A Study of the Correlation between the Bulkiness of peri-Substituents and the Distortion of a Naphthalene Ring
Abstract
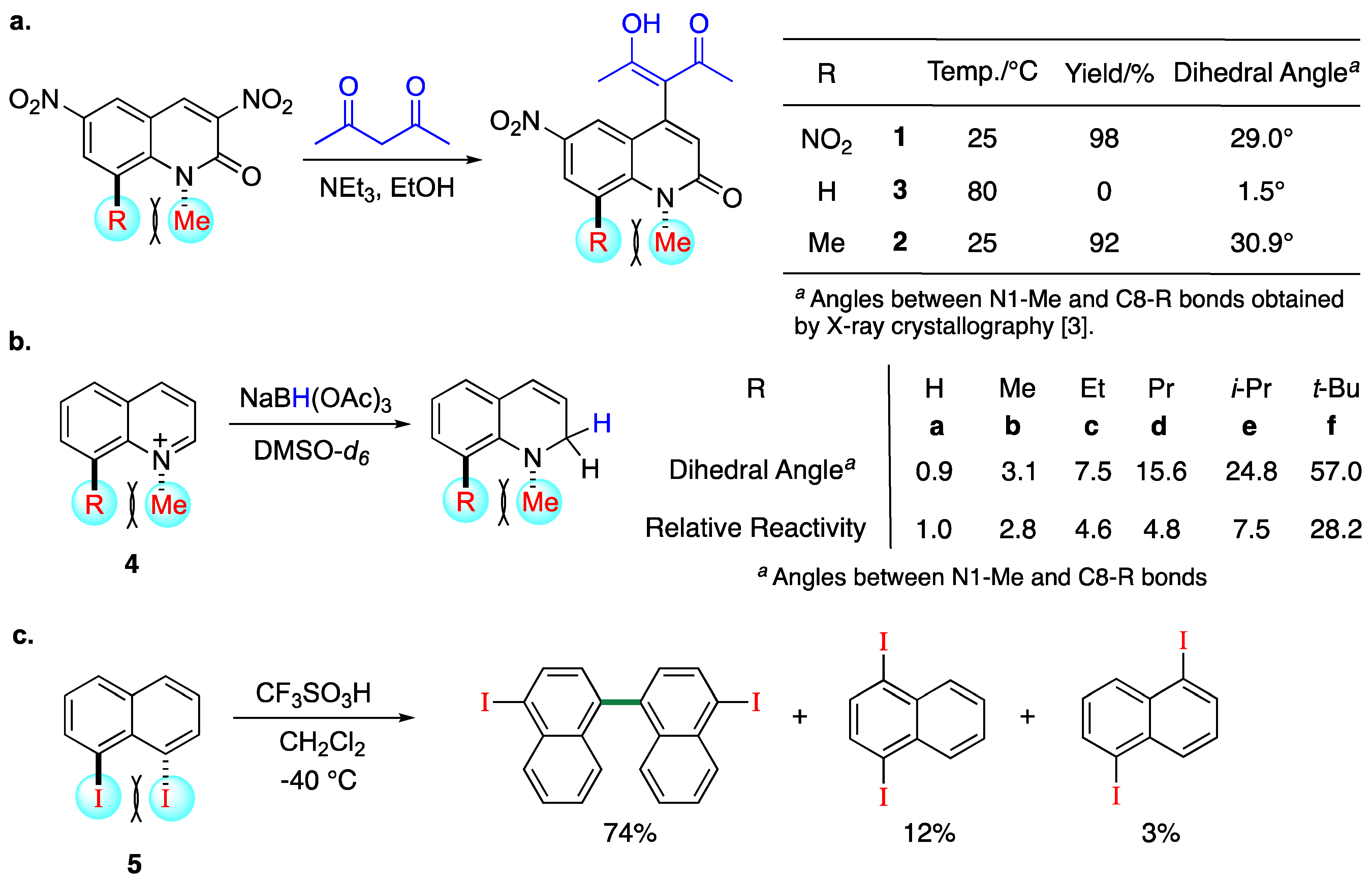
1. Introduction
2. Results and Discussion
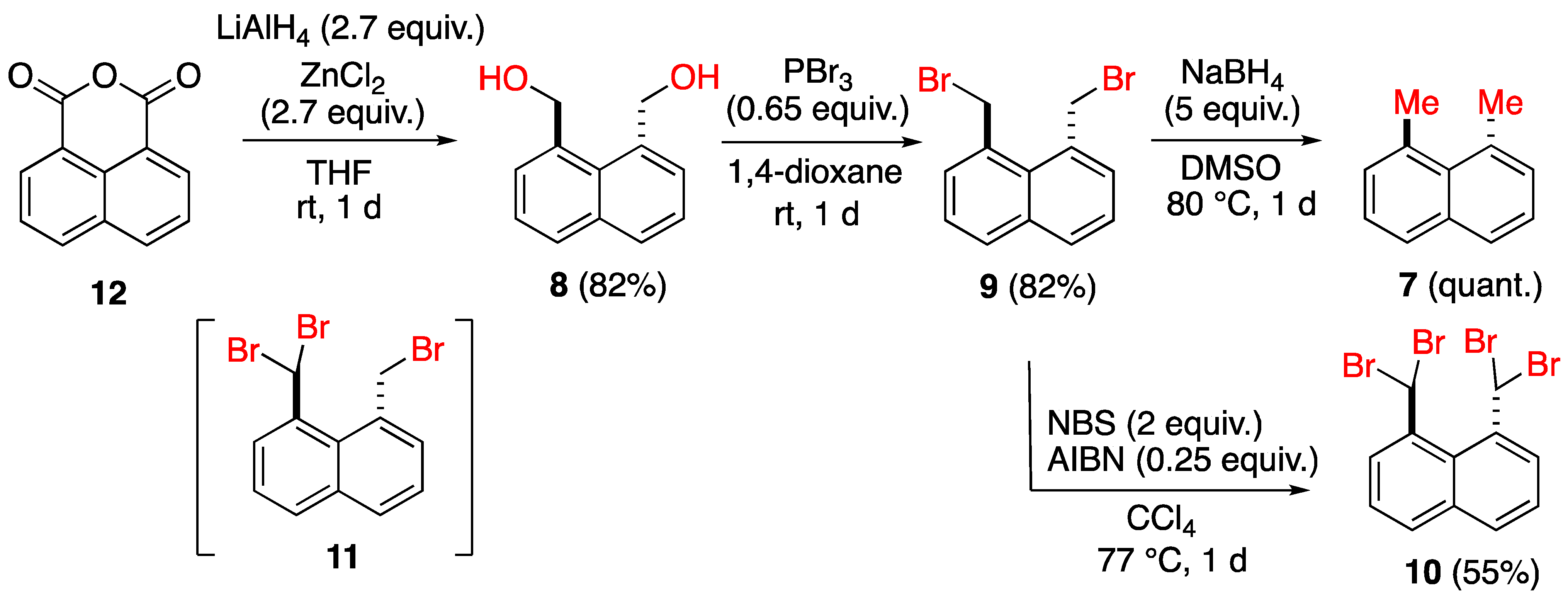
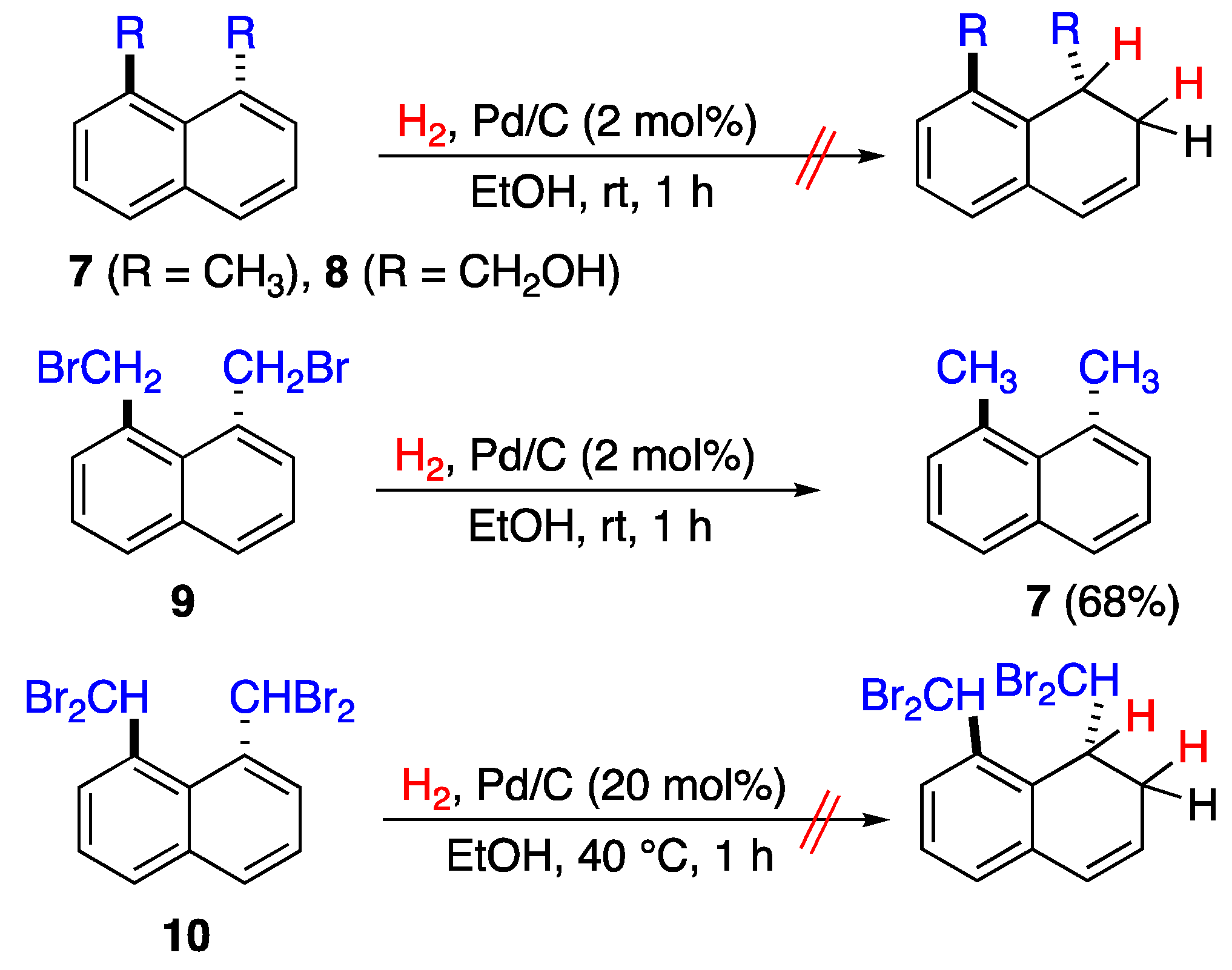
2.1. Synthesis of 1,8-Disubstituted Naphthalenes
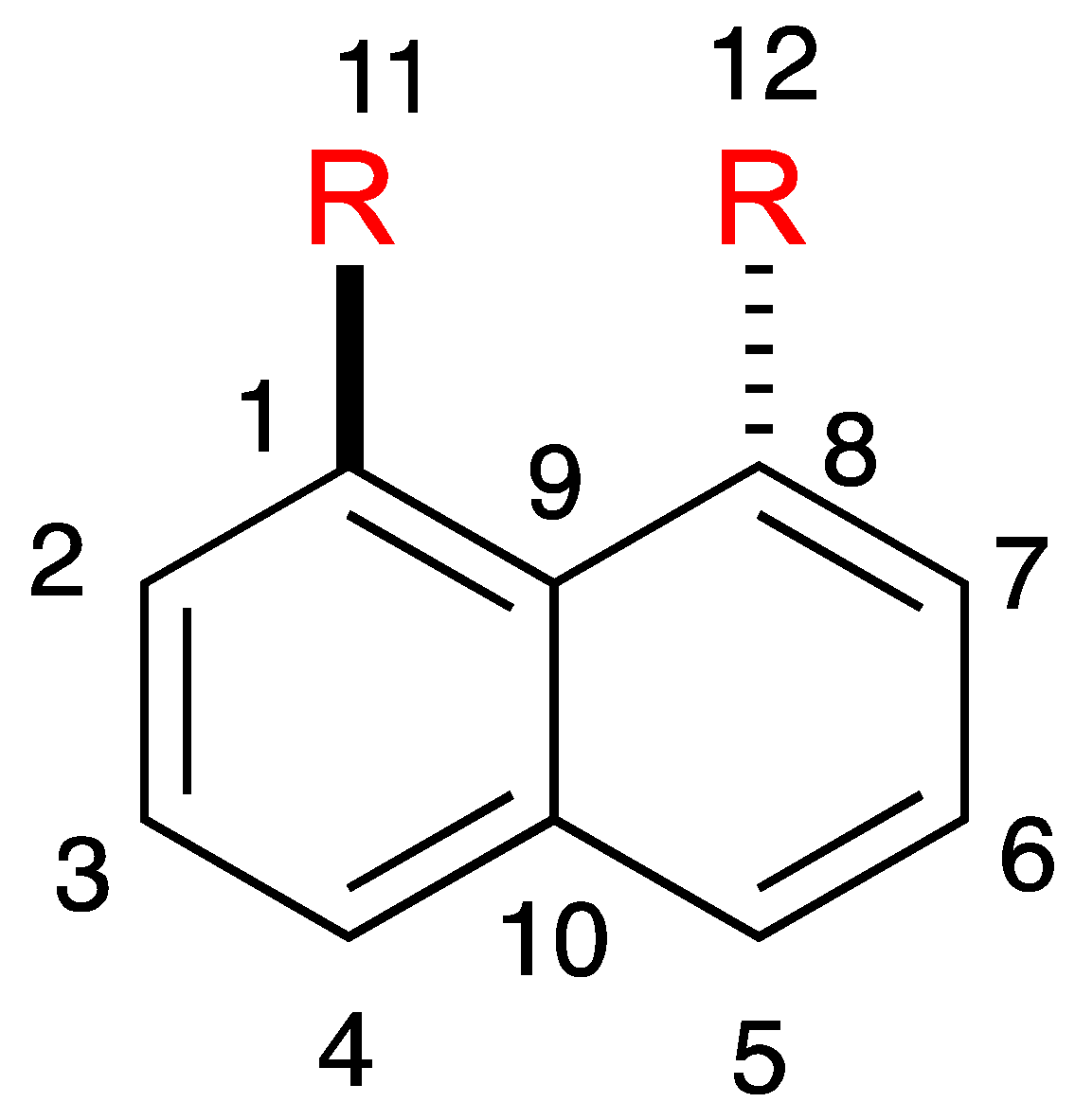
2.2. Crystal Structure Study
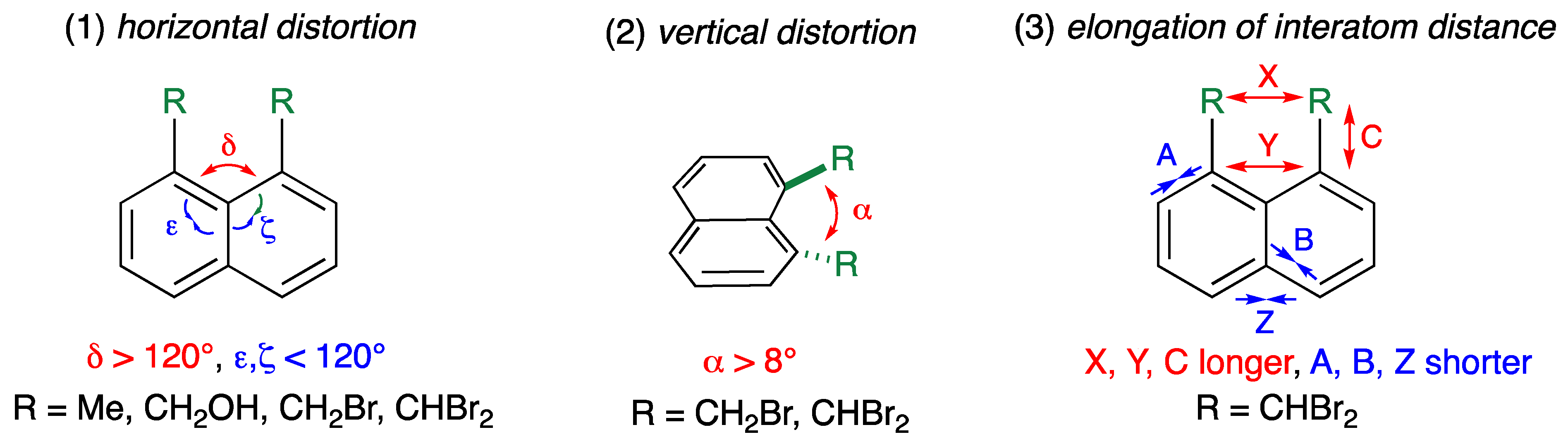
2.2.1. Vertical Distortion
2.2.2. Horizontal Distortion
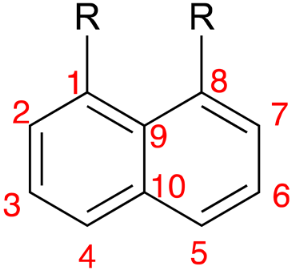
2.2.3. Bond Length and Interatom Distance
2.2.4. Mechanism of Distortion
2.3. Evaluation of the Reactivity
2.3.1. Nitration Reaction
2.3.2. Hydrogenation Reaction
2.3.3. Another Effect of a Bromo Group
2.3.4. NIC S Calculations
3. Materials and Methods
- Preparation of 1,8-dimethylnaphthalene (7) [18]
- Preparation of 1,8-bis(hydroxymethyl)naphthalene (8) [18]
- Preparation of 1,8-bis(bromomethyl)naphthalene (9) [18]
- Preparation of 1,8-bis(dibromomethyl)naphthalene (10)
- Nitration reaction for peri-substituted naphthalenes
- Hydrogenation reaction for peri-substituted naphthalenes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, X.; Kobiro, K.; Asahara, H.; Kakiuchi, K.; Sugimoto, R.; Saigo, K.; Nishiwaki, N. Reactive 2-quinolones dearomatized by steric repulsion between 1-methyl and 8-substituted groups. Tetrahedron 2013, 69, 4624–4630. [Google Scholar] [CrossRef]
- Fan, W.; Winands, T.; Doltsinis, L.N.; Li, Y.; Wang, Z. A decatwistacene with an overall 170° torsion. Angew. Chem. Int. Ed. 2017, 56, 15373–15377. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hiroto, S.; Lee, S.; Son, M.; Hisaki, I.; Yoshida, T.; Kim, D.; Kobayashi, N.; Shinokubo, H. Synthesis of highly twisted and fully π-conjugated porphyrinic oligomers. J. Am. Chem. Soc. 2015, 137, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, E.; Qu, R.; Yamago, S. Bromination of cycloparaphenylenes: Strain-induced site-selective bis-addition and its application for kate-stage functionalization. Angew. Chem. Int. Ed. 2017, 56, 10428–10432. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Yokoyama, S.; Asahara, H.; Nishiwaki, N. Non-electronic aromatic ring activation by simple steric repulsion between substituents in 1-methylquinolinium salt systems. Bull. Chem. Soc. Jpn. 2020, 93, 50–57. [Google Scholar] [CrossRef]
- Iwai, K.; Nishiguchi, N.; Nishiwaki, N. Halo-Jacobsen rearrangement induced by steric repulsion between peri-iodo groups. J. Org. Chem. 2023, 88. in press. [Google Scholar] [CrossRef]
- Schweizer, W.B.; Procter, G.; Kaftory, M.; Dunitz, J.D. Structural studies of 1,8-disubstituted naphthalenes as probes for nucleophile-electrophile interactions. Helv. Chim. Acta 1978, 61, 2783–2808. [Google Scholar] [CrossRef]
- Schumann, H.; Dechert, S.; Hummert, M.; Lange, K.C.H.; Schutte, S.; Wassermann, B.C.; Köhler, K.; Eichhorn, J. Intramolecularly nitrogen-stabilized organoaluminum compounds containing naphthyl, benzyl, and phenyl ligands. Z. Anorg. Allg. Chem. 2004, 630, 1196–1204. [Google Scholar] [CrossRef]
- Pla, D.; Sadek, O.; Cadet, S.; Mestre-Voegtlé, B.; Gras, E. Naphthylaminoborane: From structural switches to frustrated Lewis pair reactivity. Dalton Trans. 2015, 44, 18340–18346. [Google Scholar] [CrossRef]
- Pascal, R.A. Twisted acenes. Chem. Rev. 2006, 106, 4809–4819. [Google Scholar] [CrossRef]
- Balasubramaniyan, V. Peri-Interactions in naphthalene derivatives. Chem. Rev. 1966, 66, 567–641. [Google Scholar] [CrossRef]
- Bristow, J.C.; Naftalin, I.; Cliff, S.V.A.; Yang, S.; Carraveta, M.; Heinmaa, I.; Stern, R.; Wallis, J.D. Modelling of an aza-Michael reaction from crystalline naphthalene derivatives containing peri-peri interactions: Very long N-C bonds? Cryst. Eng. Commun. 2020, 22, 6783–6795. [Google Scholar] [CrossRef]
- Hoefelmeyer, J.D.; Schulte, M.; Tschinkl, M.; Gabbaï, F.P. Naphthalene derivatives peri-substituted by group 13 elements. Coord. Chem. Rev. 2002, 235, 93–103. [Google Scholar] [CrossRef]
- Kilian, P.; Knight, F.R.; Woollins, J.D. Naphthalene and related systems peri-substituted by group 15 and 16 elements. Chem. Eur. J. 2011, 17, 2302–2328. [Google Scholar] [CrossRef]
- Yang, J.; Horst, M.; Werby, S.H.; Cegelski, L.; Burns, N.Z.; Xia, Y. Bicyclohexene-peri-naphthalenes: Scalable synthesis, diverse functionalization, efficient polymerization, and facile mechanoactivation of their polymers. J. Am. Chem. Soc. 2020, 142, 14619–14626. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Q.; Liu, Z.; Wei, Y.; Li, B.; Wang, F. Mild and Efficient Synthesis of 1,8-naphthalide and 1,8-naphthalenedimethanol. Synth. Commun. 2004, 34, 2269–2275. [Google Scholar] [CrossRef]
- Kamada, T.; Gama, Y.; Wasada, N. Synthesis of the 1H,5H-naphthol[1,8-ef][1,3]dithiochin system. Bull. Chem. Soc. Jpn. 1989, 62, 3024–3025. [Google Scholar] [CrossRef]
- Nagao, Y.; Yoshida, T.; Arimitsu, K.; Kozawa, K. Synthesis and properties of dicarboximide derivatives of perylene and azaperylene. Heterocycles 2010, 80, 1197–1213. [Google Scholar] [CrossRef]
- Hauptmann, S.; Hunger, P.; Blaskovits, A. Synthesis and properties of naphthalene-1,8-dicarboxaldehyde. J. Prakt. Chem. 1986, 37, 72–77. [Google Scholar] [CrossRef]
- Robert, J.B.; Sherfinski, J.S.; Marsh, R.E.; Roberts, J.D. 1,8-Interactions in naphthalene derivatives. An x-ray structure determination and nuclear magnetic resonance studies of l,8-di(bromomethyl)naphthalene. J. Org. Chem. 1974, 39, 1152–1156. [Google Scholar] [CrossRef]
- Davies, A.; Warren, K.D. Nitration of dimethylnaphtalene in acetic anhydride. J. Chem. Soc. B 1969, 873–878. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Arey, J. Synthesis and identification of dimethylnitronaphthalenes and ethylnitronaphthalenes to aid in their analysis in ambient air. Polycyclic Aromat. Compd. 2007, 27, 211–237. [Google Scholar] [CrossRef]
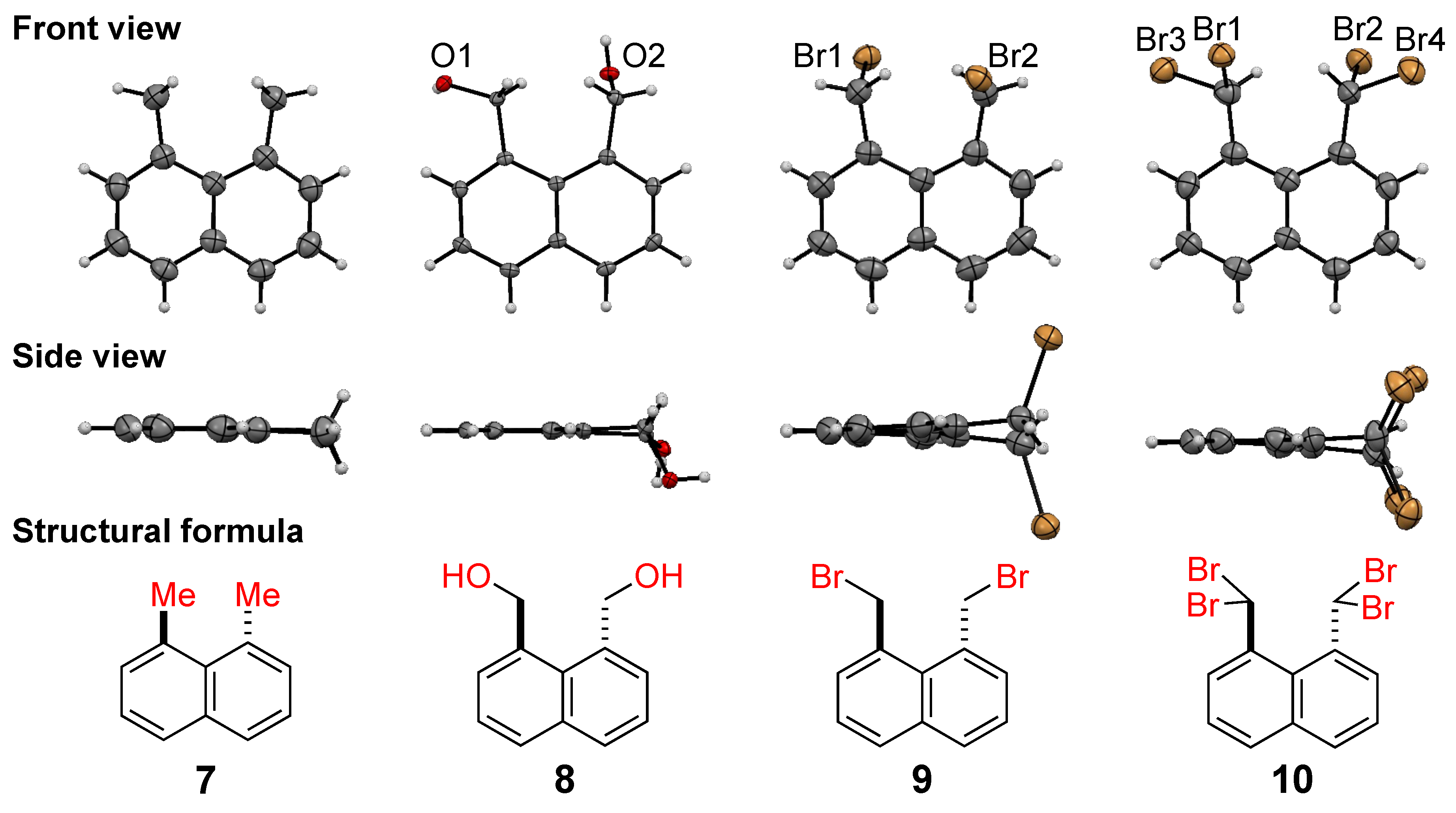
| 7 (R = CH3) | 8 (R = CH2OH) | 9 (R = CH2Br) | 10 (R = CHBr2) | |
|---|---|---|---|---|
| Empirical formula | C12H12 | C12H12O2 | C12H10Br2 | C12H8Br4 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n | C2/c | P21/n |
| ɑ (Å) | 9.5783(6) | 8.4875(4) | 23.3250(11) | 10.1755(4) |
| b (Å) | 6.8687(4) | 4.8113(2) | 7.5802(3) | 6.7237(2) |
| c (Å) | 13.2814(10) | 22.4730(9) | 12.3748(6) | 19.3037(8) |
| β (°) | 92.066(7) | 94.379(4) | 95.479(4) | 100.207(4) |
| Z | 4 | 4 | 8 | 4 |
| Goodness-of-fit | 1.051 | 1.098 | 1.097 | 1.031 |
| R1 [I > 2σ(I)] wR2 (all data) | R1 = 0.054, wR2 = 0.160 | R1 = 0.041, wR2 = 0.110 | R1 = 0.036, wR2 = 0.107 | R1 = 0.048, wR2 = 0.132 |
| CCDC number | 2266182 | 2266183 | 2266184 | 2266185 |
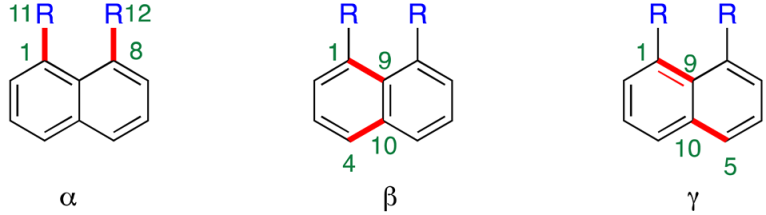 | ||||
|---|---|---|---|---|
| R | α/° | β/° | γ/° | |
| CH3 | 7 | 0.7 | 0.5 | 0.1 |
| CH2OH | 8 | 4.6 | 2.1 | 1.5 |
| CH2Br | 9 | 11.0 | 4.1 | 4.9 |
| CHBr2 | 10 | 8.3 | 3.5 | 3.1 |
 | |||||
|---|---|---|---|---|---|
| R | δ/° | ε/° | ζ/° | θ/° | |
| CH3 | 7 | 126.3(1) | 116.6(9) | 117.1(9) | 118.2(1) |
| CH2OH | 8 | 126.3(2) | 117.1(2) | 116.7(2) | 118.5(3) |
| CH2Br | 9 | 128.4(6) | 115.1(5) | 116.5(5) | 117.6(2) |
| CHBr2 | 10 | 125.5(1) | 117.2(1) | 117.3(1) | 118.8(2) |
 | ||||
|---|---|---|---|---|
| 7 (R = CH3) | 8 (R = CH2OH) | 9 (R = CH2Br) | 10 (R = CHBr2) | |
| C1–C2 | 1.375(2) | 1.380(2) | 1.380(4) | 1.37(1) |
| C2–C3 | 1.407(2) | 1.407(2) | 1.404(4) | 1.38(1) |
| C3–C4 | 1.358(2) | 1.364(2) | 1.358(4) | 1.37(1) |
| C4–C10 | 1.419(2) | 1.421(2) | 1.423(4) | 1.407(9) |
| C10–C9 | 1.442(2) | 1.435(1) | 1.430(4) | 1.434(9) |
| C9–C1 | 1.445(2) | 1.447(2) | 1.446(4) | 1.481(9) |
| C10–C5 | 1.418(2) | 1.422(2) | 1.422(4) | 1.414(9) |
| C5–C6 | 1.358(2) | 1.361(2) | 1.360(5) | 1.34(1) |
| C6–C7 | 1.409(2) | 1.407(2) | 1.400(5) | 1.41(1) |
| C7–C8 | 1.377(2) | 1.380(2) | 1.382(4) | 1.37(1) |
| C8–C9 | 1.444(2) | 1.446(2) | 1.446(4) | 1.434(9) |
 | ||||
|---|---|---|---|---|
| 7 (R = CH3) | 8 (R = CH2OH) | 9 (R = CH2Br) | 10 (R = CHBr2) | |
| C1–C8 | 2.569(2) | 2.580(2) | 2.580(4) | 2.625(9) |
| C2–C9 | 2.429(2) | 2.444(2) | 2.440(4) | 2.459(9) |
| C4–C5 | 2.442(2) | 2.440(2) | 2.445(5) | 2.413(9) |
| C11–C12 | 2.961(2) | 2.984(2) | 3.058(4) | 2.987(9) |
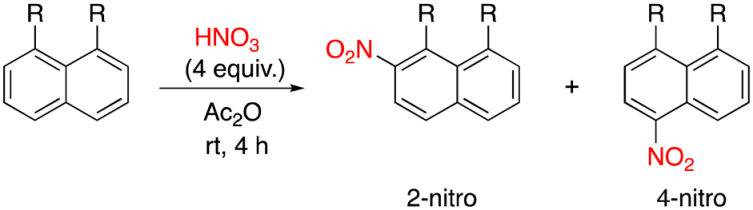 | |||||
|---|---|---|---|---|---|
| Entry | Naphthalene | Yield/% | Recovery/% | ||
| R | 2-nitro | 4-nitro | |||
| 1 | CH3 | 7 | 44 | 56 | 0 |
| 2 | CH2Br | 9 | 0 | 12 | 62 |
| 3 | CHBr2 | 10 | 0 | 0 | 61 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| R | C11–Br1 | C11–Br3 | C12–Br2 | C12–Br4 | C1–C11 | C8–C12 | ||
| CH3 | 7 | Crystal | — | — | — | — | 1.507(2) | 1.513(2) |
| calcd. | — | — | — | — | 1.517 | 1.517 | ||
| CH2Br | 9 | Crystal | 1.981(3) | — | 1.996(3) | — | 1.499(4) | 1.489(4) |
| calcd. | 2.020 | — | 2.021 | — | 1.496 | 1.496 | ||
| CHBr2 | 10 | Crystal | 1.986(8) | 1.956(7) | 1.977(7) | 1.947(8) | 1.51(1) | 1.49(1) |
| calcd. | 1.996 | 1.976 | 1.996 | 1.976 | 1.502 | 1.502 | ||
| R | HOMO | LUMO | |
|---|---|---|---|
| CH3 | 7 | −5.51 | −0.80 |
| CH2Br | 9 | −6.11 | −1.83 |
| CHBr2 | 10 | −6.29 | −2.19 |
 | |||||||
|---|---|---|---|---|---|---|---|
| R | Ring A | Ring B | |||||
| NICS(−1) | NICS(0) | NICS(1) | NICS(−1) | NICS(0) | NICS(1) | ||
| CH3 | 7 | −11.1 | −9.0 | −11.1 | −11.1 | −9.0 | −11.1 |
| CH2Br | 9 | −11.6 | −9.9 | −11.1 | −11.1 | −9.9 | −11.6 |
| CHBr2 | 10 | −11.7 | −9.3 | −10.7 | −10.6 | −9.3 | −11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, A.I.; Iwai, K.; Nishiwaki, N. A Study of the Correlation between the Bulkiness of peri-Substituents and the Distortion of a Naphthalene Ring. Molecules 2023, 28, 5343. https://doi.org/10.3390/molecules28145343
Reza AI, Iwai K, Nishiwaki N. A Study of the Correlation between the Bulkiness of peri-Substituents and the Distortion of a Naphthalene Ring. Molecules. 2023; 28(14):5343. https://doi.org/10.3390/molecules28145343
Chicago/Turabian StyleReza, Annisa Indah, Kento Iwai, and Nagatoshi Nishiwaki. 2023. "A Study of the Correlation between the Bulkiness of peri-Substituents and the Distortion of a Naphthalene Ring" Molecules 28, no. 14: 5343. https://doi.org/10.3390/molecules28145343
APA StyleReza, A. I., Iwai, K., & Nishiwaki, N. (2023). A Study of the Correlation between the Bulkiness of peri-Substituents and the Distortion of a Naphthalene Ring. Molecules, 28(14), 5343. https://doi.org/10.3390/molecules28145343







