Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction
Abstract
1. Introduction
2. Results and Discussion
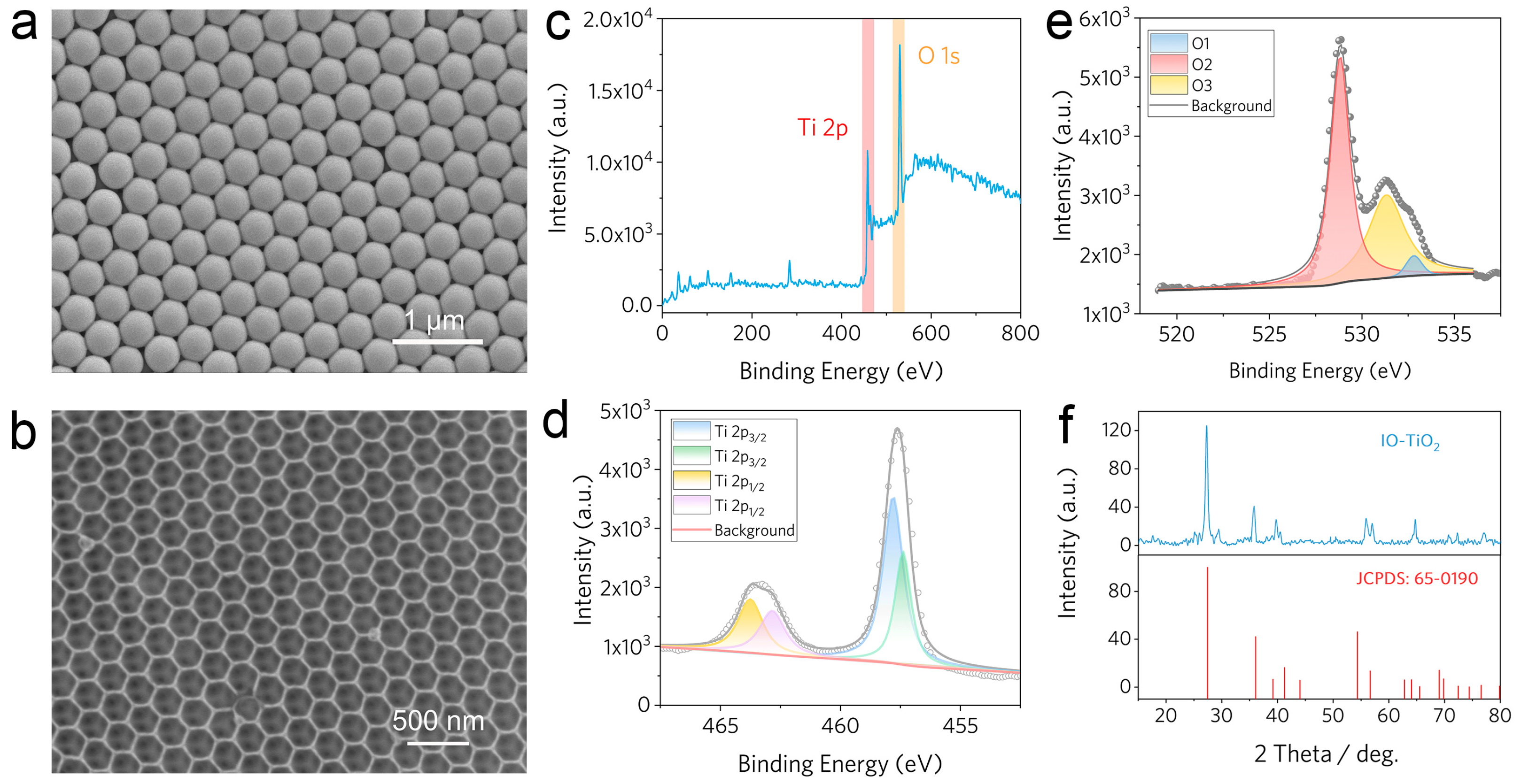
2.1. Characterizations of As-Prepared IO-TiO2
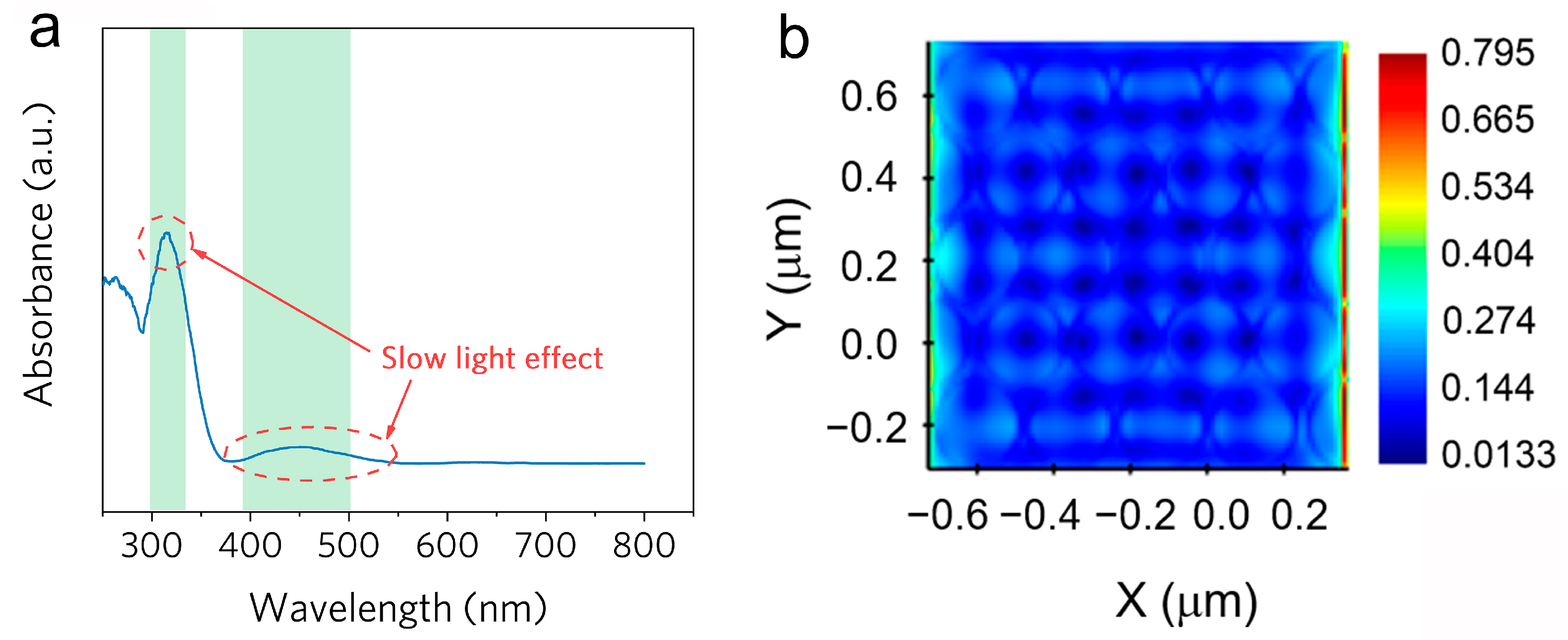
2.2. Proof of the Slow-Photon Effect
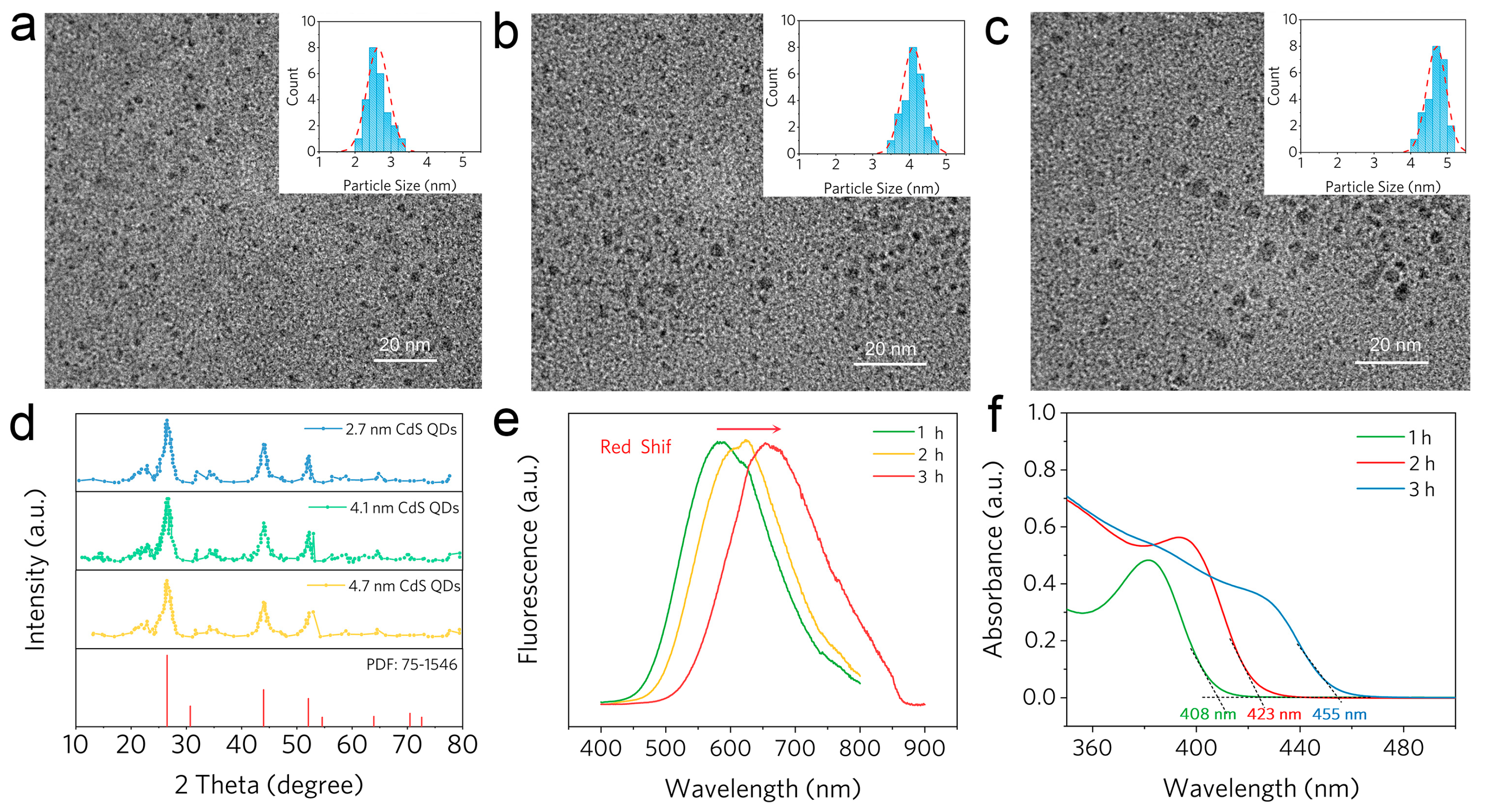
2.3. Characterizations of Various Size CdS QDs
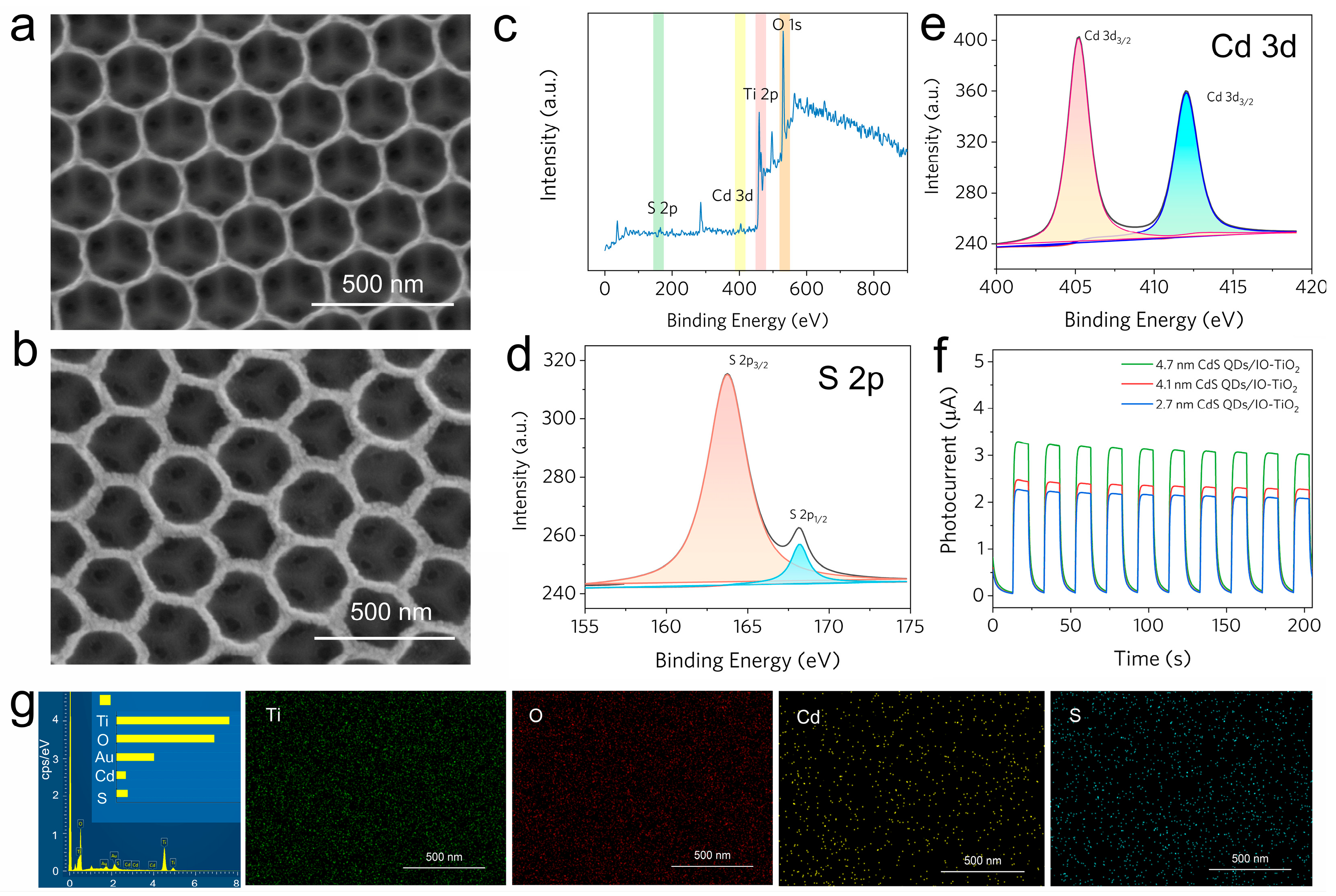
2.4. Characterizations of CdS QDs/IO-TiO2
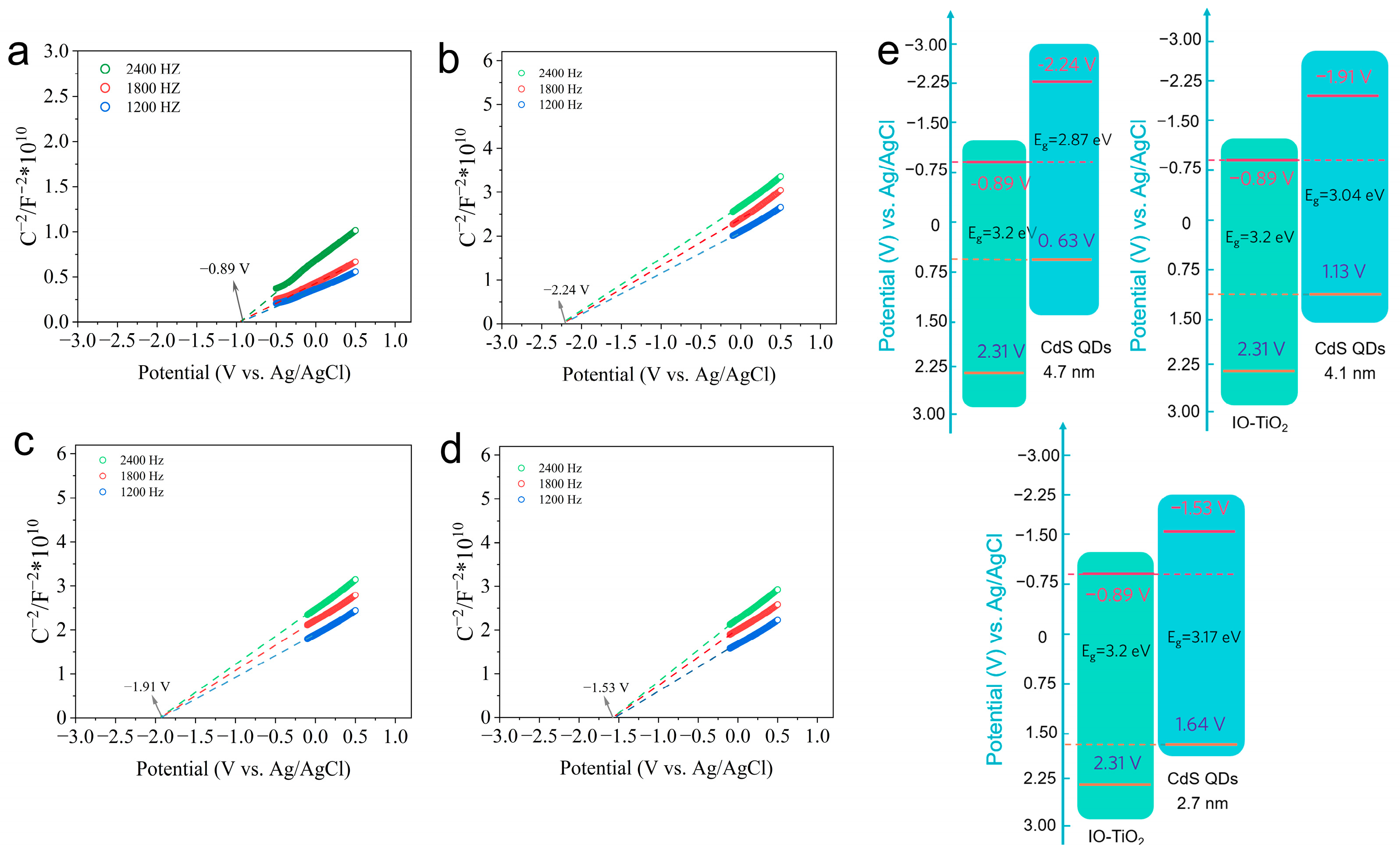
2.5. Band Structure of an Internal Electric Field
2.6. Photocatalytic Mechanism and Verification
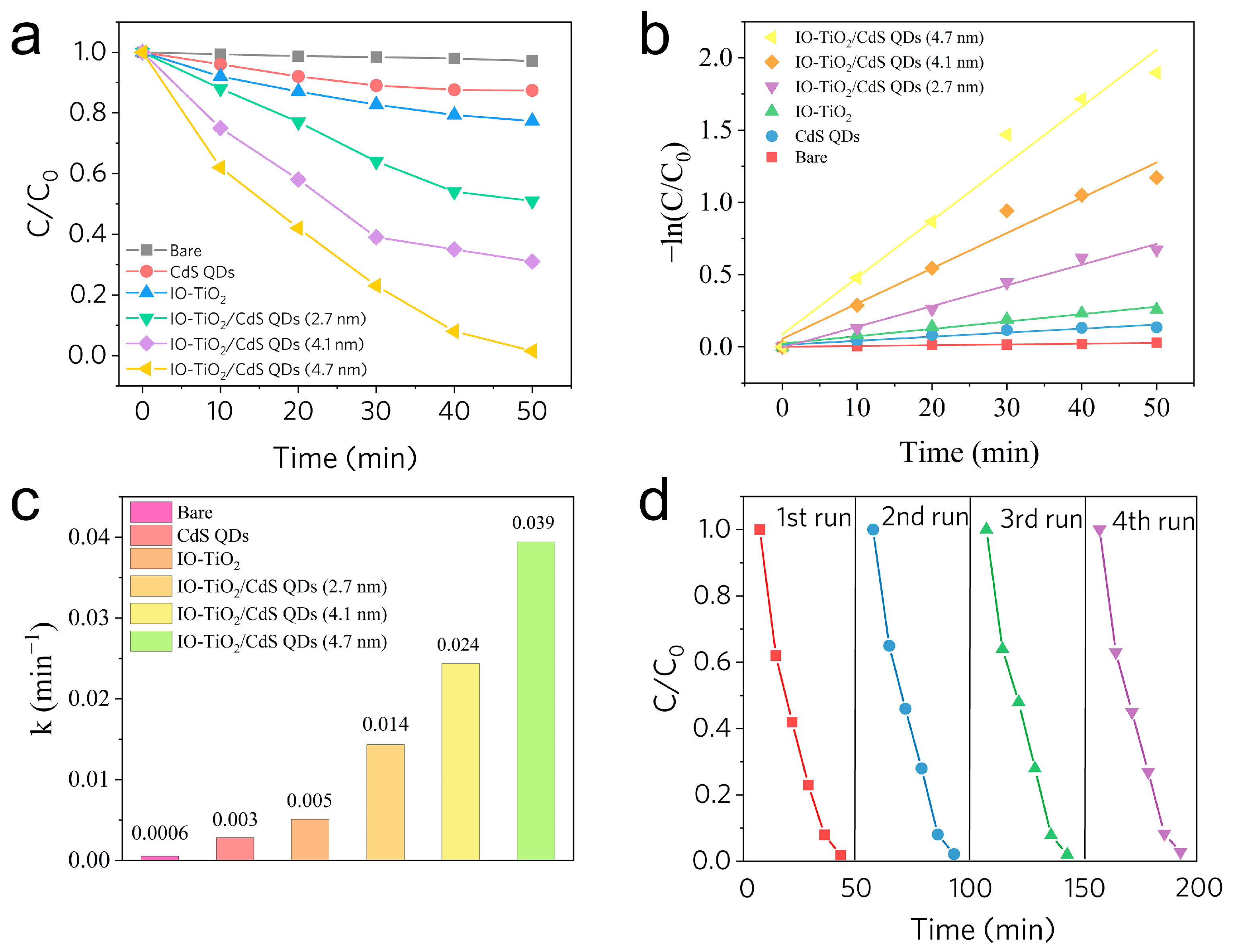
2.7. Photocatalytic Activity
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis of PS Microspheres
3.3. Assembly of Ordered PS Array Film Templates
3.4. Preparation of IO-TiO2 Structure
3.5. Synthesis of CdS QDs
3.6. Preparation of CdS QDs/IO-TiO2 Heterojunction
3.7. Measurement of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shi, W.; Liu, C.; Li, M.; Lin, X.; Guo, F.; Shi, J. Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J. Hazard. Mater. 2020, 389, 121907. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J.G. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Abdullah, C.A.C.; Chung, E.L.T.; Andou, Y. Recent progress in visible light-doped ZnO photocatalyst for pollution control. Int. J. Environ. Sci. Technol. 2023, 20, 5753–5772. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Ohno, T.; Andou, Y. Recent Progress in Photocatalytic Efficiency of Hybrid Three-Dimensional (3D) Graphene Architectures for Pollution Remediation. Top. Catal. 2022, 65, 1634–1647. [Google Scholar] [CrossRef]
- Zeng, D.; Yu, C.; Fan, Q.; Zeng, J.; Wei, L.; Li, Z.; Yang, K.; Ji, H. Theoretical and experimental research of novel fluorine doped hierarchical Sn3O4 microspheres with excellent photocatalytic performance for removal of Cr(VI) and organic pollutants. Chem. Eng. J. 2020, 391, 123607. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yang, K.; Yu, C.; Mu, P.; Yu, Z.; Lu, K.; Huang, W.; Dai, W. Photocatalytic H2O2 production and removal of Cr (VI) via a novel Lu3NbO7: Yb, Ho/CQDs/AgInS2/In2S3 heterostructure with broad spectral response. J. Hazard. Mater. 2022, 423, 127172. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Yu, C.; Fan, Q.; Liu, X. Solvothermal fabrication of Bi2MoO6 nanocrystals with tunable oxygen vacancies and excellent photocatalytic oxidation performance in quinoline production and antibiotics degradation. Chin. J. Catal. 2022, 43, 472–484. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J.G. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B 2021, 289, 120039. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, Z.; Jiang, Y.; Chu, Y.; Xu, J. Embedding CsPbBr3 perovskite quantum dots into mesoporous TiO2 beads as an S-scheme heterojunction for CO2 photoreduction. Chem. Eng. J. 2022, 433, 133762. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Sabidi, S.; Ohno, T.; Maeda, T.; Andou, Y. Cu2O/TiO2 decorated on cellulose nanofiber/reduced graphene hydrogel for enhanced photocatalytic activity and its antibacterial applications. Chemosphere 2022, 286, 131731. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J.G. TiO2–MnOx–Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2-Reduction Activity. ACS Appl. Mater. Inter. 2019, 11, 5581–5589. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J.G. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Vishnuraj, R.; Chen, S.-M.; Pullithadathil, B.; Ahmed, F.; Hasan, P.M.Z.; Bilgrami, A.L.; Kumar, S. Tailored construction of one-dimensional TiO2/Au nanofibers: Validation of an analytical assay for detection of diphenylamine in food samples. Food Chem. 2022, 380, 132052. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, M.A.; Cadús, L.E.; Morales, M.R. Tailoring materials by high-energy ball milling: TiO2 mixtures for catalyst support application. Mater. Today Chem. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Li, W.; Wang, Y.; Liao, W.; Zou, H.; Li, J.; Huang, X. Enhanced photocatalytic hydrogen evolution under visible-light using C, N co-doped mesoporous TiO2 nanocrystals templated by ionic liquids. Chem. Eng. J. 2023, 451, 138670. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, R.; Li, H.; Xu, Z.; Dai, H.; Gao, H.; Yu, H.; Wang, Z.; Wang, Y.; Liu, Y.; et al. Boosting visible light photocatalysis in an Au@TiO2 yolk-in-shell nanohybrid. Appl. Catal. B 2022, 303, 120869. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, Z.; Li, Z.; Wang, Y.; Yu, F.; Cheng, Z.; Dai, Y.; Cao, X.; Wang, Y.; Liu, Y.; et al. Double-shelled hollow nanosphere assembled by TiO2@surface sulfate functionalized CdS for boosting photocatalysis reduction of U(VI) under seawater conditions. Chem. Eng. J. 2022, 431, 133256. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, E.; Broisson, P.; Aravindakshan, N.; Wu, Z.; Cole, I.S.; Li, X.; Zhao, D.; Li, Q. Sandwich-structured TiO2 inverse opal circulates slow photons for tremendous improvement in solar energy conversion efficiency. J. Mater. Chem. A 2017, 5, 12803–12810. [Google Scholar] [CrossRef]
- Beydoun, N.; Farhat, R.; Halaoui, L.I. Enhanced Solar Light Harvesting with Q-CdTe/Se Sensitized Inverse Opal TiO2. ACS Appl. Energy Mater. 2020, 3, 3104–3119. [Google Scholar] [CrossRef]
- Maho, A.; Lobet, M.; Daem, N.; Piron, P.; Spronck, G.; Loicq, J.; Cloots, R.; Colson, P.; Henrist, C.; Dewalque, J. Photonic Structuration of Hybrid Inverse-Opal TiO2—Perovskite Layers for Enhanced Light Absorption in Solar Cells. ACS Appl. Energy Mater. 2021, 4, 1108–1119. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, W.; Tan, J.; Lee, H.; Park, J.; Moon, J. Boosting Visible Light Harvesting in p-Type Ternary Oxides for Solar-to-Hydrogen Conversion Using Inverse Opal Structure. Adv. Funct. Mater. 2019, 29, 1900194. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.-L. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B 2016, 184, 182–190. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, L.; Zhu, Q.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. Hierarchical macro-mesoporous g-C3N4 with an inverse opal structure and vacancies for high-efficiency solar energy conversion and environmental remediation. Nanoscale 2019, 11, 20638–20647. [Google Scholar] [CrossRef]
- Zalfani, M.; Van der Schueren, B.; Hu, Z.-Y.; Rooke, J.C.; Bourguiga, R.; Wu, M.; Li, Y.; Van Tendeloo, G.; Su, B.-L. Novel 3DOM BiVO4/TiO2 nanocomposites for highly enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 21244–21256. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, K.; Zhang, C.; Yang, Z.; Feng, Y.; Hao, H.; Jiang, Y.; Lou, L.-L.; Zhou, W.; Liu, S. Ternary CdS/Au/3DOM-SrTiO3 composites with synergistic enhancement for hydrogen production from visible-light photocatalytic water splitting. Appl. Catal. B 2017, 215, 74–84. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.; Liu, J.; Li, Y.; Wu, M.; Van Tendeloo, G.; Su, B.-L. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, R.; Yin, L. Slow-Photon-Effect-Induced Photoelectrical-Conversion Efficiency Enhancement for Carbon-Quantum-Dot-Sensitized Inorganic CsPbBr3 Inverse Opal Perovskite Solar Cells. Adv. Mater. 2017, 29, 1703682. [Google Scholar] [CrossRef]
- Temerov, F.; Pham, K.; Juuti, P.; Mäkelä, J.M.; Grachova, E.V.; Kumar, S.; Eslava, S.; Saarinen, J.J. Silver-Decorated TiO2 Inverse Opal Structure for Visible Light-Induced Photocatalytic Degradation of Organic Pollutants and Hydrogen Evolution. ACS Appl. Mater. Inter. 2020, 12, 41200–41210. [Google Scholar] [CrossRef]
- Wen, X.-J.; Shen, C.-H.; Fei, Z.-H.; Fang, D.; Liu, Z.-T.; Dai, J.-T.; Niu, C.-G. Recent developments on AgI based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. g-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Structures 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Pan, C.; Mao, Z.; Yuan, X.; Zhang, H.; Mei, L.; Ji, X. Heterojunction Nanomedicine. Adv. Sci. 2022, 29, 1605349. [Google Scholar] [CrossRef]
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Zhang, L.; Yang, C.; Wang, J. Recent advances on heterojunction-based photocatalysts for the degradation of persistent organic pollutants. Chem. Eng. J. 2021, 426, 130617. [Google Scholar] [CrossRef]
- Su, J.; Li, G.-D.; Li, X.-H.; Chen, J.-S. 2D/2D Heterojunctions for Catalysis. Adv. Sci. 2019, 6, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mohamed, A.R.; Ong, W.-J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem. Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999. [Google Scholar] [CrossRef]
- Min, Y.; Im, E.; Hwang, G.-T.; Kim, J.-W.; Ahn, C.-W.; Choi, J.-J.; Hahn, B.-D.; Choi, J.-H.; Yoon, W.-H.; Park, D.-S.; et al. Heterostructures in two-dimensional colloidal metal chalcogenides: Synthetic fundamentals and applications. Nano Res. 2019, 12, 1750–1769. [Google Scholar] [CrossRef]
- Sokol, K.P.; Robinson, W.E.; Warnan, J.; Kornienko, N.; Nowaczyk, M.M.; Ruff, A.; Zhang, J.Z.; Reisner, E. Bias-free photoelectrochemical water splitting with photosystem II on a dye-sensitized photoanode wired to hydrogenase. Nat. Energy 2018, 3, 944–951. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhu, Q.; Qin, W.; Han, G.; Shen, J.-R.; Zong, X.; Li, C. Spatially Separated Photosystem II and a Silicon Photoelectrochemical Cell for Overall Water Splitting: A Natural–Artificial Photosynthetic Hybrid. Angew. Chem. Int. Ed. 2016, 55, 9229–9233. [Google Scholar] [CrossRef]
- Nam, D.H.; Zhang, J.Z.; Andrei, V.; Kornienko, N.; Heidary, N.; Wagner, A.; Nakanishi, K.; Sokol, K.P.; Slater, B.; Zebger, I.; et al. Solar Water Splitting with a Hydrogenase Integrated in Photoelectrochemical Tandem Cells. Angew. Chem. Int. Ed. 2018, 57, 10595–10599. [Google Scholar] [CrossRef]
- Ng, B.-J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.-P. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yang, H.; Meng, A.; Li, Z.; Yang, L.; Wang, L.; Li, S.; Li, G.; Huang, J. Interfacial engineering improved internal electric field contributing to direct Z-scheme-dominated mechanism over CdSe/SL-ZnIn2S4/MoSe2 heterojunction for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 431, 134000. [Google Scholar] [CrossRef]
- Xu, X.; Deng, F.; Shao, P.; Dionysiou, D.D.; Luo, X.; Li, X.; Zhang, S.; Liu, X.; Liu, M. Internal electric field driving separation and migration of charge carriers via Z-scheme path in AgIn5S8/ZnO heterojunction for efficient decontamination of pharmaceutical pollutants. Chem. Eng. J. 2022, 428, 132096. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, L.; Li, Z.; Wu, W.; Bian, J.; Jing, L. Interface Modulation of FePc/Porous Ti(HPO4)2 Z-Scheme Heterojunctions with Ultrafine Ag for Efficiently Photocatalytic CO Oxidation. Small Structure 2022, 3, 2200011. [Google Scholar] [CrossRef]
- Wu, X.; Xie, S.; Liu, C.; Zhou, C.; Lin, J.; Kang, J.; Zhang, Q.; Wang, Z.; Wang, Y. Ligand-Controlled Photocatalysis of CdS Quantum Dots for Lignin Valorization under Visible Light. ACS Catal. 2019, 9, 8443–8451. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Q.; Huang, S.; Zheng, J.; Guan, X.; Patterson, R.; Kim, J.; Shi, L.; Lin, C.-H.; Lei, Q.; et al. Flexible and efficient perovskite quantum dot solar cells via hybrid interfacial architecture. Nat. Commun. 2021, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.D.; Bettis Homan, S.; Kodaimati, M.; He, C.; Nepomnyashchii, A.B.; Swenson, N.K.; Lian, S.; Calzada, R.; Weiss, E.A. Electronic Processes within Quantum Dot-Molecule Complexes. Chem. Rev. 2016, 116, 12865–12919. [Google Scholar] [CrossRef]
- Xu, L.; Aumaitre, C.; Kervella, Y.; Lapertot, G.; Rodríguez-Seco, C.; Palomares, E.; Demadrille, R.; Reiss, P. Increasing the Efficiency of Organic Dye-Sensitized Solar Cells over 10.3% Using Locally Ordered Inverse Opal Nanostructures in the Photoelectrode. Adv. Funct. Mater. 2018, 28, 1706291. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef]
- Lv, C.; Lan, X.; Wang, L.; Dai, X.; Zhang, M.; Cui, J.; Yuan, S.; Wang, S.; Shi, J. Rapidly and highly efficient degradation of tetracycline hydrochloride in wastewater by 3D IO-TiO2-CdS nanocomposite under visible light. Environ. Technol. 2021, 42, 377–387. [Google Scholar] [CrossRef]
- Veerathangam, K.; Pandian, M.S.; Ramasamy, P. Size-dependent photovoltaic performance of cadmium sulfide (CdS) quantum dots for solar cell applications. J. Alloys Compd. 2018, 735, 202–208. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.-N.; Li, J.-J.; Zhao, J.-W. The effect of core size on the fluorescence emission properties of CdTe@CdS core@shell quantum dots. J. Lumin. 2018, 199, 216–224. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, X.; Xi, J.; Ji, Z. The promising photoanode of Pt coupled TiO2 NFs/CdS QDs with enhanced photoelectrochemical performance. J. Alloys Compd. 2019, 790, 900–908. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, S.; Chang, X.; Hu, J.; Wang, X. Effect of the combination of inverse opal photon superficial areaic crystal and heterojunction on active free radicals and its effect on the mechanism of photocatalytic removal of organic pollutants. Ceram. Int. 2023, 49, 27107–27116. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Jung, N.; Yu, Y.-J.; Oh, J.-E.; Kim, M.-D. Plasmonic Pt nanoparticles triggered efficient charge separation in TiO2/GaN NRs hybrid heterojunction for the high performance self-powered UV photodetectors. Appl. Surf. Sci. 2022, 594, 153474. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.-F.; Hu, Z.-Y.; Liu, J.; Li, Y.; Hu, J.; Van Tendeloo, G.; Chen, L.-H.; Su, B.-L. Size effect of bifunctional gold in hierarchical titanium oxide-gold-cadmium sulfide with slow photon effect for unprecedented visible-light hydrogen production. J. Colloid Interface Sci. 2021, 604, 131–140. [Google Scholar] [CrossRef]
- Aboulaich, A.; Billaud, D.; Abyan, M.; Balan, L.; Gaumet, J.-J.; Medjadhi, G.; Ghanbaja, J.; Schneider, R. One-Pot Noninjection Route to CdS Quantum Dots via Hydrothermal Synthesis. ACS Appl. Mater. Inter. 2012, 4, 2561–2569. [Google Scholar] [CrossRef] [PubMed]


| Heating Duration (h) | 1 | 2 | 3 |
|---|---|---|---|
| λcut-off (nm) | 408 | 423 | 452 |
| Eg (eV) | 3.17 | 3.04 | 2.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.-B.; Bao, N.; Zhang, Q.; Ding, S.-N. Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction. Molecules 2023, 28, 5437. https://doi.org/10.3390/molecules28145437
Zhu L-B, Bao N, Zhang Q, Ding S-N. Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction. Molecules. 2023; 28(14):5437. https://doi.org/10.3390/molecules28145437
Chicago/Turabian StyleZhu, Li-Bang, Ning Bao, Qing Zhang, and Shou-Nian Ding. 2023. "Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction" Molecules 28, no. 14: 5437. https://doi.org/10.3390/molecules28145437
APA StyleZhu, L.-B., Bao, N., Zhang, Q., & Ding, S.-N. (2023). Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction. Molecules, 28(14), 5437. https://doi.org/10.3390/molecules28145437








