Research on Improved MOF Materials Modified by Functional Groups for Purification of Water
Abstract
1. Introduction
2. Several Advantages of MOFs Modified by Functional Groups as Adsorbents
2.1. Large Specific Surface Area
2.2. Porous and Adjustable Structure
2.3. Coordinated Unsaturated Metal Sites
3. Synthesis of MOFs Modified by Functional Groups
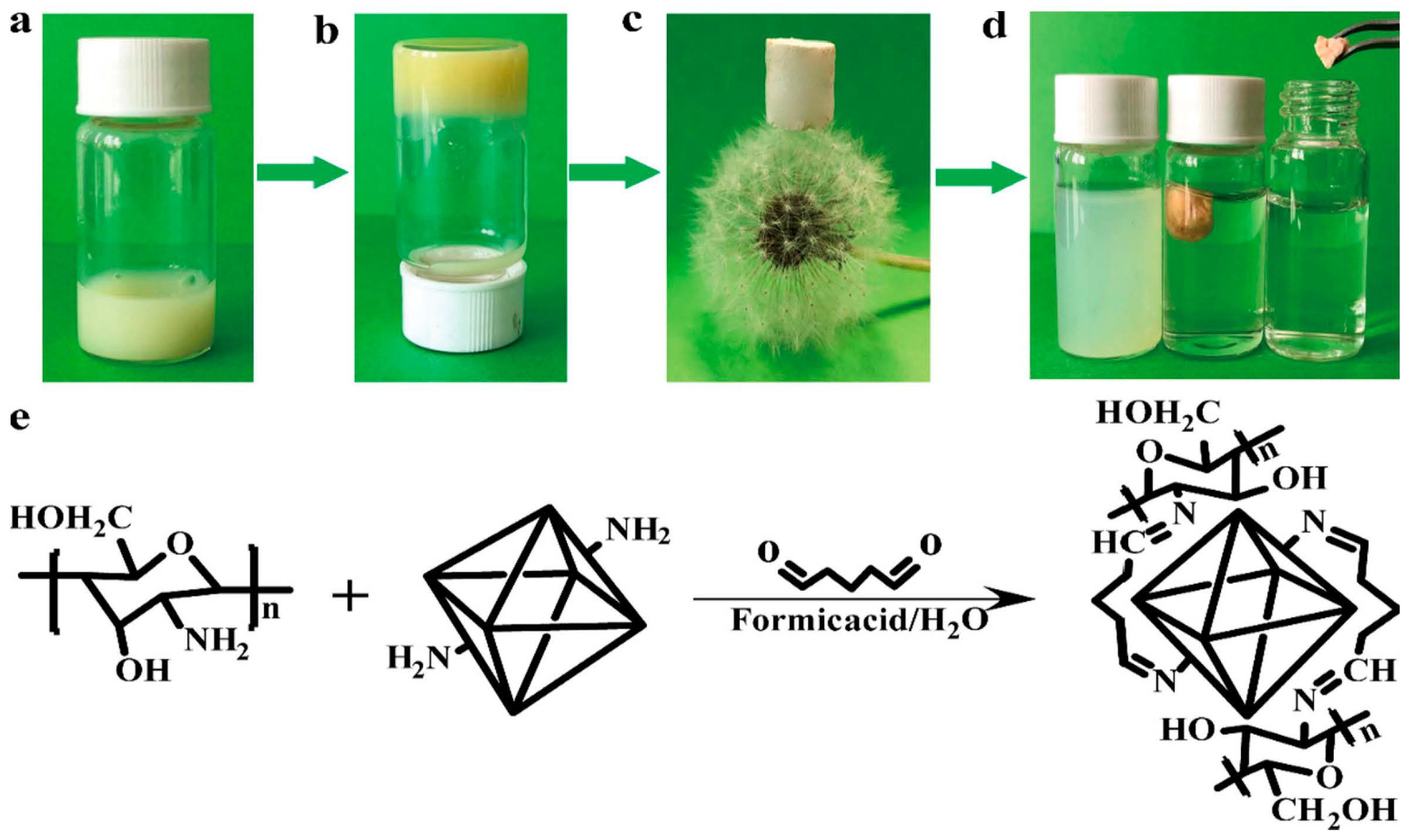
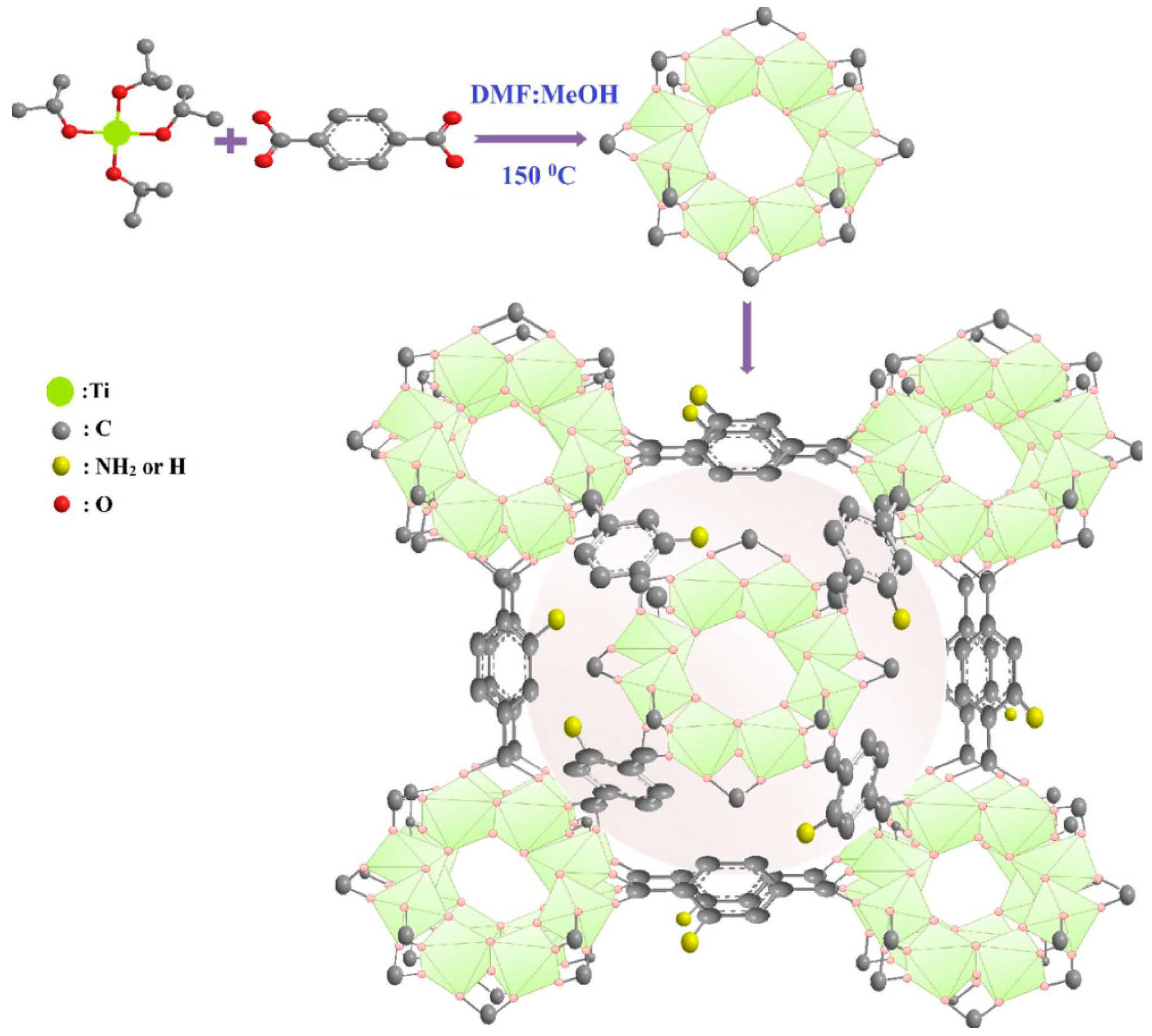
3.1. Direct Synthesis Using Functionalized Linkers
3.2. Post-Synthetic Modification
3.3. Other Means
4. Mechanism of Contaminant Adsorption in MOFs Modified by Functional Groups
4.1. Electrostatic Interaction
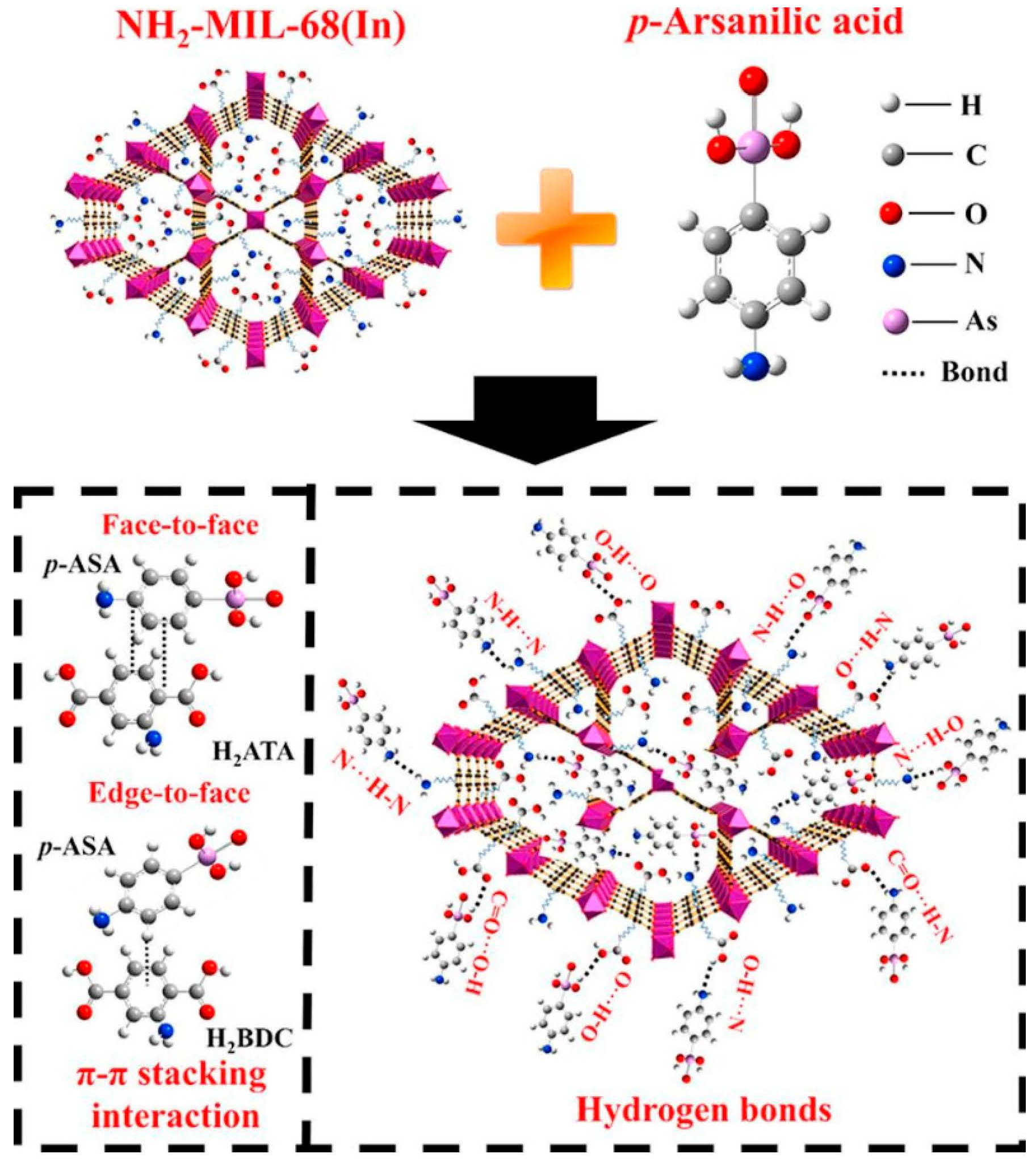
4.2. Hydrogen-Bond Interaction
4.3. π–π. Interaction
4.4. Hydrophobic Interaction
4.5. Lewis Acid–Base Interaction
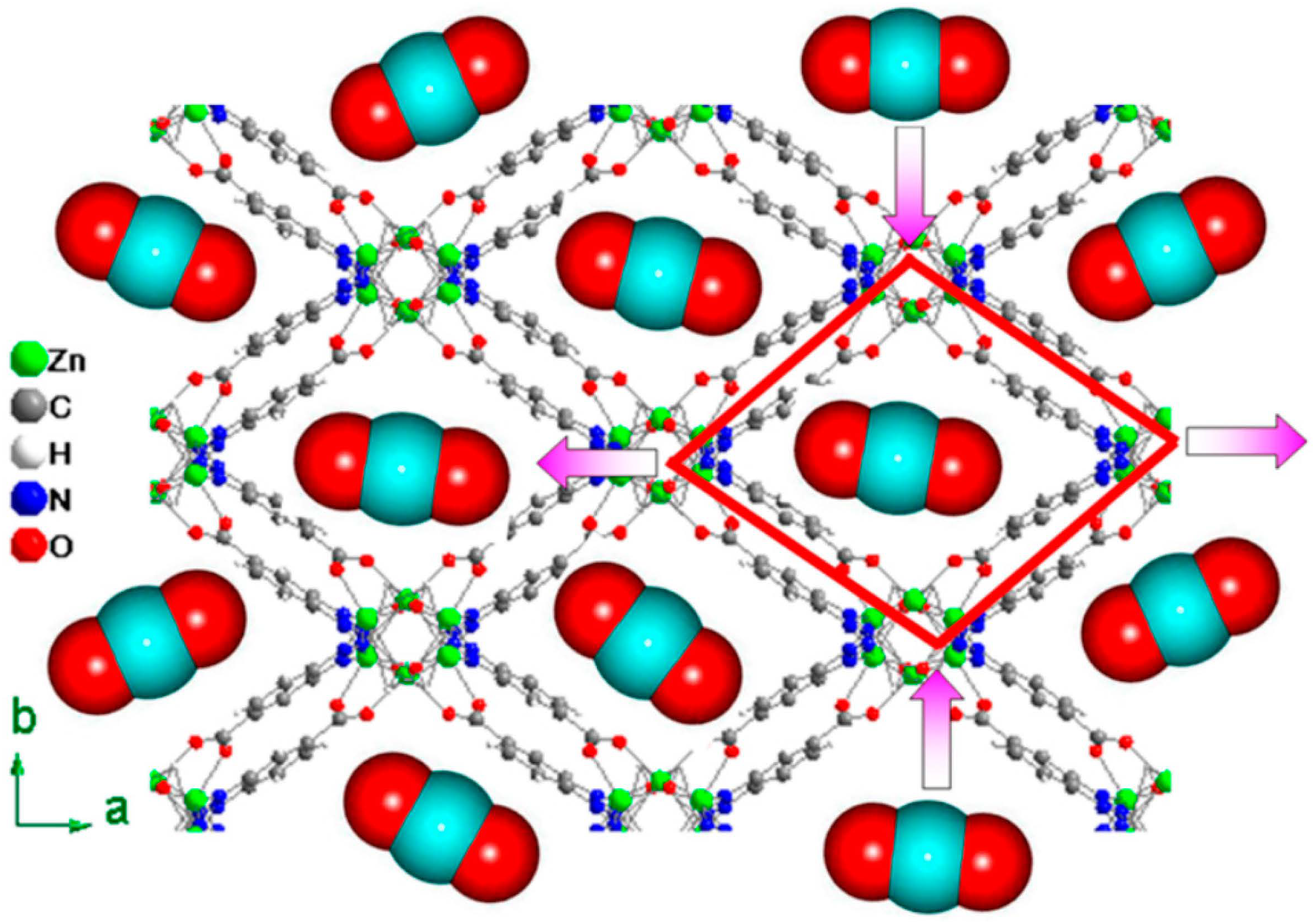
5. Application of MOFs by Modified Functional Groups in Water Purification
5.1. N-Containing Group
5.1.1. -NH2
5.1.2. -NMe3+
5.2. S-Containing Group
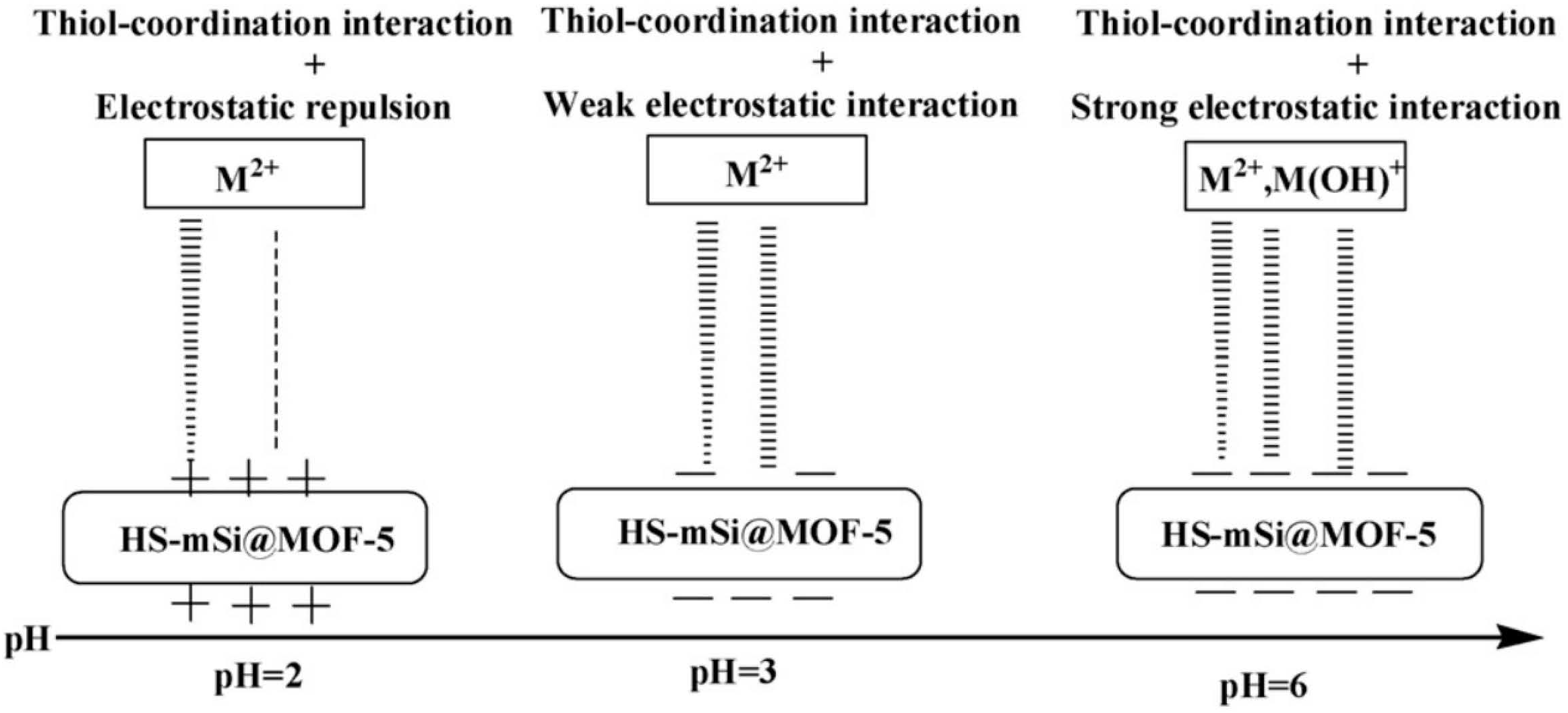
5.2.1. -SH
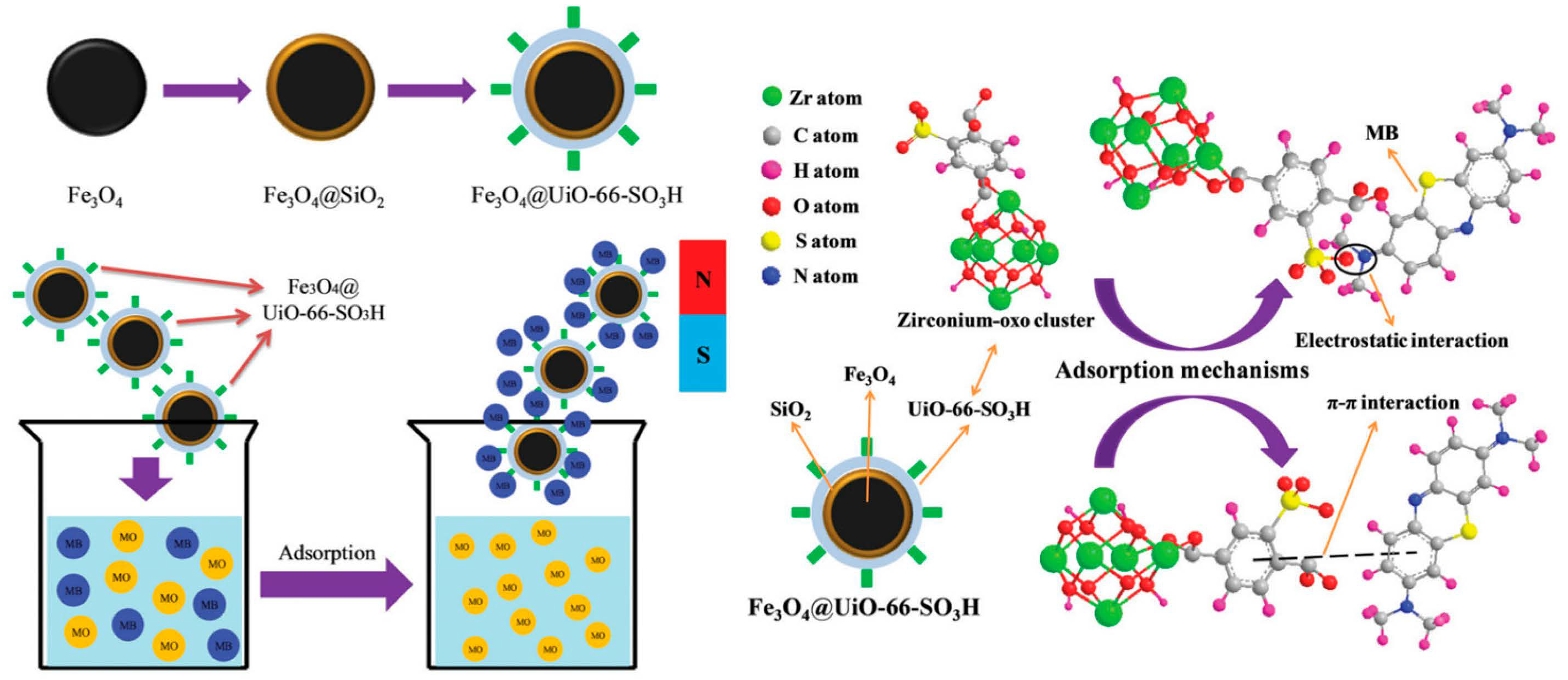
5.2.2. -SO3H
5.2.3. -SO4
5.3. -OH and -COOH
5.3.1. -OH
5.3.2. -COOH
5.4. Halogen Groups
6. Summary and Expectation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, N.; Gevenga, C.; Echeverria, J. Managing Water for People and Nature. Science 2001, 292, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Vorosmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Rolston, A. Social changes affect water quality too. Nature 2016, 536, 396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Kim, K.H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef]
- Lv, Z.; Liang, C.; Cui, J.; Zhang, Y.; Xu, S. A facile route for the synthesis of mesoporous melamine-formaldehyde resins for hexavalent chromium removal. RSC Adv. 2015, 5, 18213–18217. [Google Scholar] [CrossRef]
- Li, L.L.; Feng, X.Q.; Han, R.P.; Zang, S.Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Energy-efficient sonochemical approach for the preparation of nanohybrid composites from graphene oxide and metal-organic framework. Inorg. Chem. Commun. 2019, 102, 185–191. [Google Scholar] [CrossRef]
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Amide-Functionalized Metal-Organic Framework for High Efficiency and Fast Removal of Pb(II) from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3170–3178. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpaa, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Nthunya, L.; Masheane, M.; George, M.; Kime, M.-B.; Mhlanga, S. Removal of Fe and Mn From Polluted Water Sources in Lesotho Using Modified Clays. J. Water Chem. Technol. 2019, 41, 81–86. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Musyoka, N.; Dladla, B.S.; Nxumalo, E.N.; Mhlanga, S.D. Microwave-assisted synthesis of coal fly ash-based zeolites for removal of ammonium from urine. RSC Adv. 2020, 10, 2416–2427. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’ keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974–987. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-Based Metal-Organic Framework with High Methane and Hydrogen Storage Capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’ keeffe, M.; Kim, J.; Yaghi, O.M. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, 988–997. [Google Scholar] [CrossRef]
- Chen, J.W.; Li, K.; Yang, J.; Gu, J.L. Hierarchical large-pore MOFs templated from poly(ethylene oxide)-b-polystyrene diblock copolymer with tuneable pore sizes. Chem. Commun. 2022, 58, 10028–10031. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’ keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.L.; Chen, K.J.; Xing, H.B.; Yang, Q.W.; Krishna, R.; Bao, Z.B.; Wu, H.; Zhou, W.; Dong, X.L.; Han, Y.; et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation. Angew. Chem. Int. Edit. 2010, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H. Precise Construction of MOFs with Catalytic Functions: Selective Regulators for Hydrogenation Reactions. Acta Phys.-Chim. Sin. 2016, 32, 2645–2646. [Google Scholar]
- Ji, P.F.; Manna, K.; Lin, Z.K.; Feng, X.Y.; Urban, A.; Song, Y.; Lin, W.B. Single-Site Cobalt Catalysts at New Zr12(μ3-O)8(μ3-OH)8(μ2-OH)6 Metal-Organic Framework Nodes for Highly Active Hydrogenation of Nitroarenes, Nitriles, and Isocyanides. J. Am. Chem. Soc. 2017, 12, 12234–12242. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, L.R.; Liang, Y.Y.; He, J.P.; Zhao, J.X.; Chen, J.H. Metal-Organic-Framework-Derived Fe-N/C Electrocatalyst with Five-Coordinated Fe-Nx Sites for Advanced Oxygen Reduction in Acid Media. ACS Catal. 2017, 7, 1655–1663. [Google Scholar] [CrossRef]
- Shen, J.Q.; Liao, P.Q.; Zhou, D.D.; He, C.T.; Wu, J.X.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Modular and Stepwise Synthesis of a Hybrid Metal-Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. Efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energ. 2017, 210, 317–326. [Google Scholar] [CrossRef]
- Zou, L.F.; Zhou, H.C. Hydrogen Storage in Metal-Organic Frameworks. In Nanostructured Materials for Next-Generation Energy Storage and Conversion; Chen, Y.P., Bashir, S., Liu, J.L., Eds.; Springer: Berlin, Heidelberg, Germany, 2017; Volume 22, pp. 143–170. [Google Scholar]
- Liu, D.M.; Wu, H.H.; Wang, S.Z.; Xie, Z.G.; Li, J.; Lin, W.B. A high connectivity metal-organic framework with exceptional hydrogen and methane uptake capacities. Chem. Sci. 2012, 3, 3032–3037. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.P.V.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2015, 112, 1105–1125. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Zapata, F.; Lin, G.; Qian, G.; Lobkovsky, E.B. Luminescent Open Metal Sites within a Metal-Organic Framework for Sensing Small Molecules. Adv. Mater. 2010, 19, 1693–1696. [Google Scholar] [CrossRef]
- Liu, T.Z.; Rong, H.; Zhang, X.; Zhang, K.L.; Liu, Y.; Zhang, X.B.; Bai, R.Y.; Li, D.; Yang, Y.H. Metal-Organic Framework Nanomaterials as Novel Signal Probes for Electron Transfer Mediated Ultrasensitive Electrochemical Immunoassay. Anal. Chem. 2016, 88, 12516–12523. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid Phase Adsorption by Microporous Coordination Polymers: Removal of Organosulfur Compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939. [Google Scholar] [CrossRef]
- Herm, Z.R.; Wiers, B.M.; Mason, J.A.; Baten, J.M.V.; Hudson, M.R.; Zajdel, P.; Brown, C.R.; Masciocchi, N.; Krishna, R.; Long, J.R. Separation of Hexane Isomers in a Metal-Organic Framework with Triangular Channels. Science 2013, 340, 960–964. [Google Scholar] [CrossRef]
- Das, S.; Xu, S.X.; Ben, T.; Qiu, S.L. Chiral Recognition and Separation by Chirality-Enriched Metal-Mrganic Frameworks. Angew. Chem. Int. Edit. 2018, 57, 8629–8633. [Google Scholar] [CrossRef]
- Ryu, D.W.; Lee, W.R.; Lee, J.W.; Yoon, J.H.; Kim, H.C.; Koh, E.K.; Hong, C.S. Magnetic metal-organic framework constructed from a paramagnetic metalloligand exhibiting a significant sorption and reversible magnetic conversions. Chem. Commun. 2009, 46, 8779–8781. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kong, C.L.; Zhang, Q.J.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincǎ, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Ma, L.L.; Zhong, C.L.; An, X.H.; Liu, D.H. Enhancement of CO2/N2 Mixture Separation Using the Thermodynamic Stepped Behavior of Adsorption in Metal-Organic Frameworks. J. Phys. Chem. C 2011, 115, 2790–2797. [Google Scholar] [CrossRef]
- Li, H.L.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Draznieks, C.M.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. Int. Edit. 2004, 116, 6456–6461. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Vitillo, J.G.; Regli, L.; Chavan, S.; Ricchiardi, G.; Spoto, G.; Dietzel, P.D.C.; Bordiga, S.; Zecchina, A. Role of Exposed Metal Sites in Hydrogen Storage in MOFs. J. Am. Chem. Soc. 2008, 130, 8386–8396. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials 2020, 10, 2274. [Google Scholar] [CrossRef]
- Lee, Y.R.; Cho, S.M.; Ahn, W.S. Effects of polydimethylsiloxane coating of Ni-MOF-74 on CH4 storage. Korean J. Chem. Eng. 2018, 35, 1542–1546. [Google Scholar] [CrossRef]
- Wang, G.F.; Graham, E.; Zheng, S.L.; Zhu, J.X.; Zhu, R.L.; He, H.P.; Sun, Z.M.; Mackinnon, I.D.R.; Xi, Y.F. Diatomite-Metal-Organic Framework Composite with Hierarchical Pore Structures for Adsorption/Desorption of Hydrogen, Carbon Dioxide and Water Vapor. Materials 2020, 13, 4700. [Google Scholar] [CrossRef]
- Schulte, Z.M.; Kwon, Y.H.; Han, Y.; Liu, C.; Li, L.; Yang, Y.H.; Jarvi, A.G.; Saxena, S.; Veser, G.; Johnson, J.K.; et al. H2/CO2 separations in multicomponent metal-adeninate MOFs with multiple chemically distinct pore environments. Chem. Sci. 2020, 11, 12807. [Google Scholar] [CrossRef]
- Habib, N.; Durak, O.; Zeeshan, M.; Uzun, A.; Keskin, S. A novel IL/MOF/polymer mixed matrix membrane having superior CO2/N2 selectivity. J. Membr. Sci. 2022, 658, 120712. [Google Scholar] [CrossRef]
- Altintas, C.; Keskin, S. Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations. ACS Sustain. Chem. Eng. 2019, 7, 2739–2750. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, A.R.; Kim, M.J.; Yoon, T.U.; Kim, J.H.; Bae, Y.S. Low-temperature Cu(I) loading on a mesoporous Metal-Organic framework for adsorptive separation of C3H6/C3H8 mixtures. Microporous Mesoporous Mater. 2019, 279, 271–277. [Google Scholar] [CrossRef]
- Yue, Y.F.; Rabone, J.A.; Liu, H.J.; Mahurin, S.M.; Li, M.R.; Wang, H.L.; Lu, Z.L.; Chen, B.L.; Wang, J.H.; Fang, Y.X.; et al. A Flexible Metal-Organic Framework: Guest Molecules Controlled Dynamic Gas Adsorption. J. Phys. Chem. C 2015, 119, 9442–9449. [Google Scholar] [CrossRef]
- Liang, H.; Liu, R.P.; An, X.Q.; Hu, C.Z.; Zhang, X.W.; Liu, H.J. Bimetal-organic frameworks with coordinatively unsaturated metal sites for highly efficient Fenton-like catalysis. Chem. Eng. J. 2021, 414, 128669. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhou, Y.X.; Wang, H.W.; Lu, J.L.; Uchida, T.; Xu, Q.; Yu, S.H.; Jiang, H.L. Multifunctional PdAg@MIL-101 for One-Pot Cascade Reactions: Combination of Host-Guest Cooperation and Bimetallic Synergy in Catalysis. ACS Catal. 2015, 5, 2062–2069. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, D. De facto methodologies toward the synthesis and scale-up production of UiO-66-type metal-organic frameworks and membrane materials. Dalton Trans. 2015, 44, 19018–19040. [Google Scholar] [CrossRef]
- Peikert, K.; Hoffmann, F.; Fröba, M. Amino substituted Cu3(BTC)2: A new metal-organic framework with a versatile functionality. Chem. Commun. 2012, 48, 11196–11198. [Google Scholar] [CrossRef]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Ferey, G.; Chang, J.S. Porous chromium terephthalate MIL- 101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.; Camp, J.S. Advances, updates, and analytics for the computation-ready, experimental metal-organic framework database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998. [Google Scholar] [CrossRef]
- Jeong, W.; Lim, D.-W.; Kim, S.; Harale, A.; Yoon, M.; Suh, M.P.; Kim, J. Modeling adsorption properties of structurally deformed metal-organic frameworks using structure-property map. Proc. Natl. Acad. Sci. USA 2017, 114, 7923–7928. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive denitrogenation of model fuel by functionalized UiO-66 with acidic and basic moieties. Chem. Eng. J. 2017, 321, 40–47. [Google Scholar] [CrossRef]
- Sarker, M.; Shin, S.; Jhung, S.H. Synthesis and functionalization of porous Zr-diaminostilbenedicarboxylate metal-organic framework for storage and stable delivery of ibuprofen. ACS Omega 2019, 4, 9860–9867. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.M.H.; Bhadra, B.N.; Jhung, S.H. Removal of nitrogen-containing compounds from microalgae derived biofuel by adsorption over functionalized metal organic frameworks. Fuel 2020, 280, 118622. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Carboxylic-acid-functionalized UiO-66-NH2: A promising adsorbent for both aqueous-and non-aqueous-phase adsorptions. Chem. Eng. J. 2018, 331, 124–131. [Google Scholar] [CrossRef]
- Bernt, S.; Guillerm, V.; Serre, C.; Stock, N. Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid. Chem. Commun. 2011, 47, 2838–2840. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-M.; Kim, H.-Y.; Ahn, W.-S. Friedel-Crafts acylation of p-xylene over sulfonated zirconium terephthalates. Catal. Lett. 2014, 144, 817–824. [Google Scholar] [CrossRef]
- Huang, M.; Chang, G.; Su, Y.; Xing, H.; Zhang, Z.; Yang, Y.; Ren, Q.; Bao, Z.; Chen, B. A metal-organic framework with immobilized Ag(I) for highly efficient desulfurization of liquid fuels. Chem. Commun. 2015, 51, 12205–12207. [Google Scholar] [CrossRef]
- She, H.; Ma, X.; Chang, G. Highly efficient and selective removal of N-heterocyclic aromatic contaminants from liquid fuels in a Ag(I) functionalized metal-organic framework: Contribution of multiple interaction sites. J. Colloid Interface Sci. 2018, 518, 149–155. [Google Scholar] [CrossRef]
- Valadi, F.M.; Ekramipooya, A.; Gholami, M.R. Selective separation of Congo Red from a mixture of anionic and cationic dyes using magnetic-MOF: Experimental and DFT study. J. Mol. Liq. 2020, 318, 114051. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 1–3, 52–62. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.W.; Yin, N. Efficient Removal of Pb(II) and Cd(II) Using NH2-Functionalized Zr-MOFs via Rapid Microwave-Promoted Synthes. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; El-Mehalmey, W.A.; Haikal, R.R.; Safy, M.E.A.; Amin, M.; Shatla, H.R.; Karakalos, S.G.; Alkordi, M.H. Tuning the Chemical Environment within the UiO-66-NH2 Nanocages for Charge-Dependent Contaminant Uptake and Selectivity. Inorg. Chem. 2019, 58, 15078–15087. [Google Scholar] [CrossRef]
- Abdollahi, N.; Moussavi, G.; Giannakis, S. A review of heavy metals’ removal from aqueous matrices by Metal-Organic Frameworks (MOFs): State-of-the art and recent advances. J. Environ. Chem. Eng. 2022, 10, 107394. [Google Scholar] [CrossRef]
- Planas, N.; Dzubak, A.L.; Poloni, R.; Lin, L.-C.; McManus, A.; McDonald, T.M.; Neaton, J.B.; Long, J.R.; Smit, B.; Gagliardi, L. The Mechanism of Carbon Dioxide Adsorption in an Alkylamine-Functionalized Metal-Organic Framework. J. Am. Chem. Soc. 2013, 135, 7402–7405. [Google Scholar] [CrossRef]
- Hasan, Z.; Tong, M.; Jung, B.K.; Ahmed, I.; Zhong, C.; Jhung, S.H. Adsorption of Pyridine over Amino-Functionalized Metal-Organic Frameworks: Attraction via Hydrogen Bonding versus Base–Base Repulsion. J. Phys. Chem. C 2014, 118, 21049–21056. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Adsorptive denitrogenation of model fuel with CuCl-loaded metal-organic frameworks (MOFs). Chem. Eng. J. 2014, 251, 35–42. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yan, X.-P. Metal-organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Yang, H.; Petit, C.; Hsu, F.-K. Removing oil droplets from water using a copper-based metal organic frameworks. Chem. Eng. J. 2014, 249, 293–301. [Google Scholar] [CrossRef]
- Yang, C.; Kaipa, U.; Mather, Q.Z.; Wang, X.; Nesterov, V.; Venero, A.F.; Omary, M.A. Fluorous Metal-Organic Frameworks with Superior Adsorption and Hydrophobic Properties toward Oil Spill Cleanup and Hydrocarbon Storage. J. Am. Chem. Soc. 2011, 133, 18094–18097. [Google Scholar] [CrossRef]
- Ania, C.O.; Bandosz, T.J. Importance of Structural and Chemical Heterogeneity of Activated Carbon Surfaces for Adsorption of Dibenzothiophene. Langmuir 2005, 21, 7752–7759. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.; Li, H.; Ding, W.; Chao, Y.; Wu, P.; Xun, S.; Zhang, M.; Li, H. Few-layered graphene-like boron nitride induced a remarkable adsorption capacity for dibenzothiophene in fuels. Green Chem. 2015, 17, 1647–1656. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Ionic liquids supported on metal- organic frameworks: Remarkable adsorbents for adsorptive desulfurization. Chem. Eur. J. 2014, 20, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, S.S.; Yu, H.H.; Zeng, F.M.; Li, X.; Su, Z.M. Covalently crosslinked zirconium-based metal-organic framework aerogel monolith with ultralow-density and highly efficient Pb(II) removal. J. Colloid Interface Sci. 2020, 561, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Azmi, L.H.M.; Williams, D.R.; Ladewig, B.P. Polymer-Assisted Modification of Metal-Organic Framework MIL-96(Al): Influence on Particle Size, Crystal Morphology and Perfluorooctanoic Acid (PFOA) Removal. Chemosphere 2020, 262, 128072. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Savunthari, K.V.; Chandrasekar, N.; Balaji, R.; Selvarajan, E. Removal of environmental contaminants of emerging concern using metal-organic framework composite. Environ. Technol. Innov. 2022, 25, 102216. [Google Scholar] [CrossRef]
- Park, J.M.; Jhung, S.H. A remarkable adsorbent for removal of bisphenol S from water: Aminated metal-organic framework, MIL-101-NH2. Chem. Eng. J. 2020, 396, 125224. [Google Scholar] [CrossRef]
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr (VI) on nano UiO-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Baghelani, Y.M. Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework. Environ. Nanotechnol. Monit. Manag. 2017, 7, 89–96. [Google Scholar]
- Lv, Y.C.; Zhang, R.S.; Zeng, S.L.; Liu, K.Y.; Huang, S.Y.; Liu, Y.F.; Xu, P.F.; Lin, C.X.; Cheng, Y.J.; Liu, M.H. Removal of p-arsanilic acid by an amino-functionalized indium-based metal-organic framework: Adsorption behavior and synergetic mechanism. Chem. Eng. J. 2018, 339, 359–368. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Beygi, T.M. Anionic dyes removal from aqueous solution using TMU-16 and TMU-16-NH2 as isoreticular nanoporous metal organic frameworks. J. Taiwan Inst. Chem. Eng. 2016, 66, 164–171. [Google Scholar] [CrossRef]
- Haque, E.; Lo, V.; Minett, A.I.; Harris, A.T.; Church, T.L. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal-organic framework, amino-MIL-101(Al). J. Mater. Chem. A 2014, 2, 193. [Google Scholar] [CrossRef]
- Liu, H.C.; Chen, L.G.; Ding, J. Adsorption behavior of magnetic amino-functionalized metal-organic framework for cationic and anionic dyes from aqueous solution. RSC Adv. 2016, 6, 48884. [Google Scholar] [CrossRef]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef]
- Wu, G.G.; Ma, J.P.; Li, S.; Wang, S.S.; Jiang, B.; Luo, S.Y.; Li, J.H.; Wang, X.Y.; Guan, Y.F.; Chen, L.X. Cationic metal-organic frameworks as an efficient adsorbent for the removal of 2,4-dichlorophenoxyacetic acid from aqueous solutions. Environ. Res. 2020, 186, 109542. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, C.; Xia, Y. Efficient adsorption and removal of diclofenac sodium from water with quaternary ammonium functionalized metal-organic frameworks. Chin. J. Chromatogr. 2018, 36, 222–229. [Google Scholar]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ai, Y.J.; Alsaedi, A.; Hayat, T.; Wang, X.K. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801. [Google Scholar] [CrossRef]
- Singh, N.; Srivastava, I.; Dwivedi, J.; Sankararamakrishnan, L. Ultrafast removal of ppb levels of Hg(II) and volatile Hg(0) using post modified metal organic framework. Chemosphere 2020, 270, 129490. [Google Scholar] [CrossRef]
- Zhang, J.M.; Xiong, Z.H.; Li, C.; Wu, C.S. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution. J. Mol. Liq. 2016, 221, 43–50. [Google Scholar] [CrossRef]
- Moradi, S.E.; Dadfarnia, S.; Shabani, A.M.H.; Emami, S. Microwave-enhanced Fenton-like degradation by surface-modified metal-organic frameworks as a promising method for removal of dye from aqueous samples. Turk. J. Chem. 2017, 41, 426–439. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, G.Q.; Chen, H.H.; Hu, X.Y.; Niu, Z.; Ma, S.Q. Functionalized metal-organic framework as a new platform for efficient and selective removal of cadmium(II) from aqueous solution. J. Mater. Chem. A 2015, 3, 15292–15298. [Google Scholar] [CrossRef]
- Liu, J.M.; Cui, H.H.; Li, J.H.; Chen, M.C. A research on the cadmium ions adsorption of Sulfhydryl- and sulfo-functionalized UiO-66 with silica layer from water. J. Environ. Chem. Eng. 2021, 9, 104621. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Ma, H.; Wang, Z.H.; Zhao, N.; Wang, S. Fabrication of Fe3O4@UiO-66-SO3H core-shell functional adsorbents for highly selective and efficient removal of organic dyes. New J. Chem. 2019, 43, 7770. [Google Scholar] [CrossRef]
- Kang, C.F.; Peng, Y.G.; Tang, Y.Z.; Huang, H.L.; Zhong, C.L. Sulfate-Rich Metal-Organic Framework for High Efficiency and Selective Removal of Barium from Nuclear Wastewater. Ind. Eng. Chem. Res. 2017, 56, 13866–13873. [Google Scholar] [CrossRef]
- Peng, Y.G.; Huang, H.L.; Liu, D.H.; Zhong, C.L. Radioactive Barium Ion Trap Based on Metal-Organic Framework for Efficient and Irreversible Removal of Barium from Nuclear Wastewater. ACS Appl. Mater. Interfaces 2016, 8, 8527–8535. [Google Scholar] [CrossRef]
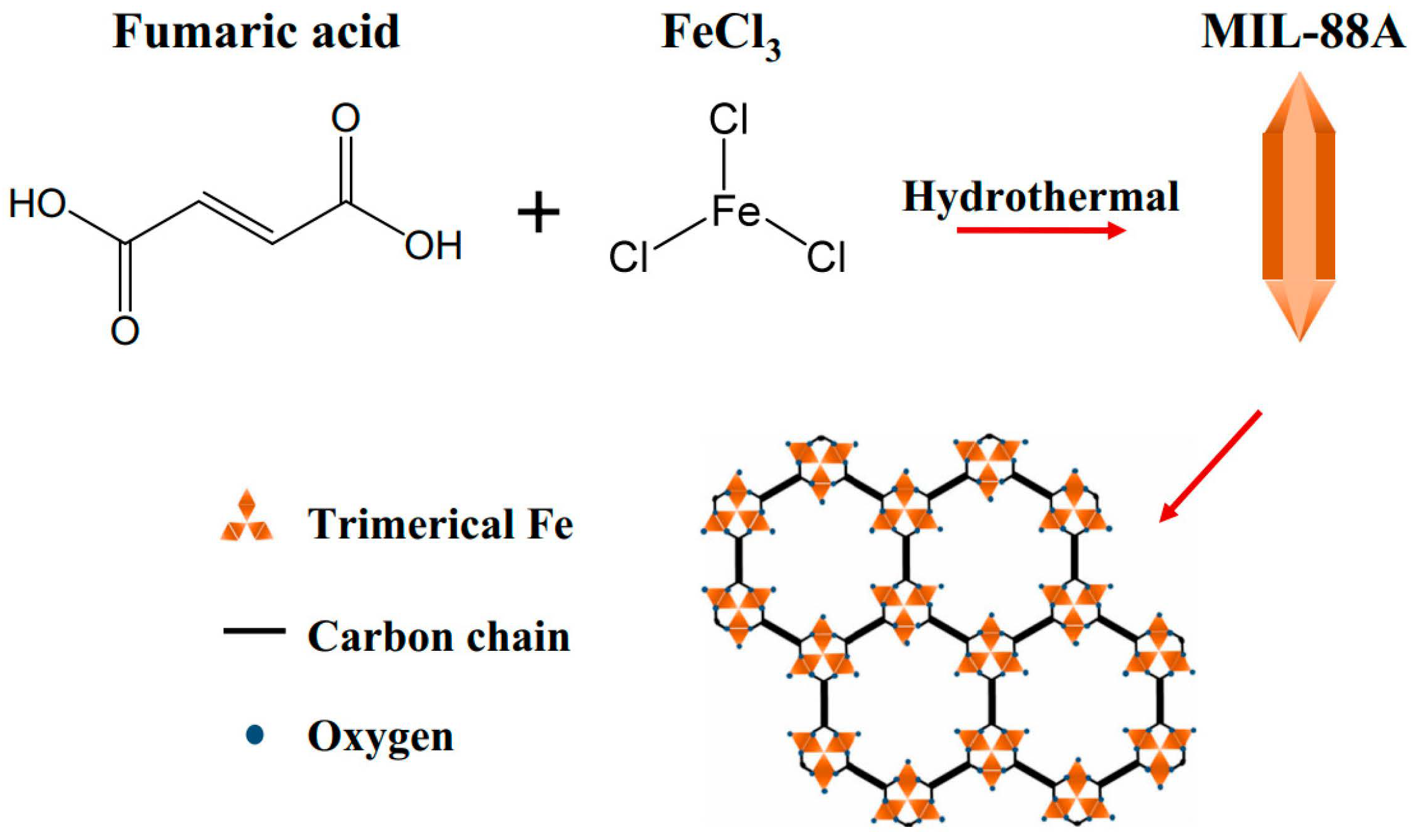
- Wu, H.; Ma, M.D.; Gai, W.Z.; Yang, H.X.; Zhou, J.G.; Cheng, Z.X.; Xu, P.G.; Deng, Z.Y. Arsenic removal from water by metal-organic framework MIL-88A microrods. Environ. Sci. Pollut. Res. 2018, 25, 27196–27202. [Google Scholar] [CrossRef]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D.B.; Yan, M. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial UiO-66-(OH)2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Zhu, G.C.; Lin, J.L.; Yuan, Q.; Wang, X.F.; Zhao, Z.L.; Hursthouse, A.S.; Wang, Z.H.; Li, Q.B. A biochar supported magnetic metal organic framework for the removal of trivalent antimony. Chemosphere 2021, 282, 131068. [Google Scholar] [CrossRef]
- Ren, L.; Zhao, X.D.; Liu, B.S.; Huang, H.L. Synergistic effect of carboxyl and sulfate groups for effective removal of radioactive strontium ion in a Zr-metal-organic framework. Water Sci. Technol. 2021, 83, 2001–2011. [Google Scholar] [CrossRef]
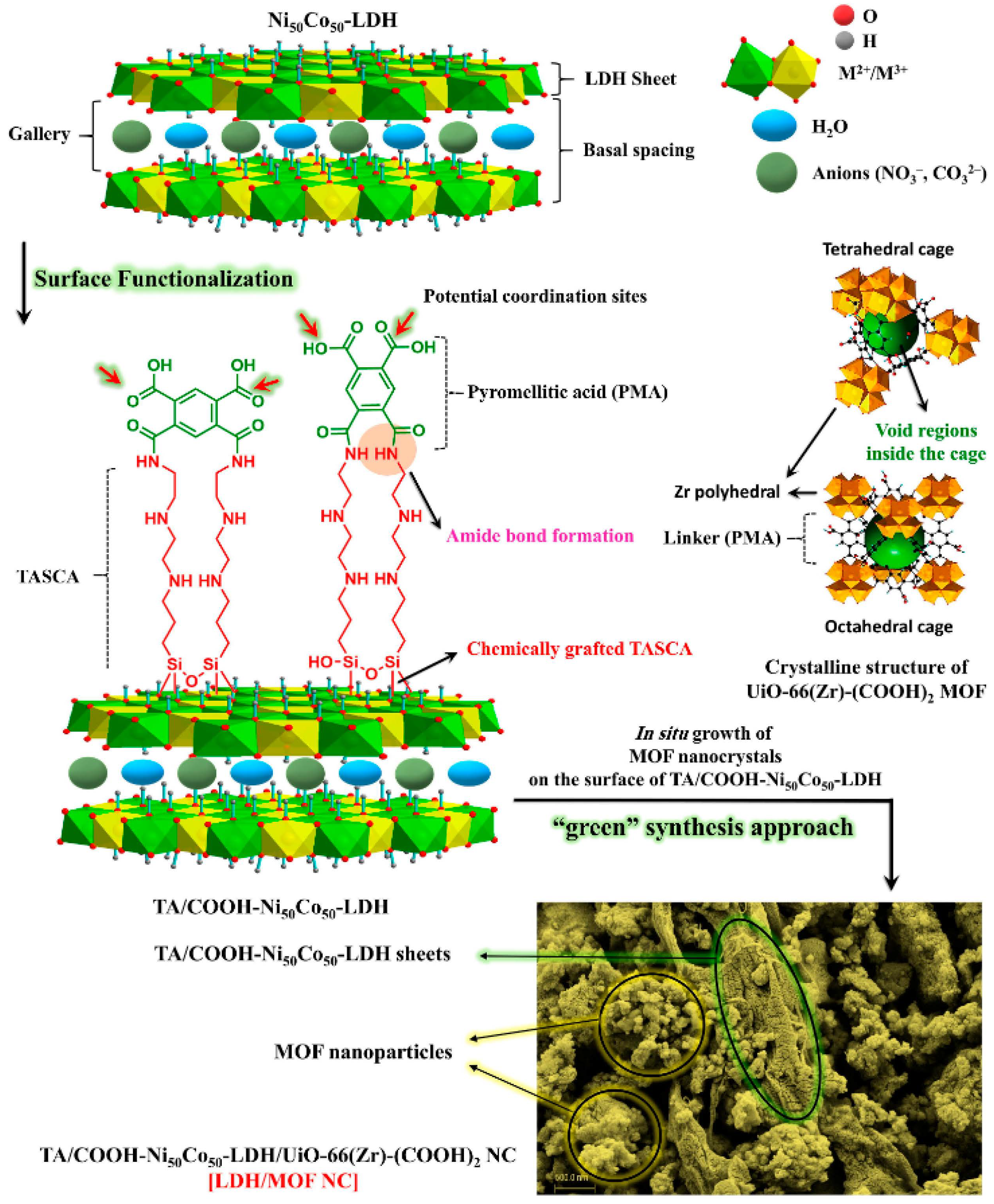
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Shirazian, S. A water-stable functionalized NiCo-LDH/MOF nanocomposite: Green synthesis, characterization, and its environmental application for heavy metals adsorption. Arab. J. Chem. 2021, 14, 103052. [Google Scholar] [CrossRef]
- Sini, K.; Bourgeois, D.; Idouhar, M.; Carboni, M.; Meyer, D. Metal-organic framework sorbents for the removal of perfluorinated compounds in an aqueous environment. New J. Chem. 2018, 42, 17889–17894. [Google Scholar] [CrossRef]
- Amador, R.N.; Cirre, L.; Carboni, M.; Meyer, D. BTEX removal from aqueous solution with hydrophobic Zr metal organic frameworks. J. Environ. Manag. 2018, 214, 17–22. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Mo, X.-J.; Zheng, S.-R.; Jin, X.; Gao, Y.; Cai, S.-L.; Fan, J.; Zhang, W.-G. Hydrolytically Stable Nanotubular Cationic Metal-Organic Framework for Rapid and Efficient Removal of Toxic Oxo-Anions and Dyes from Water. Inorg. Chem. 2019, 58, 2899–2909. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal-organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]


| MOF | Introduced Group | Contaminant | Adsorbing Capacity(mg·g−1) | Main Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|
| UiO-66 | -NH2 | Pb2+ | 102.03 | Lewis acid–base interaction | [83] |
| MIL-96 | -NH2 | PFOA | 340.00 | Electrostatic interaction | [84] |
| Cu-BTC | -NH2 | IBF ACE | 187.97 125.45 | Electrostatic interaction, Hydrophobic interaction | [85] |
| MIL-101 | -NH2 | BPS | 513.00 | Hydrogen-bond interaction | [86] |
| UiO-66 | -NH2 | Cr6+ | 32.36 | Electrostatic interaction | [87] |
| TMU-16 | -NH2 | Cd2+ | 126.60 | Lewis acid–base interaction | [88] |
| MIL-68(In) | -NH2 | p-ASA | 401.60 | π–π interaction, Hydrogen-bond interaction | [89] |
| TMU-16 | -NH2 | MO | 393.70 | Electrostatic interaction, Hydrogen-bond interaction | [90] |
| MIL-101(Al) | -NH2 | MB | 762.00 | Electrostatic interaction | [91] |
| MIL-101(Al) | -NH2 | MG IC | 274.40 135.00 | Electrostatic interaction, Hydrogen-bond interaction, π–π interaction, Hydrophobic interaction | [92] |
| MIL-125(Ti) | -NH2 | BR46 BB41 MB | 1296.00 1257.00 862.00 | Hydrogen-bond interaction | [93] |
| UiO-66 | -NMe3+ | 2,4-d | 279.00 | Electrostatic interaction, π–π interaction | [94] |
| MIL-101(Cr) | -NMe3+ | DCF | 310.60 | Electrostatic interaction, π–π interaction | [95] |
| Cu-MOF | -SH | Hg2+ | 714.29 | Lewis acid–base interaction | [96] |
| UiO-66 | -SH | Hg2+ | 784.30 | Lewis acid–base interaction | [97] |
| MIL-88A | -SH | Hg2+ | 1111.10 | Lewis acid–base interaction | [98] |
| MOF-5 | -SH | Pb2+ Cd2+ | 312.50 65.20 | Electrostatic interaction, Lewis acid–base interaction | [99] |
| MIL-100(Fe) | -SO3H | MO | 99.9% | Hydrogen-bond interaction, π–π interaction | [100] |
| UiO-66 | -SO3H | DCF | 263.00 | Electrostatic interaction, π–π interaction | [101] |
| Cu-BTC | -SO3H | Cd2+ | 88.70 | Lewis acid–base interaction, Electrostatic interaction | [102] |
| UiO-66 | -SO3H | Cd2+ | 409.96 | Lewis acid–base interaction, Electrostatic interaction | [103] |
| UiO-66 | -SO3H | MB | 297.30 | Lewis acid–base interaction, π–π interaction | [104] |
| Zr-BTC | -SO4 | Ba2+ | 181.80 | Lewis acid–base interaction | [105] |
| MOF-808 | -SO4 | Ba2+ | 131.10 | Lewis acid–base interaction | [106] |
| MIL-88A | -OH | As5+ | 145.00 | Lewis acid–base interaction | [107] |
| MIL-101 | -OH | NAP | 185.00 | Hydrogen-bond interaction | [108] |
| MIL-101 | -OH | PCMX KET NAP | 79.00 80.00 152.00 | Electrostatic interaction, Hydrogen-bond interaction | [109] |
| UiO-66 | -OH | MB TC | 96.69 37.96 | Lewis acid–base interaction, Hydrogen-bond interaction | [110] |
| UiO-66 | -COOH | Sb3+ | 56.49 | Electrostatic interaction | [111] |
| Zr-BTC | -COOH | Sr2+ | 67.50 | Electrostatic interaction | [112] |
| UiO-66-(Zr) | -COOH | Hg2+ Ni2+ | 509.80 439.00 | Electrostatic interaction | [113] |
| UiO-66 | -F | PFOA | 470.00 | Hydrophobic interaction | [114] |
| UiO-66 | -F | B | 76.30 | π–π interaction, Hydrophobic interaction | [115] |
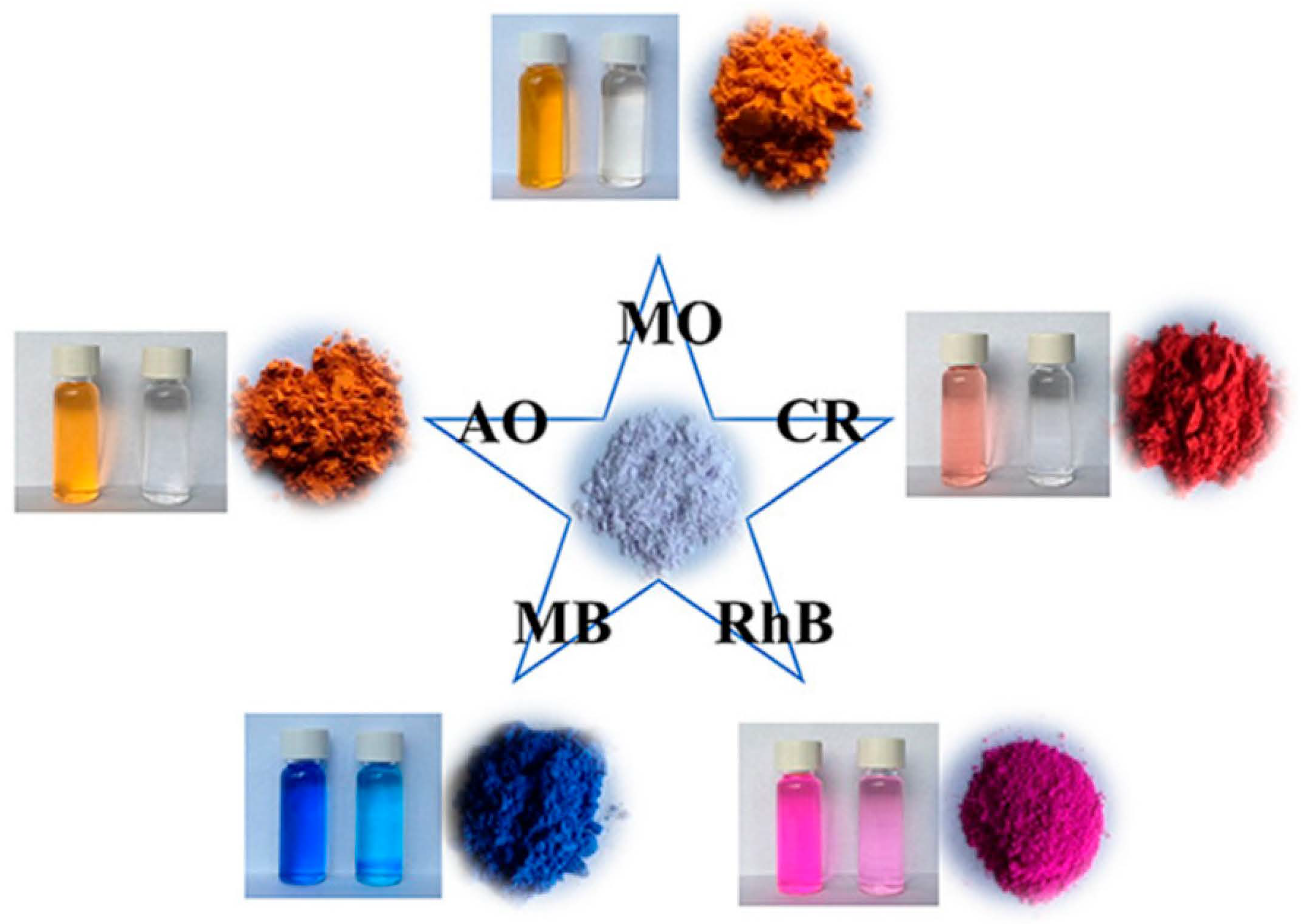
| SCNU-Z1 | -Cl | CrO42− Cr2O72− MnO4− ReO4− MO AO CR MB | 126.00 241.00 292.00 318.00 285.00 180.00 585.00 262.00 | Hydrophobic interaction | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, Y. Research on Improved MOF Materials Modified by Functional Groups for Purification of Water. Molecules 2023, 28, 2141. https://doi.org/10.3390/molecules28052141
Liu J, Wang Y. Research on Improved MOF Materials Modified by Functional Groups for Purification of Water. Molecules. 2023; 28(5):2141. https://doi.org/10.3390/molecules28052141
Chicago/Turabian StyleLiu, Junyan, and Yang Wang. 2023. "Research on Improved MOF Materials Modified by Functional Groups for Purification of Water" Molecules 28, no. 5: 2141. https://doi.org/10.3390/molecules28052141
APA StyleLiu, J., & Wang, Y. (2023). Research on Improved MOF Materials Modified by Functional Groups for Purification of Water. Molecules, 28(5), 2141. https://doi.org/10.3390/molecules28052141








