Silver-Catalyzed Decarboxylative Acylation of Isocyanides Accesses to α-Ketoamides with Air as a Sole Oxidant
Abstract
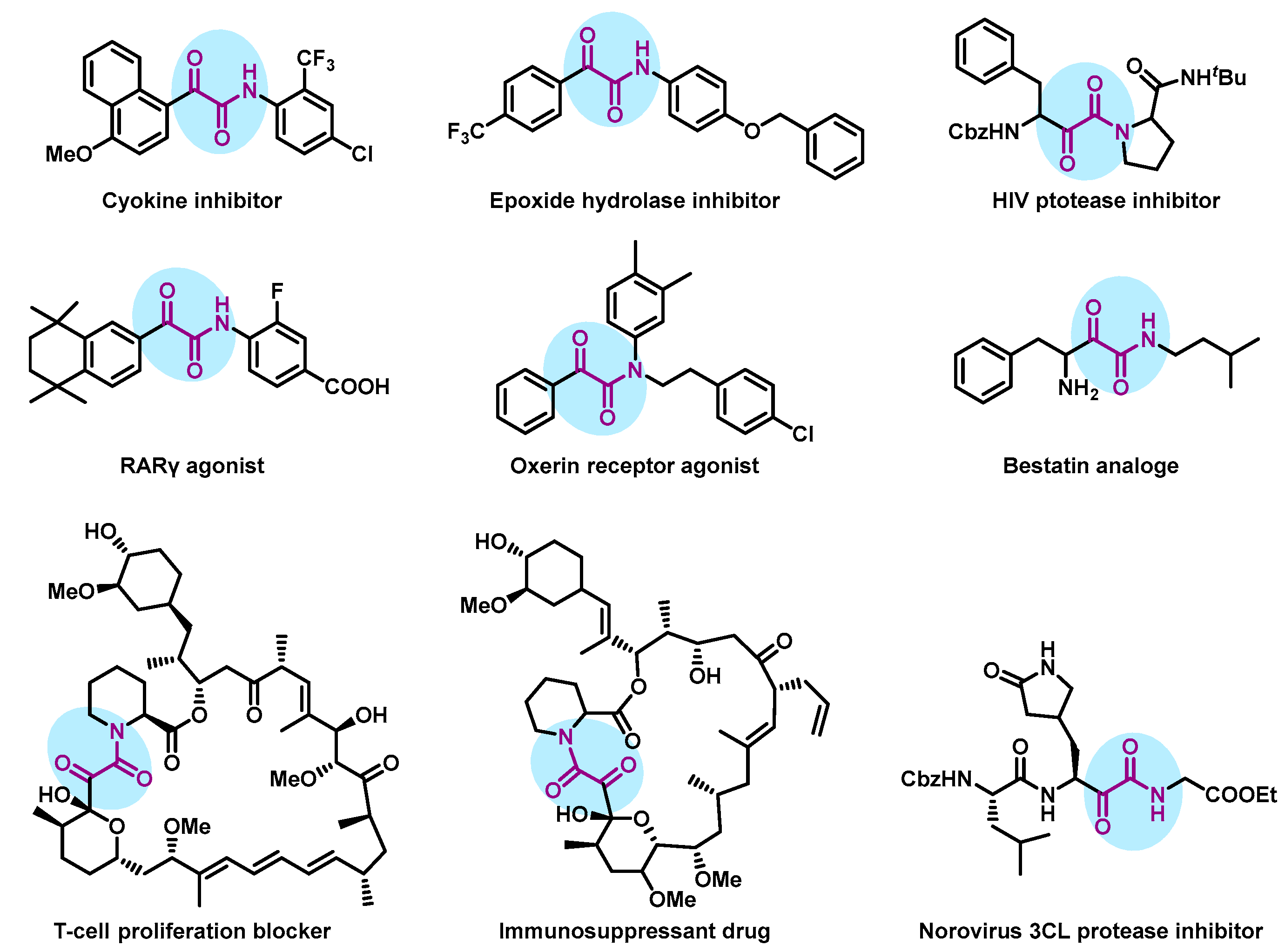
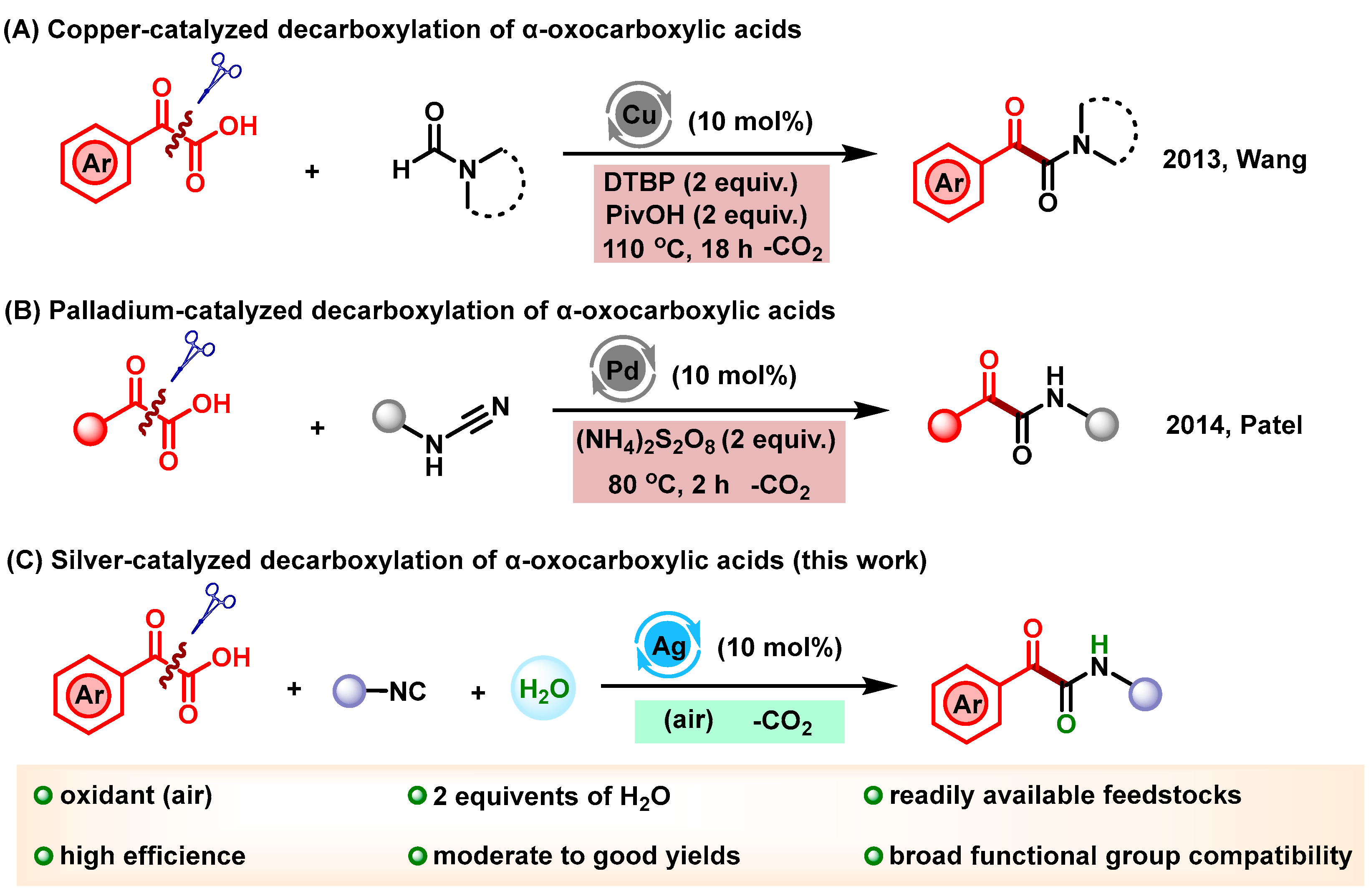
1. Introduction
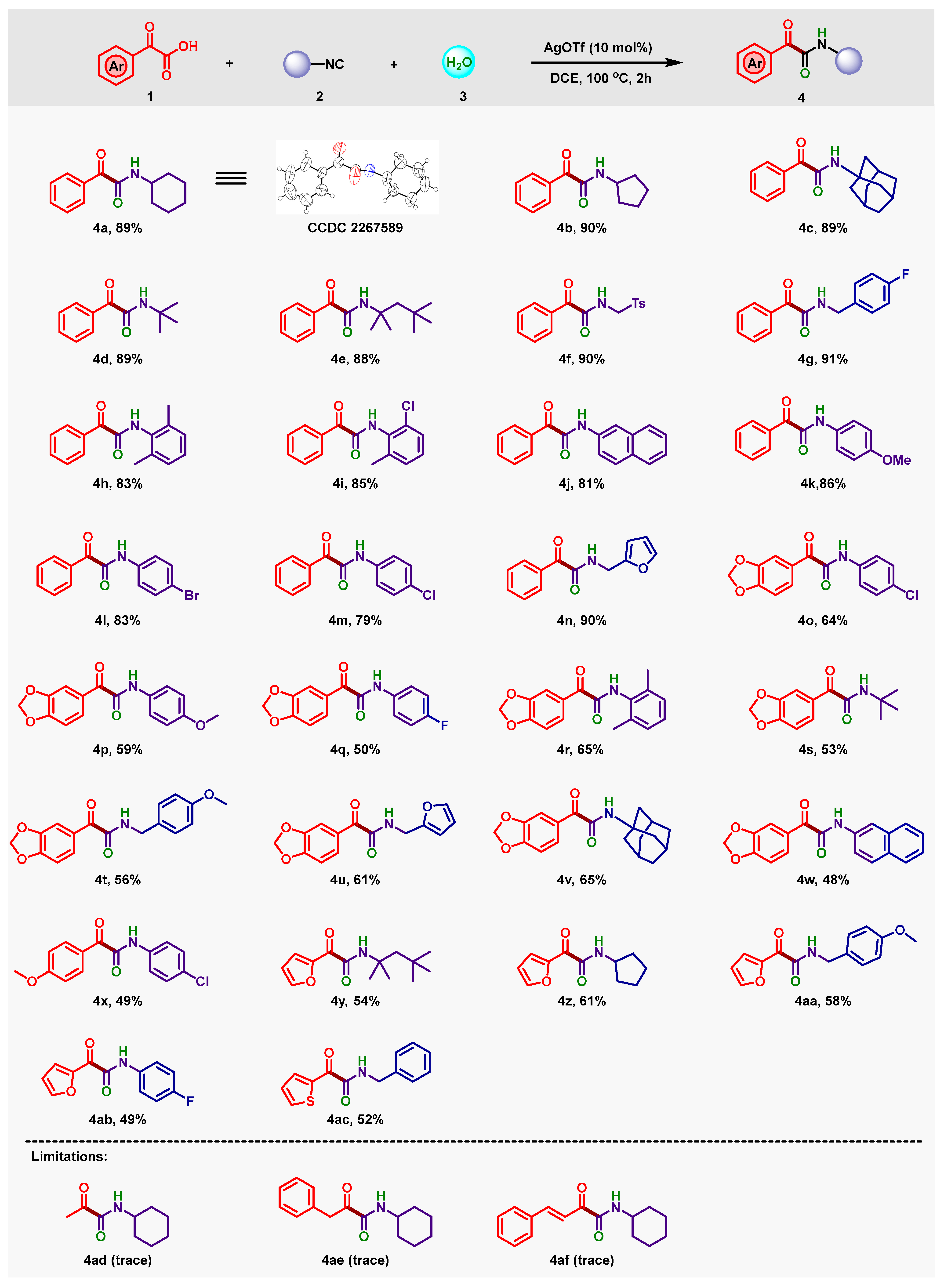
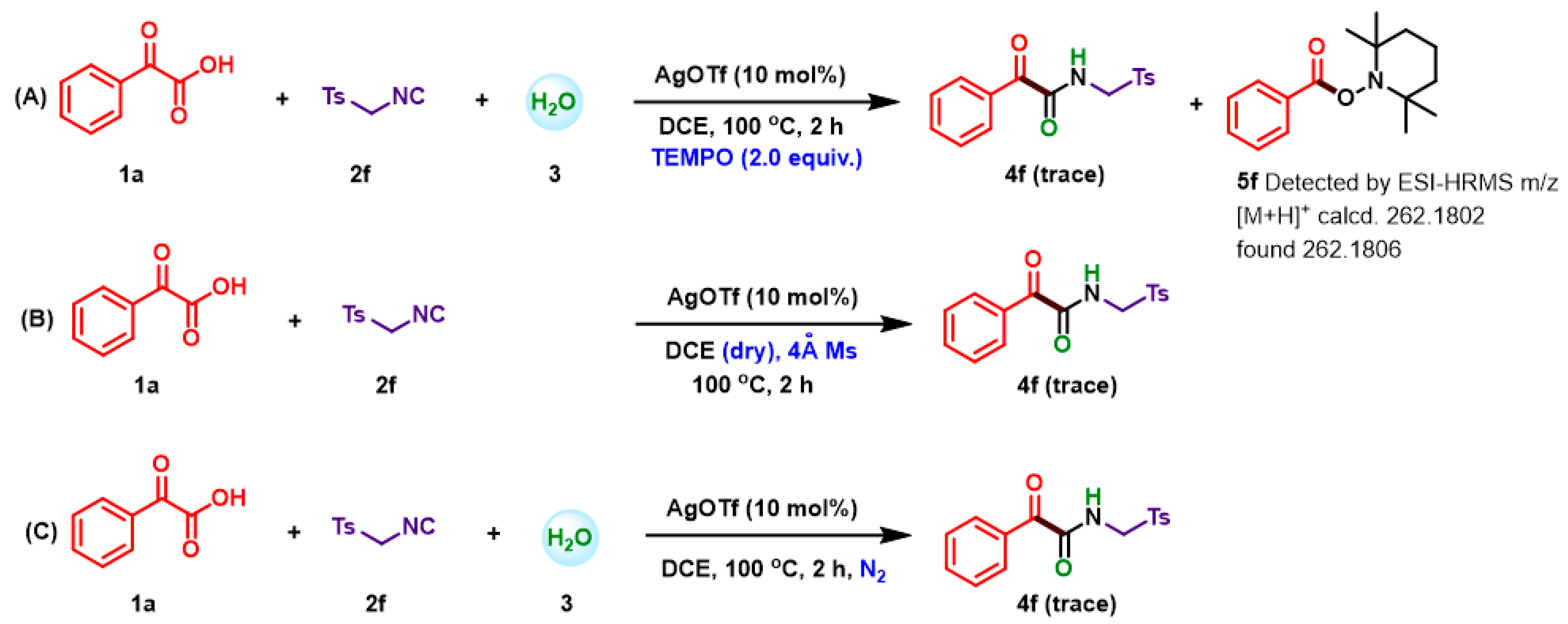
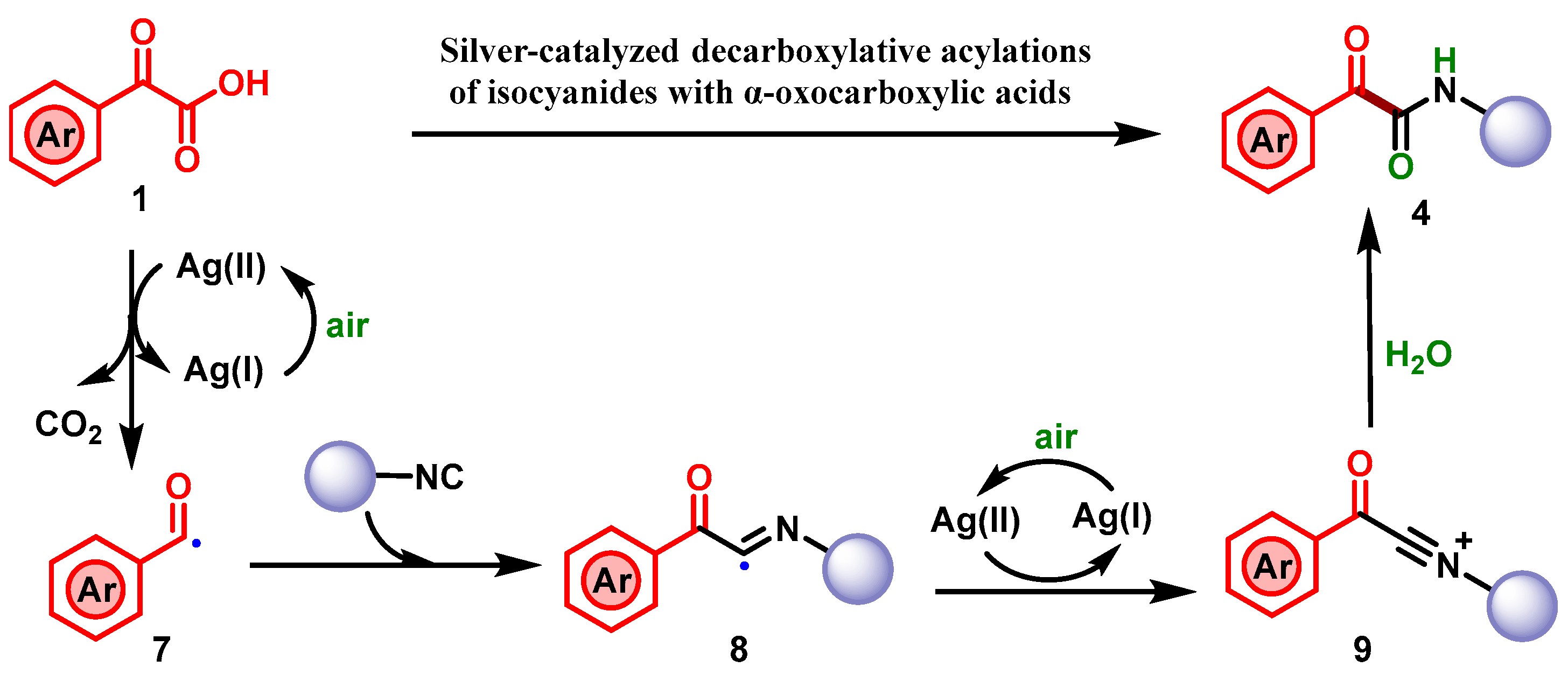
2. Results
3. Materials and Methods
3.1. Analytical Techniques
3.2. Synthetic Procedures for the Synthesis of Compound 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cai, W.; Qiao, X.; Zhang, H.; Li, B.; Guo, J.; Zhang, L.; Chen, W.-W.; Zhao, B. Asymmetric biomimetic transamination of α-keto amides to peptides. Nat. Commun. 2021, 12, 5174–5182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, B.; Chen, Y.; Chan, J.F.-W.; Yuan, S.; Ye, H.; Nie, L.; Zhou, J.; Wu, Y.; Wu, M.; et al. A new class of α-ketoamide derivatives with potent anticancer and anti-SARS-CoV-2 activities. Eur. J. Med. Chem. 2021, 215, 113267–113286. [Google Scholar] [CrossRef]
- Zhou, J.; Mock, E.D.; Martella, A.; Kantae, V.; Di, X.Y.; Burggraaff, L.; Baggelaar, M.P.; Al-Ayed, K.; Bakker, A.; Florea, B.I.; et al. Activity-based protein profiling identifies α-ketoamides as inhibitors for Phospholipase A2 Group XVI. ACS Chem. Biol. 2019, 14, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vemula, S.R.; Cook, G.R. Recent advances in catalytic synthesis of α-ketoamides. ACS Catal. 2016, 6, 4920–4945. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Kusov, Y.; Nian, Y.; Ma, Q.; Wang, J.; von Brunn, A.; Leyssen, P.; Lanko, K.; Neyts, J.; et al. Alpha-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication Structurebased design, synthesis, and activity assessment. J. Med. Chem. 2020, 63, 4562–4578. [Google Scholar] [CrossRef] [PubMed]
- Robello, M.; Barresi, E.; Baglini, E.; Salerno, S.; Taliani, S.; Settimo, F.D. The alpha keto amide moiety as a privileged motif in medicinal chemistry: Current insights and emerging opportunities. J. Med. Chem. 2021, 64, 3508–3545. [Google Scholar] [CrossRef]
- Zhou, J.; Mock, E.D.; Ayed, K.A.; Di, X.Y.; Kantae, V.; Burggraaff, L.; Stevens, A.; Martella, A.; Mohr, F.; Jiang, M.; et al. Structure-activity relationship studies of α-ketoamides as inhibitors of the phospholipase A and acyltransferase (PLAAT) enzyme family. J. Med. Chem. 2020, 63, 9340–9359. [Google Scholar] [CrossRef]
- Risi, C.D.; Pollini, G.P.; Zanirato, V. Recent developments in general methodologies for the synthesis of α-ketoamides. Chem. Rev. 2016, 116, 3241–3305. [Google Scholar] [CrossRef]
- Lv, Y.; Bao, P.; Yue, H.; Li, J.-S.; Wei, W. Visible-light-mediated metal-free decarboxylative acylations of isocyanides with α-oxocarboxylic acids and water leading to α-ketoamides. Green Chem. 2019, 21, 6051–6055. [Google Scholar] [CrossRef]
- Zhao, F.; Meng, N.; Sun, T.; Wen, J.; Zhao, X.; Wei, W. Metal-free electrochemical synthesis of α-ketoamides via decarboxylative coupling of α-keto acids with isocyanides and water. Org. Chem. Front. 2021, 8, 6508–6514. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, X.; Cai, C.; Wang, L.; Gong, H. Synthesis of α-ketoamides via electrochemical decarboxylative acylation of isocyanides using α-ketoacids as an acyl source. Asian J. Org. Chem. 2022, 11, 43–47. [Google Scholar] [CrossRef]
- Papanikos, A.; Rademann, J.; Meldal, M. α-Ketocarbonyl Peptides: A General Approach to Reactive Resin-Bound Intermediates in the Synthesis of Peptide Isosteres for Protease Inhibitor Screening on Solid Support. J. Am. Chem. Soc. 2001, 123, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-G.; Zhu, H.; Fan, M.; Yan, C.; Shi, K.; Chi, X.-W.; Zou, L.-H. Copper-catalyzed coupling of anthranils and α-keto acids: Direct synthesis of α-ketoamides. Org. Biomol. Chem. 2019, 17, 5902–5907. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Wang, L. Silver-catalyzed amidation of benzoylformic acids with tertiary amines via selective carbon–nitrogen bond cleavage. Org. Biomol. Chem. 2013, 11, 3649–3654. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L.-N.; Duan, X.-H. Copper-catalyzed oxidative condensation of α-oxocarboxylic acids with formamides: Synthesis of α-ketoamides. Org. Biomol. Chem. 2013, 11, 4573–4576. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Wu, Z.; Wang, Y.; Zheng, Y.; Zhao, M. Selective synthesis of aryl thioamides and aryl-α-ketoamides from α-oxocarboxylic acids and tetraalkylthiuram disulfides: An unexpected chemoselectivity from aryl sulfonyl chlorides. Org. Chem. Front. 2019, 6, 506–511. [Google Scholar] [CrossRef]
- Rodríguez, N.; Goossen, L.J. Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef]
- Weaver, J.D.; Recio, A.; Grenning, A.J.; Tunge, J.A. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 2011, 111, 1846–1913. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Fan, X. Synthesis of 2-aminobenzophenones through acylation of anilines with α-oxocarboxylic acids assisted by tert-butyl nitrite. Org. Biomol. Chem. 2018, 16, 7737–7747. [Google Scholar] [CrossRef]
- Zou, H.-X.; Li, Y.; Yang, Y.; Li, J.-H.; Xiang, J. Silver-catalyzed decarboxylative couplings of acids and anhydrides: An entry to 1,2-diketones and aryl-substituted ethanes. Adv. Synth. Catal. 2018, 360, 1439–1443. [Google Scholar] [CrossRef]
- Katiyar, S.; Kumara, A.; Sashidhara, K.V. Silver-catalyzed decarboxylative cyclization for the synthesis of substituted pyrazoles from 1,2-diaza-1,3-dienes and a-keto acids. Chem. Commun. 2022, 58, 7297–7300. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Wang, X.; Zhang, J.; Wang, X.; Hu, Y. Silver-catalyzed decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo-carboxylic acids. Org. Biomol. Chem. 2017, 15, 8929–8935. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, H.; Hu, Q.; Yang, Q.; Xu, S.; Liu, T. Silver-catalyzed decarboxylative crosscoupling of α-keto acids with alkenes giving approach to chalcones. Tetrahedron Lett. 2017, 58, 4763–4765. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Wu, Z.; Li, F.; Qin, T.; Zhang, S.; Kong, W.; Liu, L. Silver-catalyzed decarboxylative C–H functionalization of cyclic aldimines with aliphatic carboxylic acids. Chin. Chem. Lett. 2021, 32, 2777–2781. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Liu, J.; Zhao, Q.; Wang, L. Cu(II)-catalyzed decarboxylative acylation of acyl C–H of formamides with a-oxocarboxylic acids leading to α-ketoamides. Chem. Commun. 2013, 49, 3640–3642. [Google Scholar] [CrossRef] [PubMed]
- Guin, S.; Rout, S.K.; Gogoi, A.; Ali, W.; Patel, B.K. A palladium(II)-catalyzed synthesis of α-ketoamides via chemoselective aroyl addition to cyanamides. Adv. Synth. Catal. 2014, 356, 2559–2565. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Campbell, A.N.; Stahl, S.S. Overcoming the “oxidant problem”: Strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef]
- Leonardi, G.; Truscello, A.; Mondrone, G.G.; Sebastiano, R. A facile synthesis in aqueous medium of 3-hydroxy-2-pyrone from aldaric acids or their derivatives. Results Chem. 2022, 4, 100280–100284. [Google Scholar] [CrossRef]
- Panday, S.K. Advances in the mitsunobu reaction: An excellent organic protocol with versatile applications. Mini Rev. Org. Chem. 2019, 16, 127–140. [Google Scholar] [CrossRef]
- Shekuti, R.K.; Tangalipalli, S.; Dhonthulachitty, C.; Kothakapu, S.R.; Annapurna, P.D.; Neella, C.K. N-Benzoyl-4-dimethylaminopyridinium chloride: A Lewis base adduct for efficient poly and monobenzoylation. ChemistrySelect 2022, 7, e202202636. [Google Scholar]
- Tavakoli, S.D.; Prieto-Araujo, E.; Sánchez-Sánchez, E.; Gomis-Bellmunt, O. Methodology for interaction identification in modular multi-level converter-based HVDC systems. ISA Trans. 2022, 126, 300–315. [Google Scholar] [CrossRef]
- Lei, J.; Ding, Y.; Zhou, H.; Gao, X.-Y.; Cao, Y.-H.; Tang, D.-Y.; Li, H.; Xu, Z.-G.; Chen, Z.-Z. Practical synthesis of quinolone drugs via a novel TsCl-mediated domino reaction sequence. Green Chem. 2022, 24, 5755–5759. [Google Scholar] [CrossRef]
- Lei, J.; Xu, J.; Luo, Y.-F.; Li, J.; Wang, J.-Y.; Li, H.; Xu, Z.-G.; Chen, Z.-Z. A novel isocyanide/Ag2CO3-promoted addition of heteroatoms to alkynes under mild conditions. Org. Chem. Front. 2023, 10, 786–792. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Yin, F.; Su, Z.; Li, Z.; Li, C. Silver-catalyzed decarboxylative chlorination of aliphatic carboxylic acids. J. Am. Chem. Soc. 2012, 134, 4258–4263. [Google Scholar] [CrossRef]
- Varenikov, A.; Shapiro, E.; Gandelman, M. Decarboxylative halogenation of organic compounds. Chem. Rev. 2021, 121, 412–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, M.; Liu, J.; Zhang, X.-M. Tandem cycloaddition–decarboxylation of α-keto acid and isocyanide under oxidant-free conditions towards monosubstituted oxazoles. RSC Adv. 2016, 6, 73450–73453. [Google Scholar] [CrossRef]
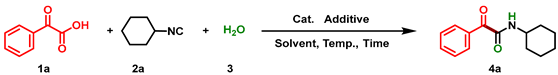 | ||||||
| Entry | Cat. | Additive | Solvent | Temp. (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | Ag2CO3 | - | MeCN | 80 | 1 | 62 |
| 2 | AgBF4 | - | MeCN | 80 | 1 | 56 |
| 3 | AgOAc | - | MeCN | 80 | 1 | 59 |
| 4 | Ag2O | - | MeCN | 80 | 1 | 51 |
| 5 | AgOTf | - | MeCN | 80 | 1 | 70 |
| 6 | AgNTf2 | - | MeCN | 80 | 1 | 54 |
| 7 | AgOTf | - | DMF | 80 | 1 | 47 |
| 8 | AgOTf | - | 1,4-Dioxane | 80 | 1 | 79 |
| 9 | AgOTf | - | DCE | 80 | 1 | 82 |
| 10 | AgOTf | - | DMSO | 80 | 1 | 61 |
| 11 | AgOTf | - | THF | 80 | 1 | 33 |
| 12 | AgOTf | - | Toluene | 80 | 1 | 28 |
| 13 | AgOTf | - | DCE | 100 | 1 | 87 |
| 14 | AgOTf | - | DCE | 120 | 1 | 86 |
| 15 | AgOTf | - | DCE | 100 | 2 | 89 |
| 16 c | AgOTf | PhI(OAc)2 | DCE | 100 | 2 | 85 |
| 17 d | AgOTf | K2S2O8 | DCE | 100 | 2 | 88 |
| 18 e | AgOTf | Oxone | DCE | 100 | 2 | 76 |
| 19 | Pd(OAc)2 | - | DCE | 100 | 2 | 23 |
| 20 | CuBr2 | - | DCE | 100 | 2 | 18 |
| 21 | FeCl2 | - | DCE | 100 | 2 | 31 |
| 22 f | AgOTf | - | DCE | 100 | 2 | 73 |
| 23 g | AgOTf | - | DCE | 100 | 2 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, X.; Chen, X.-Y.; He, Y.-T.; Lei, J.; Chen, Z.-Z.; Xu, Z.-G. Silver-Catalyzed Decarboxylative Acylation of Isocyanides Accesses to α-Ketoamides with Air as a Sole Oxidant. Molecules 2023, 28, 5342. https://doi.org/10.3390/molecules28145342
Xu J, Li X, Chen X-Y, He Y-T, Lei J, Chen Z-Z, Xu Z-G. Silver-Catalyzed Decarboxylative Acylation of Isocyanides Accesses to α-Ketoamides with Air as a Sole Oxidant. Molecules. 2023; 28(14):5342. https://doi.org/10.3390/molecules28145342
Chicago/Turabian StyleXu, Jia, Xue Li, Xing-Yu Chen, Yu-Ting He, Jie Lei, Zhong-Zhu Chen, and Zhi-Gang Xu. 2023. "Silver-Catalyzed Decarboxylative Acylation of Isocyanides Accesses to α-Ketoamides with Air as a Sole Oxidant" Molecules 28, no. 14: 5342. https://doi.org/10.3390/molecules28145342
APA StyleXu, J., Li, X., Chen, X.-Y., He, Y.-T., Lei, J., Chen, Z.-Z., & Xu, Z.-G. (2023). Silver-Catalyzed Decarboxylative Acylation of Isocyanides Accesses to α-Ketoamides with Air as a Sole Oxidant. Molecules, 28(14), 5342. https://doi.org/10.3390/molecules28145342






