.) Bak. against hepatocellular carcinoma in vitro and in vivo. Phytomed. Plus 2022, 2, 100201. [Google Scholar] [CrossRef]
- Aykan, N.F. Red Meat and Colorectal Cancer. Oncol. Rev. 2015, 9, 288. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, Q.; Yin, Z.; Shuang, J.; Bai, B.; Yu, P.; Guo, M.; Zhao, Q. Red and processed meat consumption and colorectal cancer risk: A systematic review and meta-analysis. Oncotarget 2017, 8, 83306–83314. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Heterocyclic amines and polycyclic aromatic hydrocarbons in commercial ready-to-eat meat products on UK market. Food Control 2017, 73, 306–315. [Google Scholar] [CrossRef]
- Cross, A.J.; Ferrucci, L.M.; Risch, A.; Graubard, B.I.; Ward, M.H.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R. A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Res. 2010, 70, 2406–2414. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.; Lu, Y.; Ju, Q.; Ouyang, M. Iron metabolism in colorectal cancer. Front. Oncol. 2023, 13, 1098501. [Google Scholar] [CrossRef]
- Bostan, M.; Mihaila, M.; Hotnog, C.; Bleotu, C.; Anton, G.; Roman, V.; Brasoveanu, L.I. Modulation of Apoptosis in Colon Cancer Cells by Bioactive Compounds. Color. Cancer–Pathog. Treat. 2016. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Sugherini, L.; Pompella, A.; Lodovici, M.; Caciotti, B.; Ciccoli, L.; Comporti, M. Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazine intoxication. Biochem. Pharmacol. 1997, 53, 1743–1751. [Google Scholar] [CrossRef]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, A.K.; Ghosh, D.; Dutta, M.; Mitra, E.; Dey, M.; Bhowmick, D.; Das, T.; Firdaus, S.B.; Mishra, S. Aqueous bark extract of Terminalia arjuna protects against phenylhydrazine induced oxidative damage in goat red blood cell membrane protein, phospholipid asymmetry and structural morphology: A flow cytometric and biochemical analysis. J. Pharm. Res 2014, 8, 1790–1804. [Google Scholar]
- Di Giacomo, C.; Acquaviva, R.; Lanteri, R.; Licata, F.; Licata, A.; Vanella, A. Nonproteic Antioxidant Status in Plasma of Subjects with Colon Cancer. Exp. Biol. Med. 2003, 228, 525–528. [Google Scholar] [CrossRef]
- Ofeimun, J.O.; Enwerem, J.C.; Benjamin, G. Haematological And In-Vivo Antioxidant Modulatory Activities of Justicia Secunda Vahl [Acanthaceae] Leaf Extract In Phenylhydrazine-Induced Anemic Rats. Niger. J. Pharm. 2020, 54, 84–96. [Google Scholar]
- Aloke, C.; Uche Emelike, C.; Ajuka Obasi, N.; Nkemjika Ogbu, P.; Oswald Edeogu, C.; Godwin Uzomba, C.; Ekakitie, O.; Adewale Iyaniwura, A.; Okoro, C.C.; Peter Okey, B.; et al. HPLC profiling and studies on Copaifera salikounda methanol leaf extract on phenylhydrazine-induced hematotoxicity and oxidative stress in rats. Arab. J. Chem. 2021, 14, 103428. [Google Scholar] [CrossRef]
- Jaiswal, M.; LaRusso, N.F.; Burgart, L.J.; Gores, G.J. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000, 60, 184–190. [Google Scholar]
- Benkhelifa, S.; Rafa, H.; Belhadef, S.; Ait-kaci, H.; Medjeber, O.; Belkhelfa, M.; Hetit, S.; Ait-Younes, S.; De Launoit, Y.; Moralès, O.; et al. Aberrant up-regulation of iNOS/NO system is correlated with an increased abundance of Foxp3+ cells and reduced effector/memory cell markers expression during colorectal cancer: Immunomodulatory effects of cetuximab combined with chemotherapy. Inflammopharmacology 2019, 27, 685–700. [Google Scholar] [CrossRef]
- Costa, F.P.d.; Puty, B.; Nogueira, L.S.; Mitre, G.P.; Santos, S.M.d.; Teixeira, B.J.B.; Kataoka, M.S.d.S.; Martins, M.D.; Barboza, C.A.G.; Monteiro, M.C.; et al. Piceatannol Increases Antioxidant Defense and Reduces Cell Death in Human Periodontal Ligament Fibroblast under Oxidative Stress. Antioxidants 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud Moustafa, E.; Rashed, E.R.; Rashed, R.R.; Omar, N.N. Piceatannol promotes hepatic and renal AMPK/SIRT1/PGC-1α mitochondrial pathway in rats exposed to reserpine or gamma-radiation. Int. J. Immunopathol. Pharmacol. 2021, 35, 205873842110161. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Miura, Y.; Yagasaki, K. Antiproliferative and Anti-Invasive Effect of Piceatannol, a Polyphenol Present in Grapes and Wine, against Hepatoma AH109A Cells. J. Biomed. Biotechnol. 2012, 2012, 672416. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Tongson, J.; Kim, K.-H.; Park, Y. Piceatannol attenuates fat accumulation and oxidative stress in steatosis-induced HepG2 cells. Curr. Res. Food Sci. 2020, 3, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, S.A.; Azab, S.S.; Elsherbİny, D.A.; El-demerdash, E. Piceatannol ameliorates cisplatin-induced histological and biochemical alterations in rats kidney. Int. J. Pharm. Pharm. Sci. 2017, 9, 305. [Google Scholar] [CrossRef]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Bardhan, K.; Liu, K. Epigenetics and colorectal cancer pathogenesis. Cancers 2013, 5, 676–713. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu, S., II; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, W.I.; Bae, J.H.; Cho, M.K.; Lee, S.H.; Nam, H.S.; Choi, I.H.; Cho, S.W. Overexpression of Nrf2 promotes colon cancer progression via ERK and AKT signaling pathways. Ann. Surg. Treat. Res. 2020, 98, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Liu, Y.; Zhou, Y. Role of NRF2 in Colorectal Cancer Prevention and Treatment. Technol. Cancer Res. Treat. 2022, 21, 15330338221105736. [Google Scholar] [CrossRef] [PubMed]
- Garufi, A.; Pistritto, G.; D’Orazi, V.; Cirone, M.; D’Orazi, G. The Impact of NRF2 Inhibition on Drug-Induced Colon Cancer Cell Death and p53 Activity: A Pilot Study. Biomolecules 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Zamore, P.D.J.S. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef]
- Bandrés, E.; Cubedo, E.; Agirre, X.; Malumbres, R.; Zarate, R.; Ramirez, N.; Abajo, A.; Navarro, A.; Moreno, I.; Monzo, M. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer 2006, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef]
- Spizzo, R.; Nicoloso, M.; Lupini, L.; Lu, Y.; Fogarty, J.; Rossi, S.; Zagatti, B.; Fabbri, M.; Veronese, A.; Liu, X.; et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ. 2010, 17, 246–254. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of microRNA processing by p53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef]
- Kent, O.A.; Chivukula, R.R.; Mullendore, M.; Wentzel, E.A.; Feldmann, G.; Lee, K.H.; Liu, S.; Leach, S.D.; Maitra, A.; Mendell, J.T.; et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010, 24, 2754–2759. [Google Scholar] [CrossRef]
- Hatley, M.E.; Patrick, D.M.; Garcia, M.R.; Richardson, J.A.; Bassel-Duby, R.; Van Rooij, E.; Olson, E.N. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell 2010, 18, 282–293. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Weirauch, U.; Thomas, M.; Grünweller, A.; Hartmann, R.K.; Aigner, A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011, 71, 5214–5224. [Google Scholar] [CrossRef]
- Götte, M.; Mohr, C.; Koo, C.; Stock, C.; Vaske, A.; Viola, M.; Ibrahim, S.; Peddibhotla, S.; Teng, Y.H.; Low, J. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene 2010, 29, 6569–6580. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mo, Y.-Y. MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1Suppression of Cell Invasion and Metastasis by miR-145. Cancer Res. 2010, 70, 378–387. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Zhang, H.; Wang, H.; Qian, G.; Fan, X.; Hoffman, A.R.; Hu, J.F.; Ge, S. Putative tumor suppressor miR—145 inhibits colon cancer cell growth by targeting oncogene friend leukemia virus integration 1 gene. Cancer 2011, 117, 86–95. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, K.H.; Jeon, B.T.; Cheong, S.H.; Park, J.H.; Kim, D.H.; Kweon, H.J.; Moon, S.H. Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. J. Med. Plants Res. 2012, 6, 3072–3079. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.v.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. Apoptosis in human cancer cells. Curr. Opin. Oncol. 2004, 16, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J. Role of Apoptosis in Colon Cancer Biology, Therapy, and Prevention. Curr. Color. Cancer Rep. 2013, 9, 331–340. [Google Scholar] [CrossRef]
- Coultas, L.; Strasser, A. The role of the Bcl-2 protein family in cancer. Semin. Cancer Biol. 2003, 13, 115–123. [Google Scholar] [CrossRef]
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The Biological Activities and Therapeutic Potentials of Baicalein Extracted from Oroxylum indicum: A Systematic Review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Thushara, R.M.; Sundaram, M.S.; Hemshekhar, M.; Paul, M.; Thirunavukkarasu, C.; Basappa; Nagaraju, G.; Raghavan, S.C.; Girish, K.S.; et al. Unconjugated Bilirubin exerts Pro-Apoptotic Effect on Platelets via p38-MAPK activation. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jung, J.I.; Cho, H.J.; Her, S.; Kwon, S.-H.; Yu, R.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Inhibition of tumor progression by oral piceatannol in mouse 4T1 mammary cancer is associated with decreased angiogenesis and macrophage infiltration. J. Nutr. Biochem. 2015, 26, 1368–1378. [Google Scholar] [CrossRef]
- Wu, L.-S.; Wang, X.-W.; He, W.; Ma, X.-T.; Wang, H.-Y.; Han, M.; Li, B.-H. TRAIL inhibits platelet-induced colorectal cancer cell invasion. J. Int. Med. Res. 2019, 47, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Moon, D.-O.; Choi, Y.H.; Choi, I.-W.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. Piceatannol enhances TRAIL-induced apoptosis in human leukemia THP-1 cells through Sp1- and ERK-dependent DR5 up-regulation. Toxicol. In Vitro 2011, 25, 605–612. [Google Scholar] [CrossRef]
- Alrawi, S.J.; Stoler, D.; Carroll, R.E.; Gibbs, J.F.; Schiff, M.; Tan, D.; Dayton, M.; Anderson, G.R. New parameters in aberrant crypt foci evaluation in colon carcinogenesis (glutathione S-transferases, B-RAF mutation, genomic instability, single nucleotide polymorphism (SNP) arrays). J. Surg. Res. 2006, 130, 222–223. [Google Scholar] [CrossRef]
- Darwish, A.; Alemam, D.; Sheta, H. Role of expression of p53 and Ki67 in the progression of Wilms tumor; correlation with survival. Int. J. Cancer Biomed. Res. 2021, 5, 99–110. [Google Scholar] [CrossRef]
- Nussrat, F.L.; Ali, H.H.; Hussein, H.G.; Al-Ukashi, R.J. Immunohistochemical Expression of ki-67 and p53 in Colorectal Adenomas: A Clinicopathological Study. Oman Med. J. 2011, 229–234. [Google Scholar] [CrossRef]
- Hahn, W.C.; Weinberg, R.A. Modelling the molecular circuitry of cancer. Nat. Rev. Cancer 2002, 2, 331–341. [Google Scholar] [CrossRef]
- Lee, H.-N.; Jang, H.-Y.; Kim, H.-J.; Shin, S.-A.; Choo, G.-S.; Park, B.-K.; Kim, B.-S.; Jung, J.-Y. Induction of Apoptosis by Piceatannol in YD-15 Human Oral Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2015, 44, 975–982. [Google Scholar] [CrossRef]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.-R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N.J.M. Inhibition of Staphylococcus aureus LC 554891 by Moringa oleifera seed extract either singly or in combination with antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef]
- Nazim, M.; Aslam, D.; Khatoon, R.; Asif, D.; Chaudhary, S. Physico-chemical standardization of Hansraj (Adiantum capillus-Veneris). J. Drug Deliv. Ther. 2018, 8, 195–203. [Google Scholar] [CrossRef]
- Berger, J. Phenylhydrazine haematotoxicity. J. Appl. Biomed. 2007, 5, 125–130. [Google Scholar] [CrossRef]
- Awaad, A.; Adly, M.A.; Ellatef, M.A.A.; Foad, M.M. Comparative Expression of P53 and Survivin Proteins in Phenylhydrazine-Induced Colon Cancer of Rats and the Role of Electromagnetic Field and Broccoli Extract. Ph.D. Thesis, Sohag University, Sohag, Egypt, 2021. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Zoubi, M.S.A.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M.; et al. Albumin Nano-Encapsulation of Piceatannol Enhances Its Anticancer Potential in Colon Cancer via down Regulation of Nuclear p65 and HIF-1α. Cancers 2020, 12, 113. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Buss, H.; Chan, T.P.; Sluis, K.B.; Domigan, N.M.; Winterbourn, C.C. Protein Carbonyl Measurement by a Sensitive ELISA Method. Free Radic. Biol. Med. 1997, 23, 361–366. [Google Scholar] [CrossRef]
- Ingram, G.; Montgomery, H.A.C.; Dymock, J.F.; Henneberry, G.O.; Baker, B.E.; Forbes, J.S.; Dalladay, D.B.; Bloxam, T.W. Notes. Analyst 1961, 86, 411. [Google Scholar] [CrossRef]
- Koracevic, D. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 121–126. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Yeh, M.K.Y. Erythrocyte Glutathione Reductase. Blood 1963, 21, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Tribukait, B.; Esposti, P.L. Quantitative flow-microfluorometric analysis of the DNA in cells from neoplasms of the urinary bladder: Correlation of aneuploidy with histological grading and the cytological findings. Urol. Res. 1978, 6, 201–205. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. Bancroft’s Theory Pract. Histol. Tech. 2013, 173–186. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Gu, J. Elevated preoperative carcinoembryonic antigen (CEA) and Ki67 is predictor of decreased survival in IIA stage colon cancer. World J. Surg. 2013, 37, 208–213. [Google Scholar] [CrossRef]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165577. [Google Scholar] [CrossRef]
- Khamis, T.; Abdelalim, A.F.; Saeed, A.A.; Edress, N.M.; Nafea, A.; Ebian, H.F.; Algendy, R.; Hendawy, D.M.; Arisha, A.H.; Abdallah, S.H. Breast milk MSCs upregulated β-cells PDX1, Ngn3, and PCNA expression via remodeling ER stress/inflammatory/apoptotic signaling pathways in type 1 diabetic rats. Eur. J. Pharmacol. 2021, 905, 174188. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
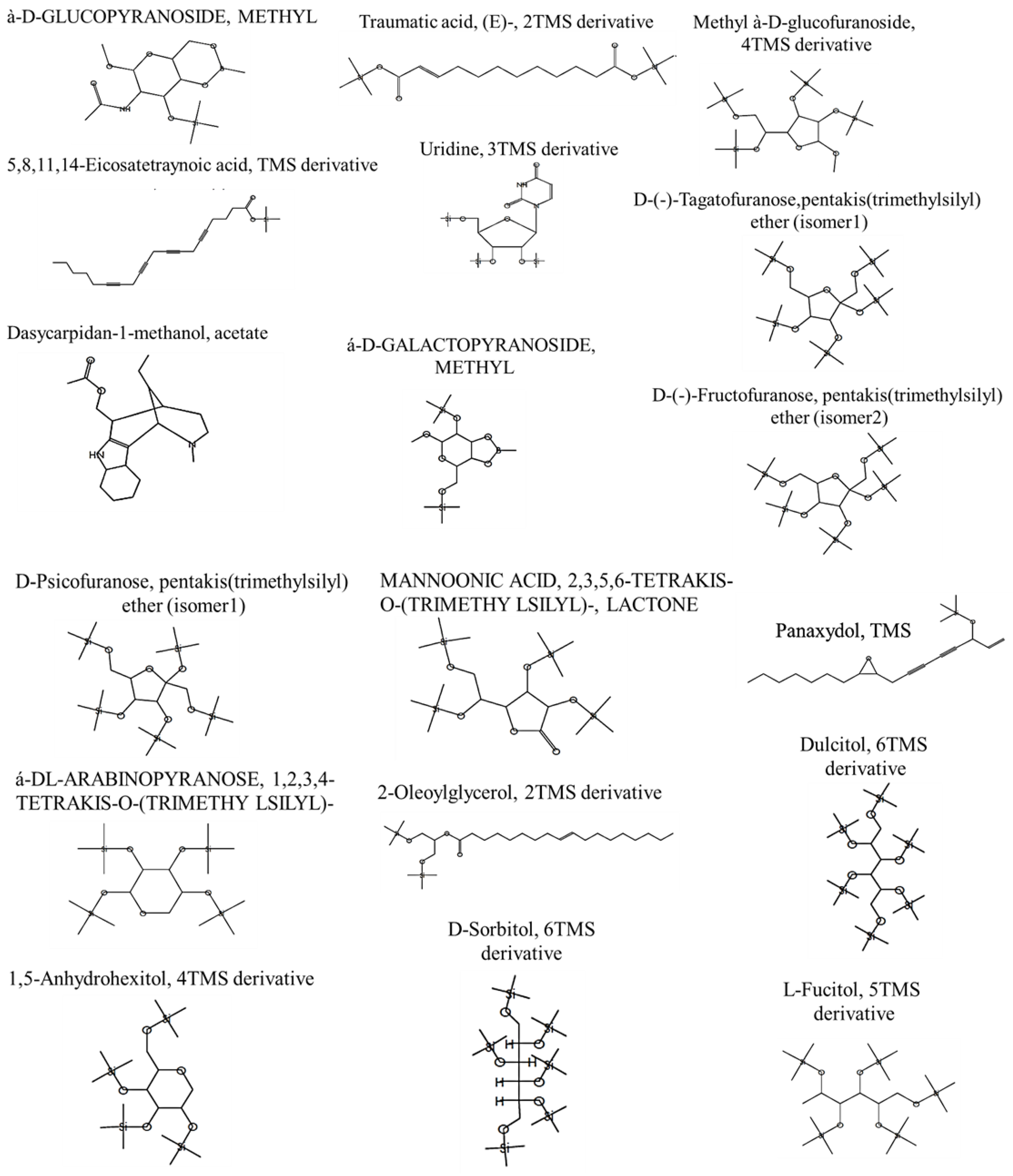



| Bioactive Compounds | RT | Area% | MF | MW | Formula | Activity—Based on the Previous Literature | References |
|---|---|---|---|---|---|---|---|
| à-D-GLUCOPYRANOSIDE, METHYL | 21.36 | 1.68 | 739 | 331 | C13H26BNO6Si | Anticancer Antioxidant | [46,47] |
| 5,8,11,14-Eicosatetraynoic acid, TMS derivative | 21.36 | 9.68 | 707 | 368 | C23H32O2Si | Anticancer | [48] |
| Dasycarpidan-1-methanol, acetate | 21.36 | 12.68 | 713 | 326 | C20H26N2O2 | Anticancer | [49] |
| Traumatic acid, (E)-, 2TMS derivative | 22.74 | 0.69 | 710 | 372 | C18H36O4Si2 | Anticancer Antioxidant | [50] |
| Panaxydol, TMS | 22.6 | 11.25 | 694 | 332 | C20H32O2Si | Anticancer | [51] |
| 2-Oleoylglycerol, 2TMS derivative | 22.88 | 0.36 | 731 | 500 | C27H56O4Si2 | Antioxidant | [52] |
| MANNOFURANOSIDE, METHYL 2,3,5,6-TETRAKIS-O-(TRIMETHY LSILYL)-, à-D- | 23.31 | 8.56 | 772 | 482 | C19H46O6Si4 | Anticancer Antimicrobial Antioxidant | [53] |
| Uridine, 3TMS derivative | 23.31 | 7.56 | 717 | 460 | C18H36N2O6Si3 | Anticancer | [54] |
| á-D-GALACTOPYRANOSIDE, METHYL | 23.07 | 15.38 | 734 | 362 | C14H31BO6Si2 | Anticancer | [55] |
| Methyl à-D-glucofuranoside, 4TMS derivative | 24.43 | 2.5 | 836 | 482 | C19H46O6Si4 | Anticancer | [56] |
| D-(-)-Tagatofuranose,pentakis(trimethylsilyl) ether (isomer1) | 24.77 | 8.77 | 765 | 540 | C21H52O6Si5 | Antioxidant | [57] |
| D-(-)-Fructofuranose, pentakis(trimethylsilyl) ether (isomer2) | 24.82 | 8.91 | 782 | 540 | C21H52O6Si5 | Antioxidant Anti-inflammatory | [58] |
| D-Psicofuranose, pentakis(trimethylsilyl) ether (isomer1) | 24.82 | 10.91 | 777 | 540 | C21H52O6Si5 | Antioxidant anticancer | [58] |
| 1,5-Anhydrohexitol, 4TMS derivative | 25.44 | 9.97 | 797 | 452 | C18H44O5Si4 | Antioxidant | [59] |
| á-DL-ARABINOPYRANOSE, 1,2,3,4-TETRAKIS-O-(TRIMETHY LSILYL)- | 25.44 | 8.97 | 807 | 438 | C17H42O5Si4 | Antioxidant anticancer | [60] |
| MANNOONIC ACID, 2,3,5,6-TETRAKIS-O-(TRIMETHY LSILYL)-, LACTONE | 26.2 | 6.2 | 728 | 466 | C18H42O6Si4 | Antioxidant anticancer | [61] |
| Dulcitol, 6TMS derivative | 27.72 | 6.27 | 759 | 614 | C24H62O6Si6 | Anti-inflammatory Anticancer | [62] |
| D-Sorbitol, 6TMS derivative | 27.72 | 6.27 | 752 | 614 | C24H62O6Si6 | Antioxidant | [63] |
| BUTANAL, 2,3,4-TRIS[(TRIMETHYLSILYL)O XY]-, (R*,R*)- | 27.72 | 6.27 | 870 | 336 | C13H32O4Si3 | Antioxidant anticancer | [64] |
| L-Fucitol, 5TMS derivative | 27.72 | 6.27 | 752 | 526 | C21H54O5Si5 | Antioxidant anticancer | [65] |
| Groups | MDA | PC | NO |
|---|---|---|---|
| Control | 12.12 ± 0.351 d | 0.837 ± 0.035 cd | 0.625 ± 0.027 c |
| AP | 11.38 ± 0.756 d | 0.773 ± 0.026 d | 0.644 ± 0.626 c |
| P | 12.00 ± 0.425 d | 0.787 ± 0.223 d | 0.740 ± 0.040 c |
| PHZ | 33.183 ± 1.359 a | 3.152 ± 0.078 a | 5.183 ± 0.252 a |
| PHZ + AP | 18.88 ± 1.339 bc | 1.830 ± 0.044 b | 0.756 ± 0.110 c |
| PHZ + P | 21.37 ± 1.644 b | 1.732 ± 0.026 b | 0.752 ± 0.045 c |
| PHZ + AP + P | 19.45 ± 1.981 b | 1.728 ± 0.030 b | 1.120 ± 0.102 b |
| PHZ + AP + P (Prophylaxis) | 16.37 ± 1.620 c | 1.163 ± 0.032 c | 0.820 ± 0.028 c |
| Groups | SOD | CAT | GST | GSH | GPx | TAC |
|---|---|---|---|---|---|---|
| Control | 459.17 ± 12.21 a | 11.80 ± 0.52 a | 12.40 ± 0.57 a | 11.17 ± 0.32 a | 31.50 ± 0.52 a | 5.13 ± 0.39 a |
| AP | 456.00 ± 12.37 a | 10.28 ± 1.07 a | 14.14 ± 0.59 a | 10.82 ± 0.58 a | 32.36 ± 2.71 a | 4.24 ± 0.94 ab |
| P | 468.17 ± 14.71 a | 11.42 ± 0.31 a | 12.97 ± 0.17 a | 10.94 ± 0.19 a | 27.67 ± 0.88 b | 5.23 ± 0.39 a |
| PHZ | 224.83 ± 9.46 c | 3.29 ± 0.77 c | 4.88 ± 0.42 c | 4.11 ± 0.63 b | 3.88 ± 0.56 d | 0.52 ± 0.12 c |
| PHZ + AP | 401.00 ± 24.75 ab | 6.86 ± 0.71 b | 10.00 ± 0.91 ab | 8.48 ± 0.59 a | 16.34 ± 2.17 c | 3.78 ± 0.30 b |
| PHZ + P | 419.83 ± 15.56 ab | 9.12 ± 0.52 a | 8.66 ± 0.64 b | 8.50 ± 0.46 a | 27.82 ± 1.65 b | 3.58 ± 0.24 b |
| PHZ + AP + P | 386.00 ± 17.89 b | 9.90 ± 0.33 a | 9.39 ± 0.43 ab | 8.28 ± 0.88 a | 29.68 ± 1.70 ab | 4.83 ± 0.34 a |
| PHZ + AP + P (Prophylaxis) | 429.17 ± 20.27 ab | 10.47 ± 0.47 a | 11.48 ± 0.41 ab | 9.65 ± 0.67 a | 32.20 ± 1.72 a | 4.30 ± 0.30 ab |
| Items | Results |
|---|---|
| Moisture content% | 9.57 ± 0.33 |
| Cold extraction | |
| Petroleum ether extracts (Cold) | 10.97 ± 0.49 |
| Chloroform extracts (Cold) | 14.75 ± 0.54 |
| Methanol extracts (Cold) | 8.25 ± 0.19 |
| Aqueous extract (cold) | 2.85 ± 0.29 |
| Hot extraction | |
| Chloroform extract (Hot) | 0.33 ± 0.02 |
| Alcoholic extract (Hot) | 17.66 ± 0.46 |
| Aqueous extract (hot) | 12.76 ± 0.82 |
| Total Ash | 7.75 ± 0.29 |
| Acid insoluble ash | 3.45 ± 0.17 |
| Water soluble ash | 8.8 ± 0.316 |
| Test for phenolic compounds | |
| Total Phenolic % | 4.6 ± 0.18 |
| pH | |
| 5% 10% | 5.62 5.48 |
| TEST FOR SAPONINS | |
| Foam Test | + |
| TEST FOR TANNINS | |
| (a) Ferric chloride reagent (b) Lead acetate test (c) Potassium dichromate test | + + + |
| TEST FOR FLAVANOIDS | |
| Shinoda Test | + |
| TEST FOR PROTEINS | |
| (a) Biuret Test (b) Xanthoproteic test | + + |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Product Size | Accession No. |
|---|---|---|---|---|
| Gapdh | GCATCTTCTTGTGCAGTGCC | GGTAACCAGGCGTCCGATAC | 91 | NM_017008.4 |
| Myc | CAACAACCGCAAATGCTCCA | AGCTACGCTTCAGCTCGTTT | 110 | NM_012603.2 |
| P53 | CCCCTGAAGACTGGATAACTGT | TCTCCTGACTCAGAGGGAGC | 75 | NM_030989.3 |
| PDCD4 | CGGCCCGAGGGGATTCTAAA | GGGTCAGTGGGGTTCACATT | 123 | NM_022265.3 |
| CK-20 | CGCATCAATACTGTGCGGTG | AGCTCCCCAGAGTGAAAACG | 91 | NM_173128.2 |
| AKT-1 | GAAGGAGAAGGCCACAGGTC | TTCTGCAGGACACGGTTCTC | 111 | NM_033230.3 |
| PI3K | CCCTGCCCCATTTCATCCTT | TGTTGTTGCCCCAGACATGA | 162 | NM_053481.2 |
| SOX2 | ACAGAGAAAACCTGAGGGCG | CATCGCCCGGAGTCTAGTTC | 173 | NM_001109181.2 |
| Nanog | TGCATTTGTCTGAGCTGGGT | ATGGAGTAGGGTGGGTGTGT | 115 | NM_001100781.1 |
| OCT4 | AAGTTGGCGTGGAGACTCTG | GGACTCCTCGGGACTAGGTT | 143 | NM_001009178.2 |
| mir-145-5P | AACCGGGTCCAGTTTTCCC | GTCGTATCCAGTGCAGGGT | ||
| U6 | GCTCGCTTCGGCAGCACA | GAGGTATTCGCACCAGAGGA | ||
| mir-145-5P stem-loop | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGAT | |||
| U6 stem-loop | AACGCTTCACGAATTTGCGTG | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, T.; Diab, A.A.-A.A.; Zahra, M.H.; El-Dahmy, S.E.; Abd Al-Hameed, B.A.; Abdelkhalek, A.; Said, M.A.; Abdellatif, H.; Fericean, L.M.; Banatean-Dunea, I.; et al. The Antiproliferative Activity of Adiantum pedatum Extract and/or Piceatannol in Phenylhydrazine-Induced Colon Cancer in Male Albino Rats: The miR-145 Expression of the PI-3K/Akt/p53 and Oct4/Sox2/Nanog Pathways. Molecules 2023, 28, 5543. https://doi.org/10.3390/molecules28145543
Khamis T, Diab AA-AA, Zahra MH, El-Dahmy SE, Abd Al-Hameed BA, Abdelkhalek A, Said MA, Abdellatif H, Fericean LM, Banatean-Dunea I, et al. The Antiproliferative Activity of Adiantum pedatum Extract and/or Piceatannol in Phenylhydrazine-Induced Colon Cancer in Male Albino Rats: The miR-145 Expression of the PI-3K/Akt/p53 and Oct4/Sox2/Nanog Pathways. Molecules. 2023; 28(14):5543. https://doi.org/10.3390/molecules28145543
Chicago/Turabian StyleKhamis, Tarek, Abd Al-Aziz Abas Diab, Mansour H. Zahra, Samih Ebrahim El-Dahmy, Basant Ahmed Abd Al-Hameed, Adel Abdelkhalek, Mahmoud A. Said, Hussein Abdellatif, Liana Mihaela Fericean, Ioan Banatean-Dunea, and et al. 2023. "The Antiproliferative Activity of Adiantum pedatum Extract and/or Piceatannol in Phenylhydrazine-Induced Colon Cancer in Male Albino Rats: The miR-145 Expression of the PI-3K/Akt/p53 and Oct4/Sox2/Nanog Pathways" Molecules 28, no. 14: 5543. https://doi.org/10.3390/molecules28145543
APA StyleKhamis, T., Diab, A. A.-A. A., Zahra, M. H., El-Dahmy, S. E., Abd Al-Hameed, B. A., Abdelkhalek, A., Said, M. A., Abdellatif, H., Fericean, L. M., Banatean-Dunea, I., Arisha, A. H., & Attia, M. S. (2023). The Antiproliferative Activity of Adiantum pedatum Extract and/or Piceatannol in Phenylhydrazine-Induced Colon Cancer in Male Albino Rats: The miR-145 Expression of the PI-3K/Akt/p53 and Oct4/Sox2/Nanog Pathways. Molecules, 28(14), 5543. https://doi.org/10.3390/molecules28145543







