Advanced Stimuli-Responsive Structure Based on 4D Aerogel and Covalent Organic Frameworks Composite for Rapid Reduction in Tetracycline Pollution
Abstract
1. Introduction
2. Results and Discussion
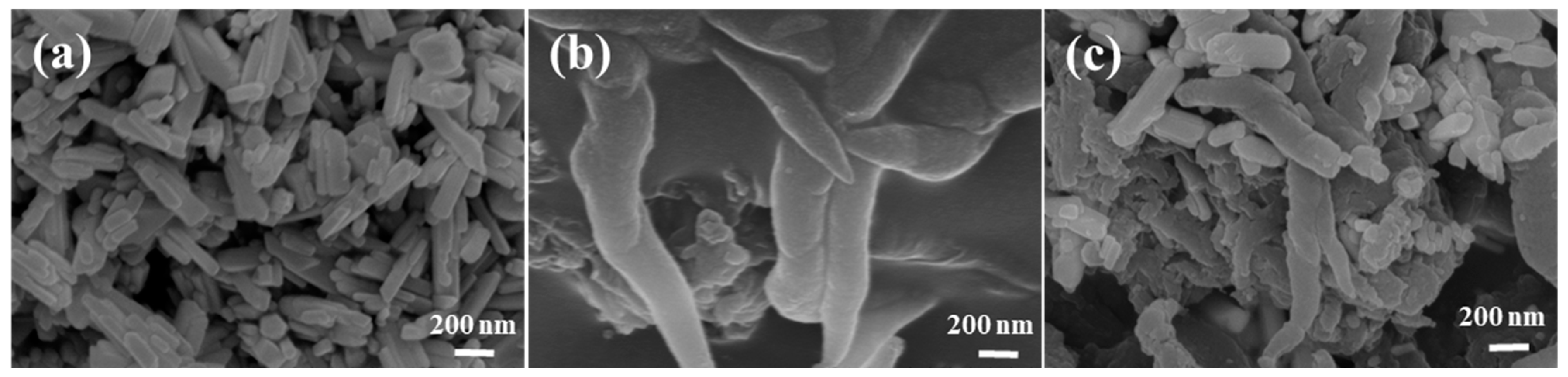
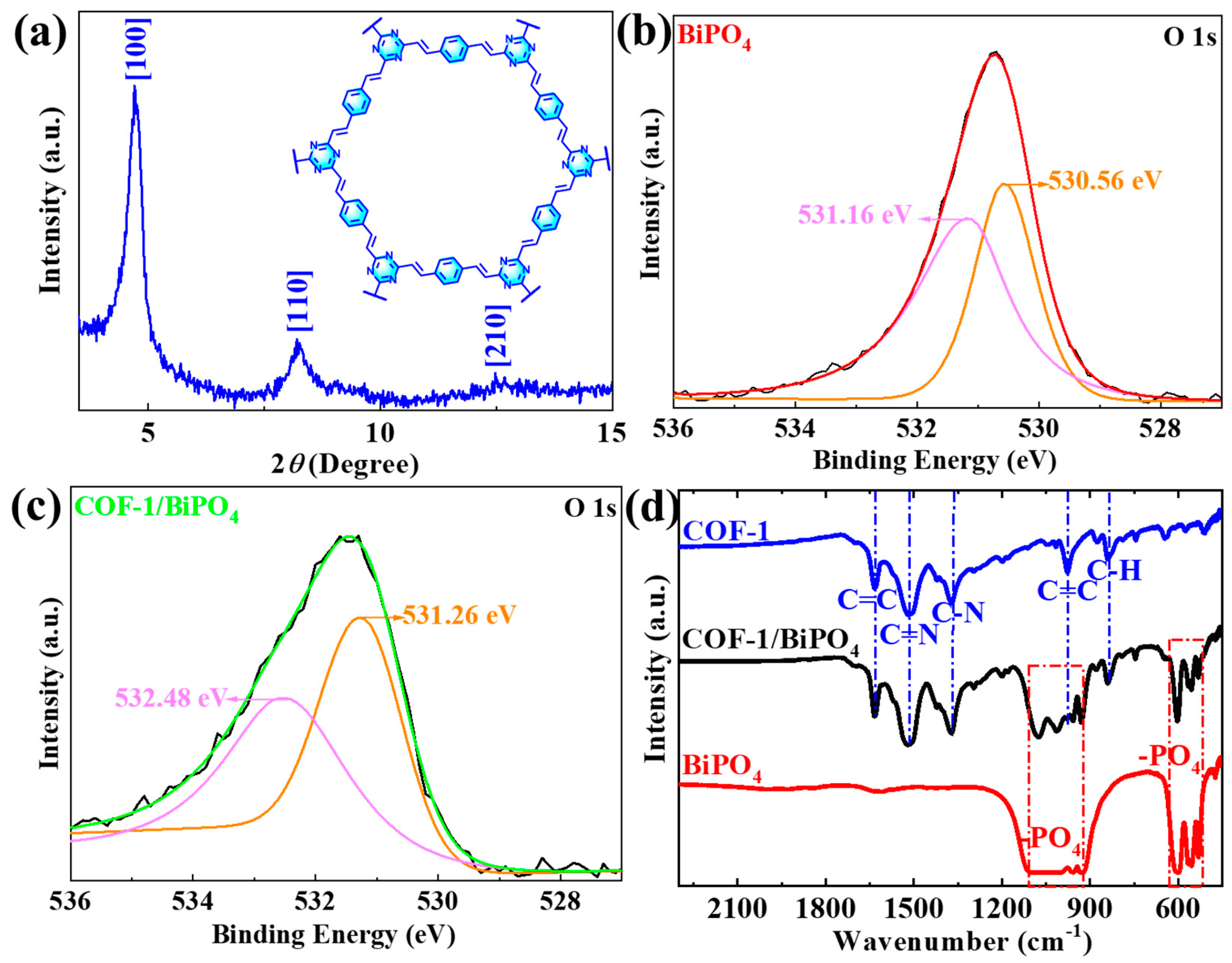
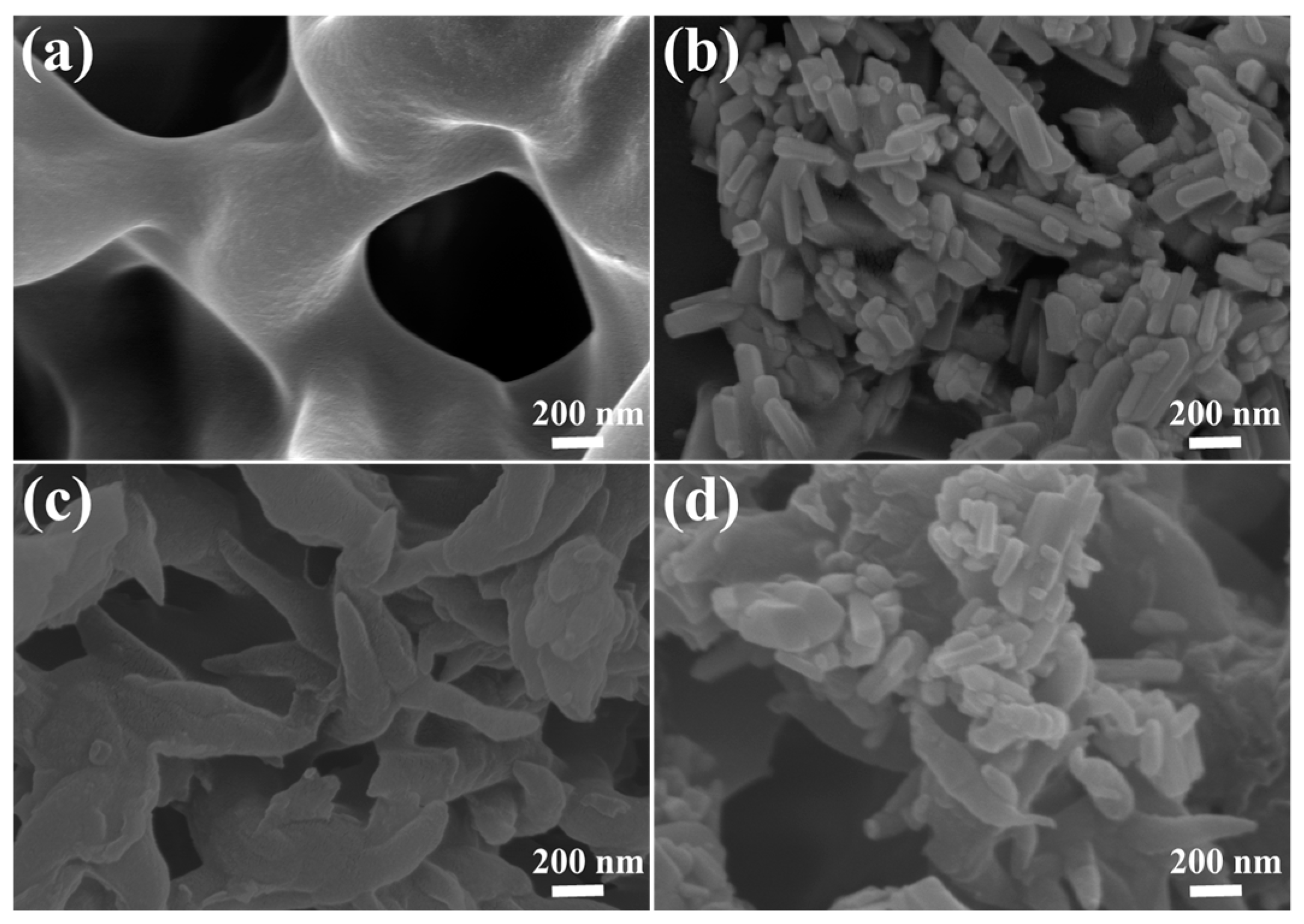
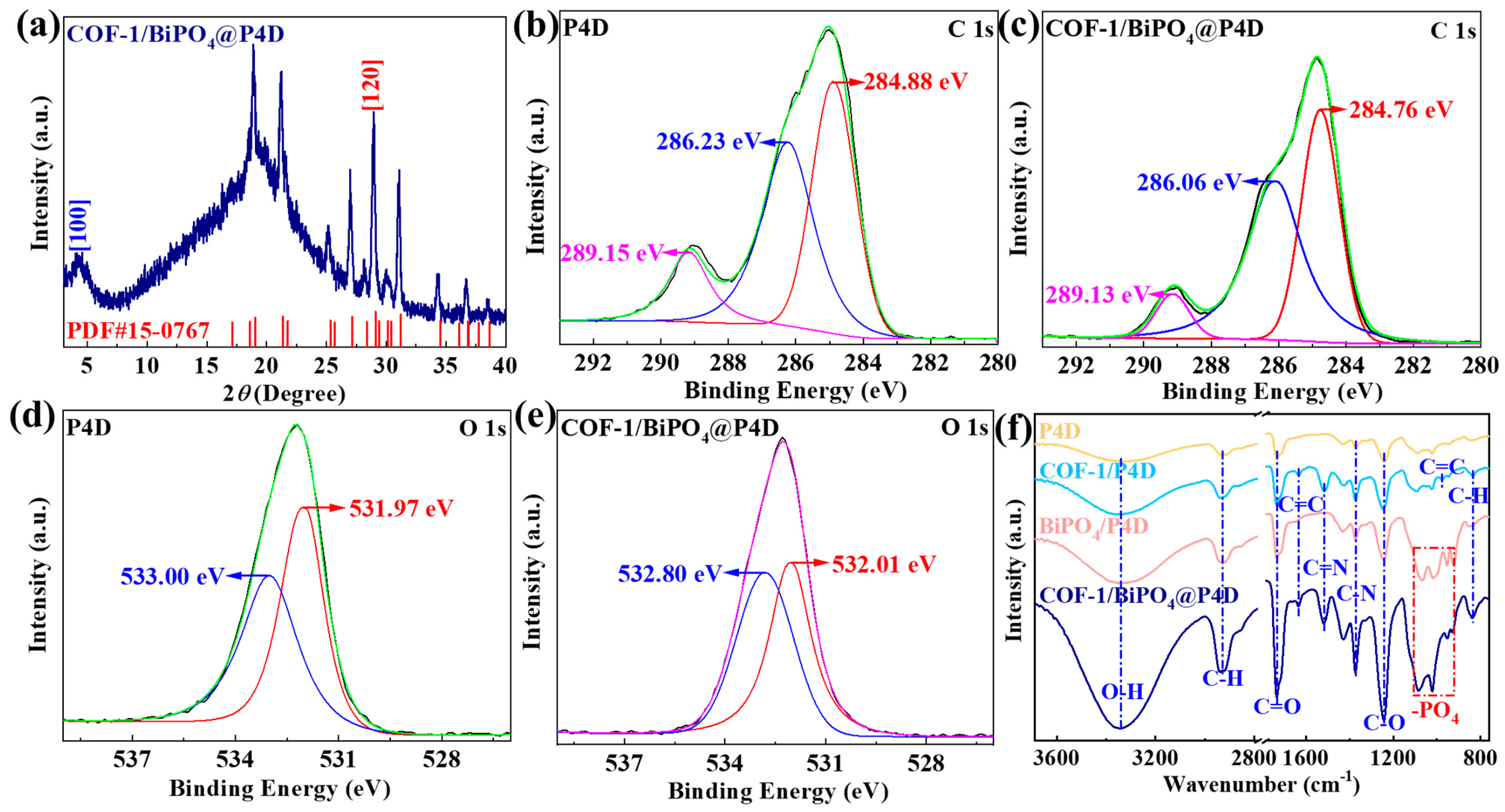
2.1. Fundamental Properties and Microstructure
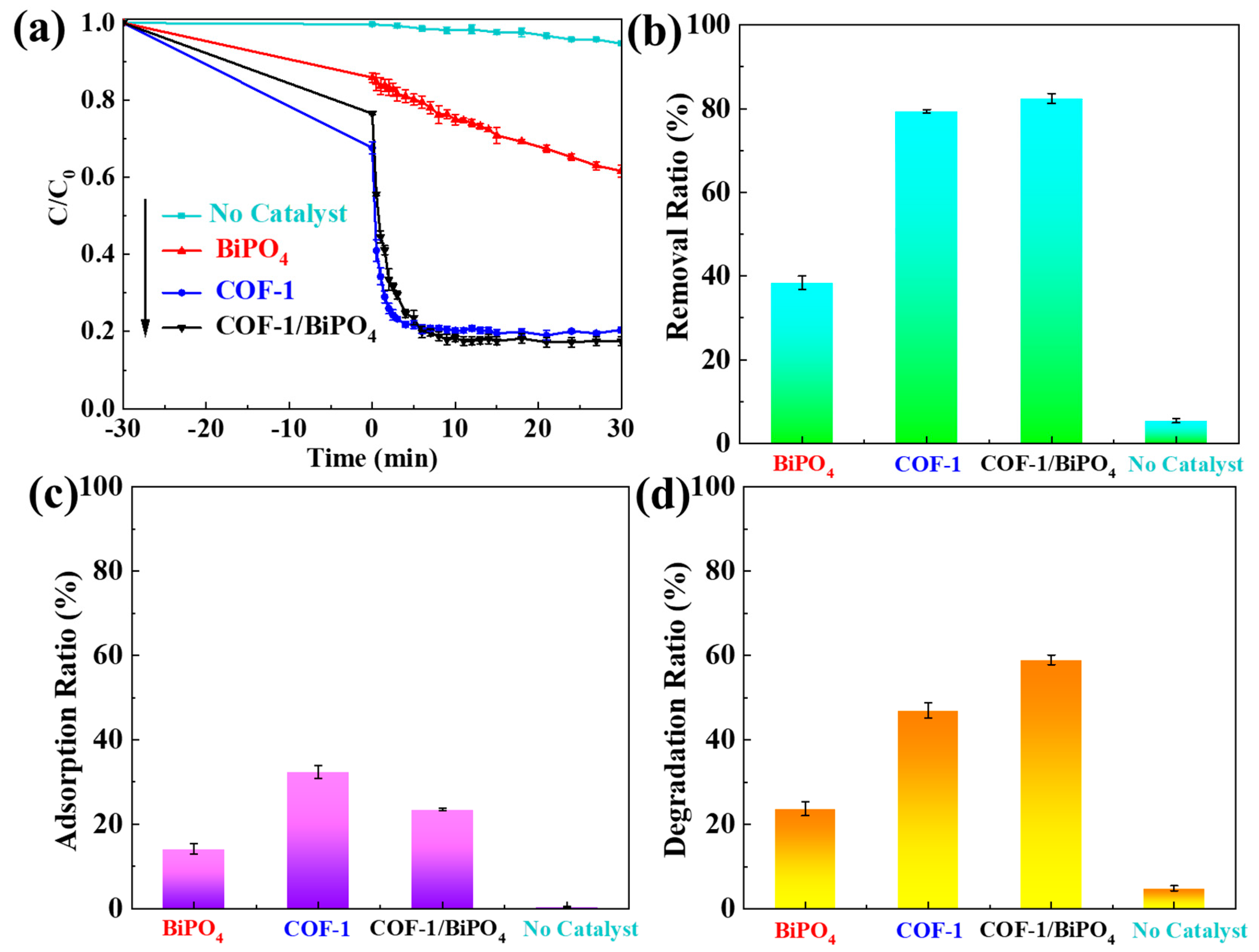
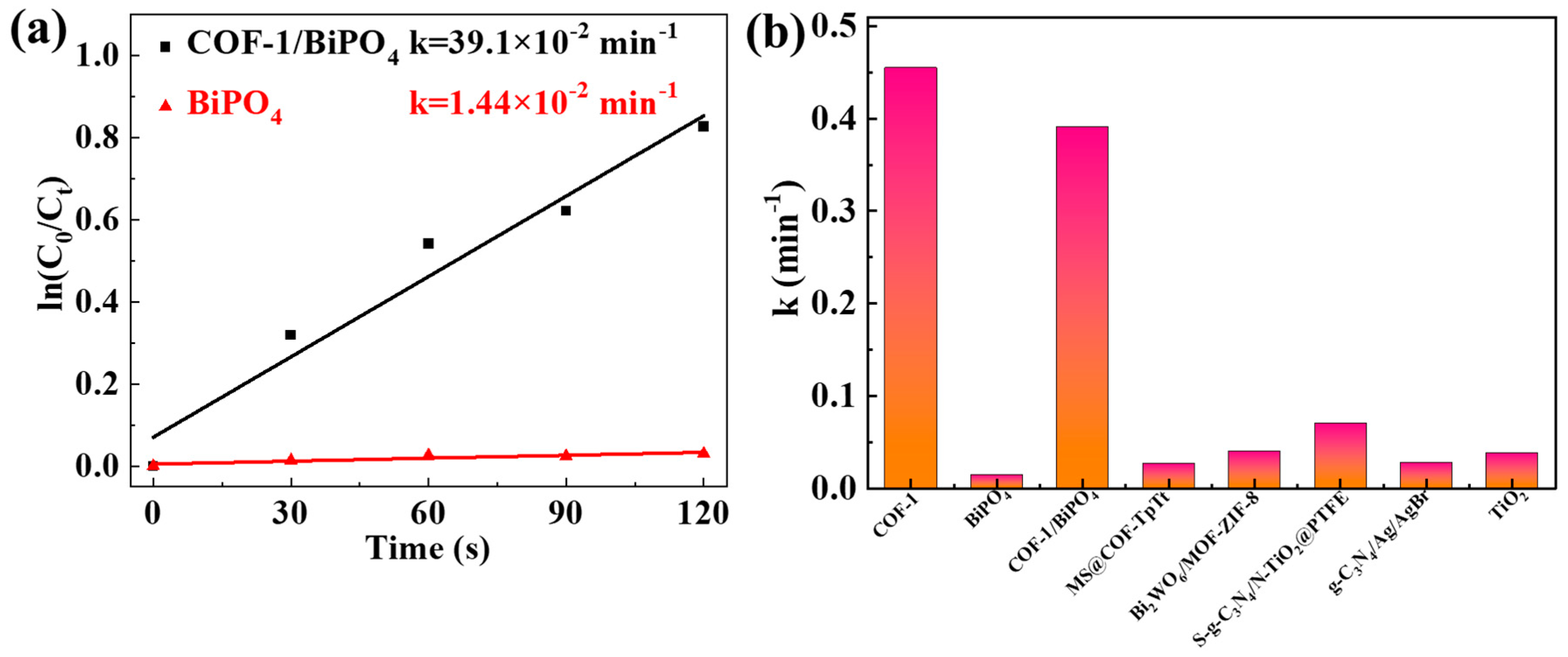
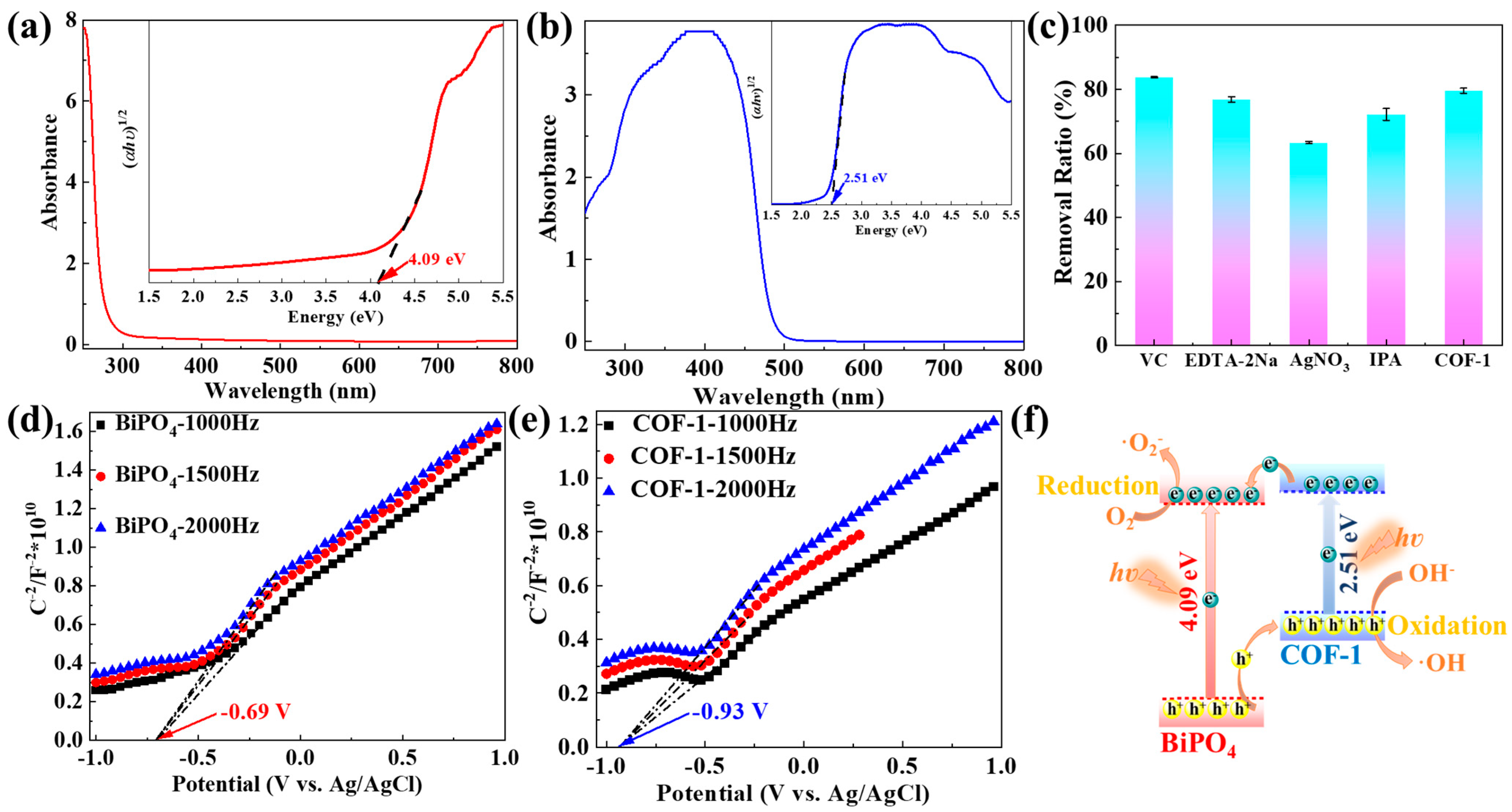
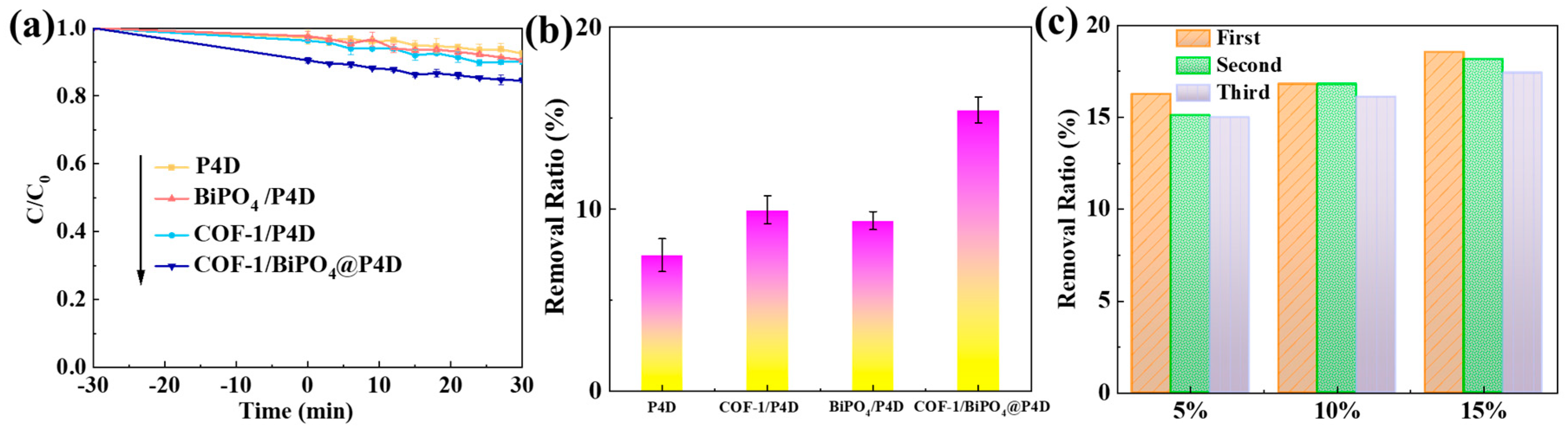
2.2. Photocatalytic Performance and Mechanism
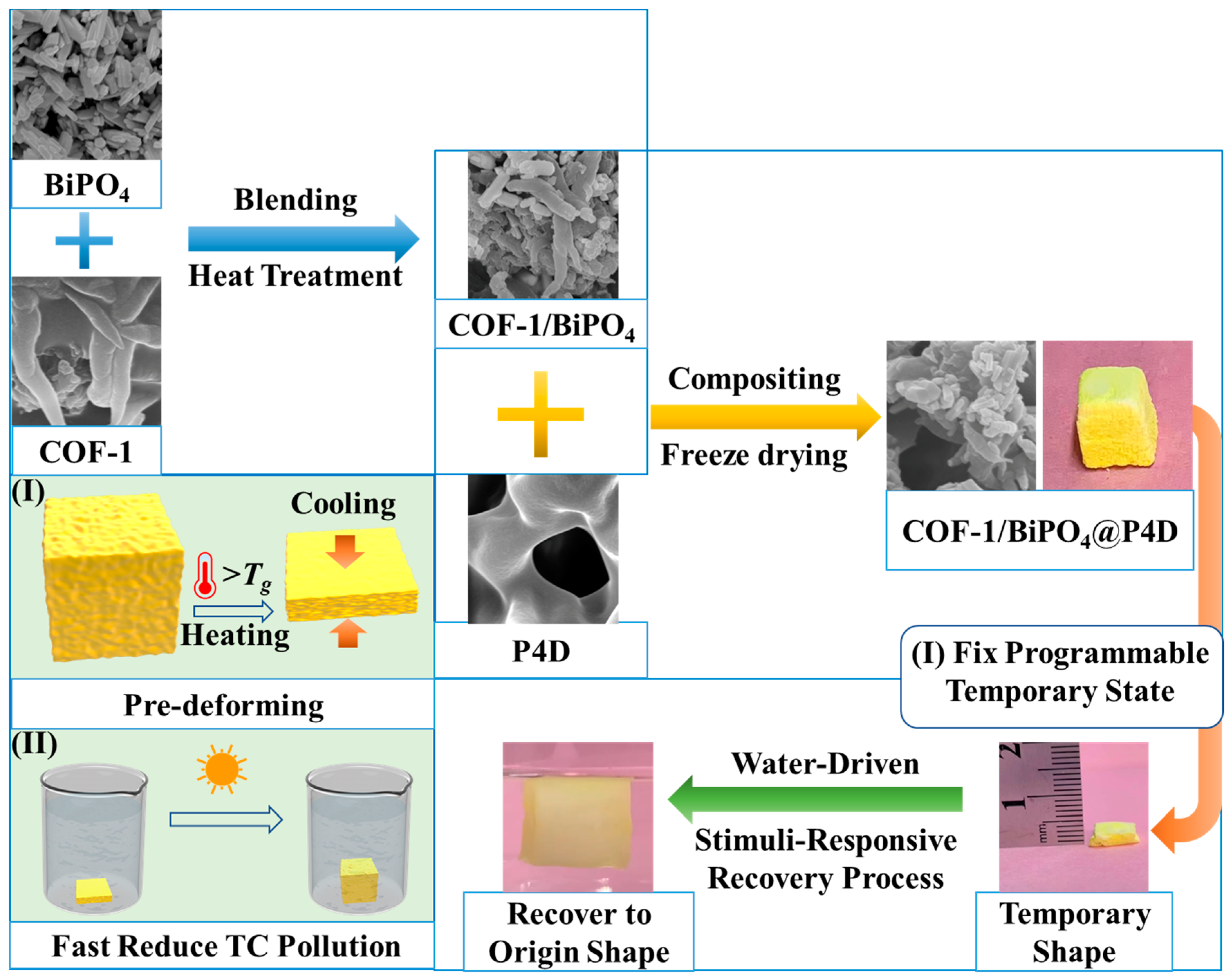
2.3. Stimuli-Responsive Photocatalytic Structure
3. Materials and Methods
3.1. Materials
3.2. Preparation of the COF-1/BiPO4@P4D
3.3. Characterization
3.4. Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Deepracha, S.; Ayral, A.; Ogawa, M. Acceleration of the photocatalytic degradation of organics by in-situ removal of the products of degradation. Appl. Catal. B Environ. 2020, 284, 119705. [Google Scholar] [CrossRef]
- Wang, W.X.; Jing, L.Q.; Qu, Y.C.; Luan, Y.B.; Fu, H.G.; Xiao, Y.C. Facile fabrication of efficient AgBr-TiO2 nanoheterostructured photocatalyst for degrading pollutants and its photogenerated charge transfer mechanism. J. Hazard. Mater. 2012, 243, 169–178. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Hua, Y.; Peia, Y.; Zhang, J.H.; Fu, M.L. In situ synthesis of TiO2@NH2-MIL-125 composites for use in combined adsorption and photocatalytic degradation of formaldehyde. Appl. Catal. B Environ. 2019, 259, 118106. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Ouachtak, H.; Malekshah, R.E.; Hafid, N.; Jada, A.; Ait Addi, A. Z-scheme g-C3N4/Fe3O4/Ag3PO4@sep magnetic nanocomposites as heterojunction photocatalysts for green malachite degradation and dynamic molecular studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131509. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Malekshah, R.E.; Alahiane, S.; Alakhras, F.; Alabbad, E.; Alghamdi, H.; Ouachtak, H.; Addi, A.A.; Jada, A. Exploring ZnO/Montmorillonite photocatalysts for the removal of hazardous RhB Dye: A combined study using molecular dynamics simulations and experiments. Mater. Today Commun. 2023, 35, 105915. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ye, X.J.; Chen, G.B.; Li, D.Z.; Meng, S.G.; Chen, S.F. Synthesis of BiPO4 by crystallization and hydroxylation with boosted photocatalytic removal of organic pollutants in air and water. J. Hazard. Mater. 2020, 399, 122999. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Ling, Q.; Liu, Y.F.; Wang, H.; Zhu, Y.F. Photocatalytic performance of BiPO4 nanorods adjusted via defects. Appl. Catal. B Environ. 2016, 187, 204–211. [Google Scholar] [CrossRef]
- Di, J.; Chen, J.; Ji, M.X.; Zhang, Q.; Xu, L.; Xia, J.X.; Li, H.M. Reactable ionic liquid induced homogeneous carbon superdoping of BiPO4 for superior photocatalytic removal of 4-chlorophenol. Chem. Eng. J. 2017, 313, 1477–1485. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Singh, A.; Dutta, D.P.; Roy, M.; Ballal, A.; Tyagi, A.K. Ag incorporated nano BiPO4: Sonochemical synthesis, characterization and improved visible light photocatalytic properties. RSC Adv. 2014, 4, 10097–10107. [Google Scholar] [CrossRef]
- Bai, J.W.; Yang, Y.; Hu, X.L.; Lu, P.; Fu, M.; Ren, X.L. Fabrication of novel organic/inorganic polyimide-BiPO4 heterojunction for enhanced photocatalytic degradation performance. J. Colloid Interface Sci. 2022, 625, 512–520. [Google Scholar] [CrossRef]
- Fellah, I.; Djellabi, R.; Amor, H.B.; Abderrahim, N.; Bianchi, C.L.; Giordana, A.; Cerrato, G.; Michele, A.D.; Hamdi, N. Visible light responsive heterostructure HTDMA-BiPO4 modified clays for effective diclofenac sodium oxidation: Role of interface inter-actions and basal spacing. J. Water Process Eng. 2022, 48, 102788. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, J.Y.; Hou, S.S.; Wu, J.X.; Wang, C.; Li, Y.M.; Jiang, G.Y.; Cui, G.Q. Novel CoAl-LDH Nanosheets/BiPO4 nanorods composites for boosting photocatalytic degradation of phenol. Pet. Sci. 2022, 19, 3080–3087. [Google Scholar] [CrossRef]
- Ben, S.K.; Gupta, S.; Harit, A.K.; Raj, K.K.; Chandra, V. Enhanced photocatalytic degradation of Reactive Red 120 dye under solar light using BiPO4@g-C3N4 nanocomposite photocatalyst. Environ. Sci. Pollut. Res. 2022, 29, 84325–84344. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Hamza, M.A.; Allam, N.K. Enhanced photoelectrochemical water splitting via engineered surface defects of BiPO4 nanorod photoanodes. Int. J. Hydrogen Energy 2021, 46, 23214–23224. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.S.; Jiang, D.L. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, M.; Lang, Z.L.; Liu, J.; Liu, M.; Chang, J.N.; Li, L.Y.; Shang, L.J.; Wang, M.; Li, S.L.; et al. Semiconductor/Covalent-Organic-Framework Z-Scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 6500–6506. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Du, L.W.; Xie, Y.; Zhang, F.H.; Li, P.; Xie, F.; Wan, X.; Pei, Q.B.; Leng, J.S.; Wang, N. Bioinspired four-dimensional polymeric aerogel with programmable temporal-spatial multiscale structure and functionality. Compos. Sci. Technol. 2021, 206, 108677. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Canova, E.; Liu, P.Y.; Wu, Z.L.; Cravotto, G. Degradation of antibiotics in wastewater: New advances in cavitational treatments. Molecules 2021, 26, 617. [Google Scholar] [CrossRef] [PubMed]
- Wammer, K.H.; Slattery, M.T.; Stemig, A.M.; Ditty, J.L. Tetracycline photolysis in natural waters: Loss of antibacterial activity. Chemosphere 2011, 85, 1505–1510. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Biotic and abiotic dissipation of tetracyclines using simulated sunlight and in the dark. Sci. Total Environ. 2018, 635, 1520–1529. [Google Scholar] [CrossRef]
- Fu, S.F.; Chen, K.Q.; Zou, H.; Xu, J.X.; Zheng, Y.; Wang, Q.F. Using calcium peroxide (CaO2) as a mediator to accelerate tetracycline removal and improve methane production during co-digestion of corn straw and chicken manure. Energy Convers. Manag. 2018, 172, 588–594. [Google Scholar] [CrossRef]
- Wu, S.Q.; Hu, H.Y.; Lin, Y.; Zhang, J.L.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Jiang, Y.H.; Li, C.S.; Fan, W.Q.; Yan, X.; Yan, M.; Shi, W.D. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Dai, X.J.; Feng, S.; Wu, W.; Zhou, Y.; Ye, Z.W.; Wang, Y.; Cao, X. Photocatalytic degradation of tetracycline by Z-Scheme Bi2WO6/ZIF-8. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2371–2383. [Google Scholar] [CrossRef]
- Song, J.H.; Zhao, K.; Yin, X.B.; Liu, Y.; Khan, I.; Liu, S.Y. Photocatalytic degradation of tetracycline hydrochloride with g-C3N4/Ag/AgBr composites. Front. Chem. 2022, 10, 1069816. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wu, Z.Z.; Liang, Y.; Wang, W.X.; Li, Y.P.; Sui, Z.Y.; Shan, L.L.; Li, C.K.; Fan, R.M.; Chen, Q. Sp2 carbon-conjugated covalent organic frameworks for efficient photocatalytic degradation and visualized pH detection. Mater. Today Chem. 2022, 25, 100962. [Google Scholar] [CrossRef]
- Lin, D.Y.; Duan, P.; Yang, W.T.; Huang, X.J.; Zhao, Y.J.; Wang, C.T.; Pan, Q.H. Facile fabrication of melamine sponge@covalent organic framework composite for enhanced degradation of tetracycline under visible light. Chem. Eng. J. 2022, 430, 132817. [Google Scholar]
- Hao, R.; Xiao, X.; Zuo, X.X.; Nan, J.M.; Zhang, W.D. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J. Hazard. Mater. 2012, 209–210, 137–145. [Google Scholar]
- Jiang, D.L.; Wang, T.Y.; Xu, Q.; Li, D.; Meng, S.C.; Chen, M. Perovskite oxide ultrathin nanosheets/g-C3N4 2D-2D heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar]
- Wang, W.; Fang, J.J.; Shao, S.F.; Lai, M.; Lu, C.H. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar]
- Yang, Y.; Zeng, Z.T.; Zhang, C.; Huang, D.L.; Zeng, G.M.; Xiao, R.; Lai, C.; Zhou, C.Y.; Guo, H.; Xue, W.J.; et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J. 2018, 349, 808–821. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.M.; Zeng, G.M.; Wang, D.B.; Niu, C.G.; Zhao, J.W.; An, H.X.; Xie, T.; Deng, Y.C. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4(040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Zhu, S.R.; Qi, Q.; Fang, Y.; Zhao, W.N.; Wu, M.K.; Han, L. Covalent Triazine Framework modified BiOBr nanoflake with enhanced photocatalytic activity for antibiotic removal. Cryst. Growth Des. 2018, 18, 883–891. [Google Scholar] [CrossRef]
- You, X.H.; Liu, F.; Jiang, G.F.; Chen, S.H.; An, B.Y.; Cui, R.L. S-g-C3N4/N−TiO2 @PTFE membrane for photocatalytic degradation of tetracycline. ChemistrySelect 2022, 7, e202203024. [Google Scholar]
- Pan, C.S.; Zhu, Y.F. New type of BiPO4 oxy-acid salt photocatalyst with high photocatalytic activity on degradation of dye. Environ. Sci. Technol. 2010, 44, 5570–5574. [Google Scholar]
- Pan, C.S.; Zhu, Y.F. Size-controlled synthesis of BiPO4 nanocrystals for enhanced photocatalytic performance. J. Mater. Chem. 2011, 21, 4235–4241. [Google Scholar] [CrossRef]
- Wang, W.X.; Liu, Y.J.; Leng, J.S. Recent developments in shape memory polymer nanocomposites: Actuation methods and mechanisms. Coord. Chem. Rev. 2016, 320–321, 38–52. [Google Scholar]
- Wang, W.X.; Liu, D.Y.; Liu, Y.J.; Leng, J.S.; Bhattacharyya, D. Electrical actuation properties of reduced graphene oxide paper/epoxy-based shape memory composites. Compos. Sci. Technol. 2015, 106, 20–24. [Google Scholar] [CrossRef]
- Wang, W.X.; Liu, X.B.; Xu, W.; Wei, H.Q.; Liu, Y.J.; Han, Y.; Jin, P.; Du, H.J.; Leng, J.S. Light-induced microfluidic chip based on shape memory gold nanoparticles/poly (vinyl alcohol) nanocomposites. Smart Mater. Struct. 2018, 27, 105047. [Google Scholar] [CrossRef]
- Wang, W.X.; Lu, H.B.; Liu, Y.J.; Leng, J.S. Sodium dodecyl sulfate/epoxy composite: Water-induced shape memory effect and its mechanism. J. Mater. Chem. A 2014, 2, 5441–5449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, W.; Liang, Y.; Du, L.; Yang, H.; Ma, H.; Cheng, H.; Yan, Y.; Shen, Y.; Chen, Q. Advanced Stimuli-Responsive Structure Based on 4D Aerogel and Covalent Organic Frameworks Composite for Rapid Reduction in Tetracycline Pollution. Molecules 2023, 28, 5505. https://doi.org/10.3390/molecules28145505
Wang W, Wang W, Liang Y, Du L, Yang H, Ma H, Cheng H, Yan Y, Shen Y, Chen Q. Advanced Stimuli-Responsive Structure Based on 4D Aerogel and Covalent Organic Frameworks Composite for Rapid Reduction in Tetracycline Pollution. Molecules. 2023; 28(14):5505. https://doi.org/10.3390/molecules28145505
Chicago/Turabian StyleWang, Wenxin, Wenjing Wang, Ying Liang, Liwen Du, Huan Yang, Haoxiang Ma, Huiting Cheng, Yaqian Yan, Yijun Shen, and Qi Chen. 2023. "Advanced Stimuli-Responsive Structure Based on 4D Aerogel and Covalent Organic Frameworks Composite for Rapid Reduction in Tetracycline Pollution" Molecules 28, no. 14: 5505. https://doi.org/10.3390/molecules28145505
APA StyleWang, W., Wang, W., Liang, Y., Du, L., Yang, H., Ma, H., Cheng, H., Yan, Y., Shen, Y., & Chen, Q. (2023). Advanced Stimuli-Responsive Structure Based on 4D Aerogel and Covalent Organic Frameworks Composite for Rapid Reduction in Tetracycline Pollution. Molecules, 28(14), 5505. https://doi.org/10.3390/molecules28145505






