Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update
Abstract
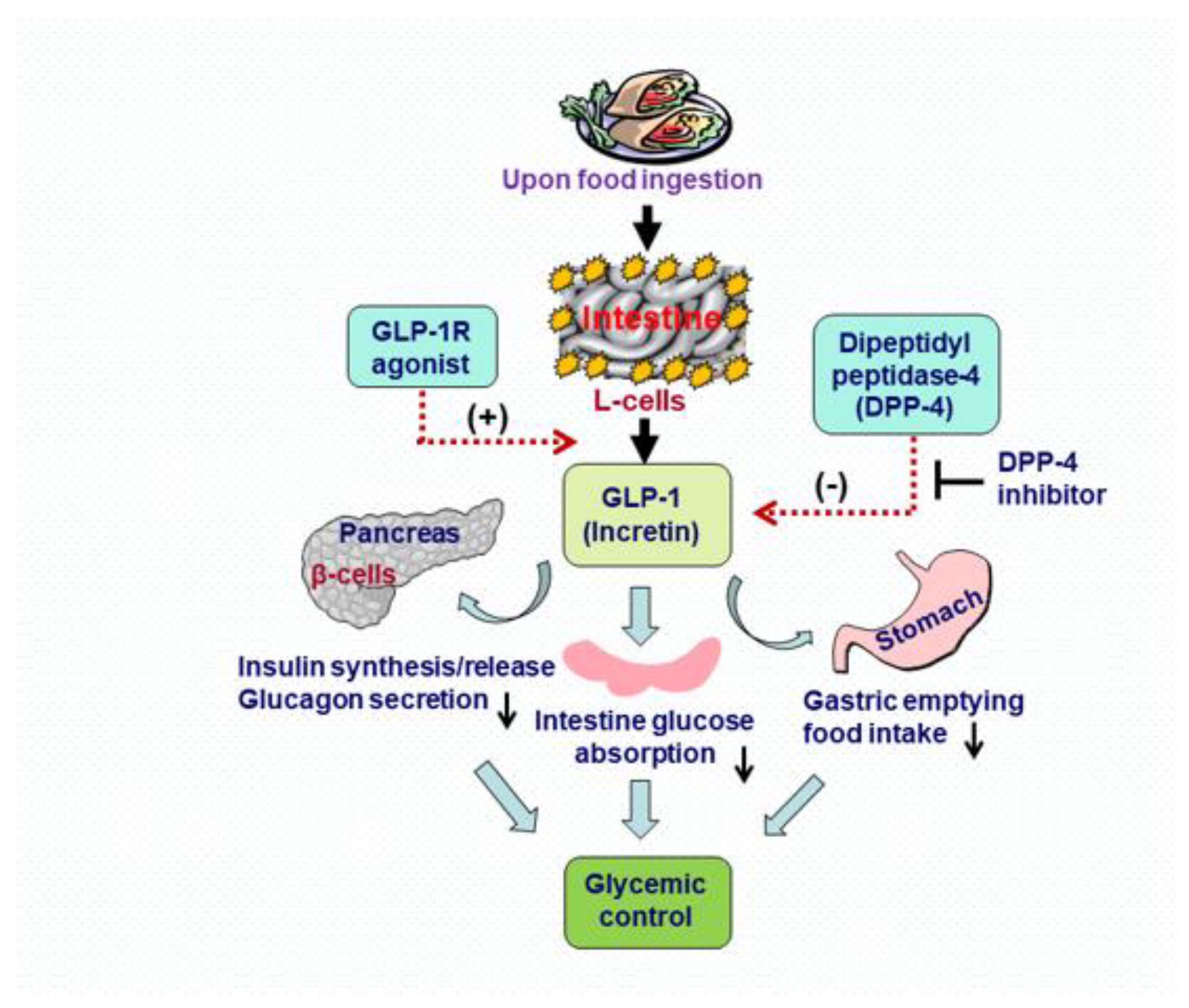
1. Introduction
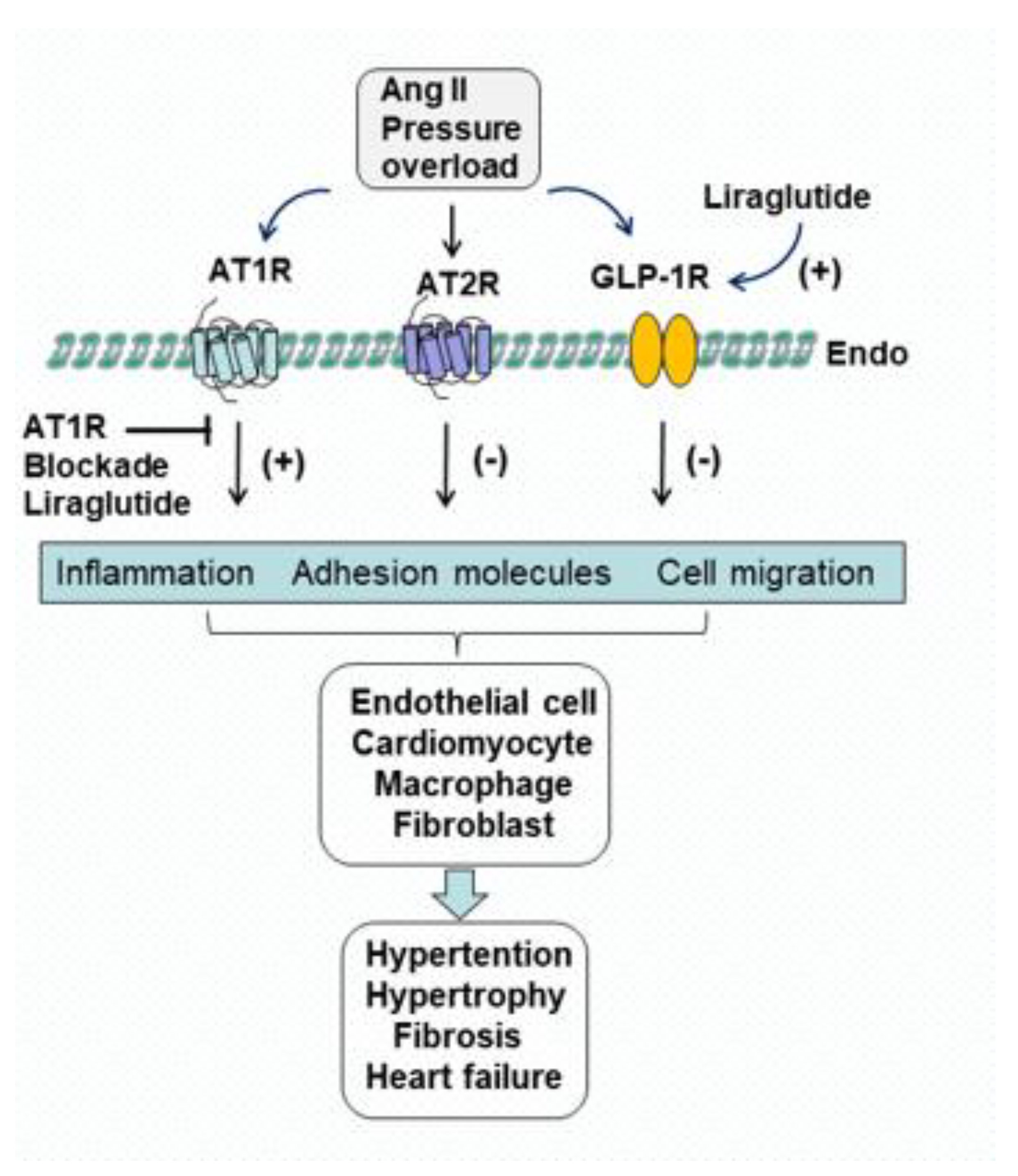
2. Effects of Liraglutide on Ang II AT1R, AT2R and GLP-1R
3. Inhibition of Inflammation and Preservation of Vascular Function with Liraglutide
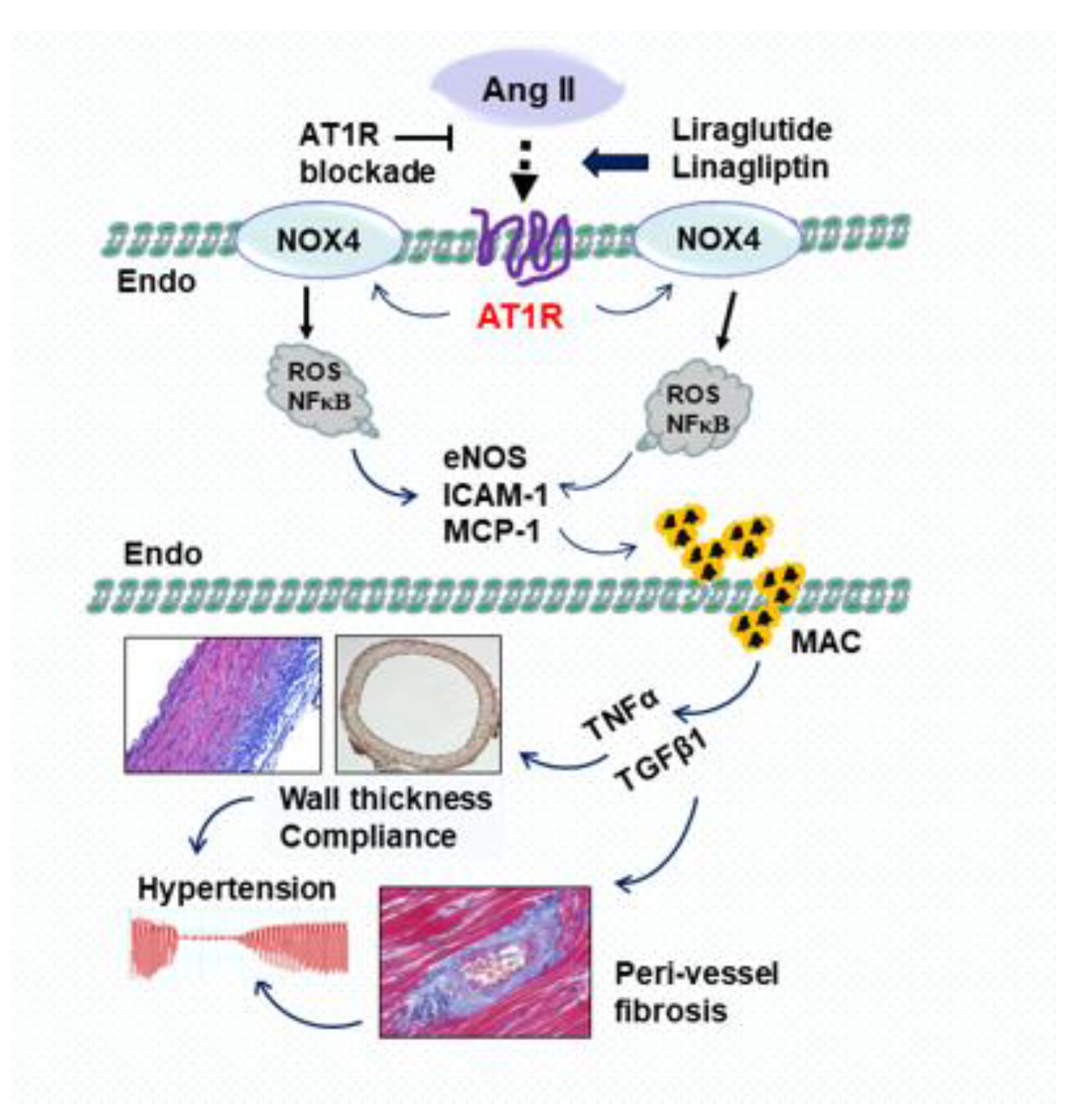
3.1. Effects of Liraglutide on Pro-Inflammatory Mediators and Oxidative Stress
3.2. Effects of Liraglutide on Vascular Morphology, Perivascular Fibrosis and Hypertension
3.3. Effects of Liraglutide on Endothelium-Dependent Vascular Relaxation
4. Attenuation of Cardiac Pathological Morphology and Improvement of Cardiac Function with Liraglutide
4.1. Effects of Liraglutide on Cardiac Interstitial Fibrosis and Mitochondrial Morphology
4.2. Effects of Liraglutide on Mammalian Target of Rapamycin and Autophagy
4.3. Effects of Liraglutide on Cardiac Hypertrophy
4.4. Effects of Liraglutide on Cardiac Function and Heart Failure
5. Future Prospect of Liraglutide Investigation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, M.G.; Nadruz, W.; Mónica, F.Z. Endothelial and vascular smooth muscle dysfunction in hypertension. Biochem. Pharmacol. 2022, 205, 115263. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; Singh, A.K.; Sessa, W.C. Targeting the vasculature in cardiometabolic disease. J. Clin. Investig. 2022, 132, e148556. [Google Scholar] [CrossRef] [PubMed]
- Halper, J. Basic components of vascular connective tissue and extracellular matrix. Adv Pharmacol. 2018, 81, 95–127. [Google Scholar] [PubMed]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Travers, J.G.; Tharp, C.A.; Rubino, M.; McKinsey, T.A. Therapeutic targets for cardiac fibrosis: From old school to next-gen. J. Clin. Investig. 2022, 132, e148554. [Google Scholar] [CrossRef]
- Halade, G.V.; Lee, D.H. Inflammation and resolution signaling in cardiac repair and heart failure. EBio Med. 2022, 79, 103992. [Google Scholar] [CrossRef]
- Poulis, N.; Martin, M.; Hoerstrup, S.P.; Emmert, M.Y.; Fioretta, E.S. Macrophage-extracellular matrix interactions: Perspectives for tissue engineered heart valve remodeling. Front. Cardiovasc. Med. 2022, 9, 952178. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Ghionzoli, N.; Del Franco, A.M.; Castiglione, V.; Aimo, A.; Giannoni, A.; Burchielli, S.; Cameli, M.; Emdin, M.; Vergaro, G. Current and emerging drug targets in heart failure treatment. Heart Fail. Rev. 2022, 27, 1119–1136. [Google Scholar] [CrossRef]
- Bai, X.J.; Hao, J.T.; Zheng, R.H.; Yan, C.P.; Wang, J.; Yang, C.H.; Zhang, W.F.; Zhao, Z.Q. Glucagon-like peptide-1 analog liraglutide attenuates pressure-overload induced cardiac hypertrophy and apoptosis through activating ATP sensitive potassium channels. Cardiovasc. Drugs Ther. 2021, 35, 87–101. [Google Scholar] [CrossRef]
- Zheng, R.H.; Zhang, W.W.; Ji, Y.N.; Bai, X.J.; Yan, C.P.; Wang, J.; Bai, F.; Zhao, Z.Q. Exogenous supplement of glucagon like peptide-1 protects the heart against aortic banding induced myocardial fibrosis and dysfunction through inhibiting mTOR/p70S6K signaling and promoting autophagy. Eur. J. Pharmacol. 2020, 883, 173318. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Powers, B.J.; Myers, E.R.; McBroom, A.J.; Sanders, G. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers for treatment of ischemic heart disease: Future research needs prioritization. Am. Heart J. 2012, 163, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Ram, C.V.S. Angiotensin receptor blockers: Current status and future prospects. Am. J. Med. 2008, 121, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [PubMed]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar] [CrossRef]
- Sanz, A.B.; Ramos, A.M.; Soler, M.J.; Sanchez-Niño, M.D.; Fernandez-Fernandez, B.; Perez-Gomez, M.V.; Ortega, M.R.; Alvarez-Llamas, G.; Ortiz, A. Advances in understanding the role of angiotensin-regulated proteins in kidney diseases. Expert Rev. Proteom. 2019, 16, 77–92. [Google Scholar] [CrossRef]
- Cheang, J.Y.; Moyle, P.M. Glucagon-like peptide-1 (GLP-1)-based therapeutics: Current status and future opportunities beyond type 2 diabetes. ChemMedChem 2018, 13, 662–671. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar]
- Zhang, L.H.; Pang, X.F.; Bai, F.; Wang, N.P.; Shah, A.I.; McKallip, R.J.; Li, X.W.; Wang, X.; Zhao, Z.Q. Preservation of glucagon-like peptide-1 level attenuates angiotensin II-induced tissue fibrosis by altering AT1/AT 2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc. Drugs Ther. 2015, 29, 243–255. [Google Scholar] [CrossRef]
- Bai, F.; Pang, X.F.; Zhang, L.H.; Wang, N.P.; McKallip, R.J.; Garner, R.E.; Zhao, Z.Q. Angiotensin II AT1 receptor alters ACE2 activity, eNOS expression and CD44-hyaluronan interaction in rats with hypertension and myocardial fibrosis. Life Sci. 2016, 153, 141–152. [Google Scholar] [CrossRef]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Hu, X.; Li, Y.; Ma, D.; Li, S.; Zhao, G.; Xie, Y.; Shu, Y.; Yang, J. Liraglutide, a glucagon-like peptide-1 receptor agonist, attenuates development of cardiac allograft vasculopathy in a murine heart transplant model. Transplantation 2019, 103, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Kshirsagar, S.; Sutariya, B.; Patel, M.; Pandey, D.; Patel, H.; Ranvir, R.; Kadam, S.; Patel, D.; et al. Coagonist of GLP-1 and glucagon decreases liver inflammation and atherosclerosis in dyslipidemic condition. Chem. Biol. Interact. 2018, 282, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Matera, M.G.; Calzetta, L.; Hanania, N.A.; Page, C.; Rossi, I.; Andreadi, A.; Galli, A.; Coppola, A.; Cazzola, M.; et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir. Med. 2019, 154, 86–92. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Lee, D.Y.; Wauquier, F.; Eid, A.A.; Roman, L.J.; Ghosh-Choudhury, G.; Khazim, K.; Block, K.; Gorin, Y.J. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: Role of mitochondrial reactive oxygen species. Biol. Chem. 2013, 288, 28668–28686. [Google Scholar] [CrossRef]
- Okabe, K.; Matsushima, S.; Ikeda, S.; Ikeda, M.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; et al. DPP (dipeptidyl peptidase)-4 inhibitor attenuates Ang II (angiotensin II)-induced cardiac hypertrophy via GLP (glucagon-like peptide)-1-dependent suppression of Nox (nicotinamide adenine dinucleotide phosphate oxidase) 4-HDAC (histone deacetylase) 4 pathway. Hypertension 2020, 75, 991–1001. [Google Scholar]
- Banks, T.E.; Rajapaksha, M.; Zhang, L.H.; Bai, F.; Wang, N.P.; Zhao, Z.Q. Suppression of angiotensin II-activated NOX4/NADPH oxidase and mitochondrial dysfunction by preserving glucagon-like peptide-1 attenuates myocardial fibrosis and hypertension. Eur. J. Pharmacol. 2022, 927, 175048. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Wang, N.P.; Erskine, J.; Zhang, W.W.; Zheng, R.H.; Zhang, L.H.; Duron, G.; Gendreau, J.; Zhao, Z.Q. Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II. J. Renin-Angiotensin-Aldosterone Syst. 2017, 18, 1470320317706653. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.F.; Zhang, L.H.; Bai, F.; Wang, N.P.; Garner, R.E.; McKallip, R.J.; Zhao, Z.Q. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Dev. Ther. 2015, 9, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.H.; Bai, X.J.; Zhang, W.W.; Wang, J.; Bai, F.; Yan, C.P.; James, E.A.; Bose, H.S.; Wang, N.P.; Zhao, Z.Q. Liraglutide attenuates cardiac remodeling and improves heart function after abdominal aortic constriction through blocking angiotensin II type 1 receptor in rats. Drug Des. Dev. Ther. 2019, 13, 2745–2757. [Google Scholar] [CrossRef] [PubMed]
- Zemse, S.M.; Hilgers, R.H.P.; Webb, R.C. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3103–H3108. [Google Scholar] [CrossRef] [PubMed]
- Soares de Moura, R.; Resende, A.C.; Emiliano, A.F.; Tano, T.; Mendes-Ribeiro, A.C.; Correia, M.L.; de Carvalho, L.C. The role of bradykinin, AT2 and angiotensin 1-7 receptors in the EDRF-dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br. J. Pharmacol. 2004, 141, 860–866. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Angiotensin II-induced signal transduction mechanisms for cardiac hypertrophy. Cells 2022, 11, 3336. [Google Scholar] [CrossRef]
- Tuohy, C.V.; Kaul, S.; Song, H.K.; Nazer, B.; Heitner, S.B. Hypertrophic cardiomyopathy: The future of treatment. Eur. J. Heart Fail. 2020, 22, 228–240. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Kurose, H. Cardiac fibrosis and fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Cowling, R.T.; Kupsky, D.; Kahn, A.M.; Daniels, L.B.; Greenberg, B.H. Mechanisms of cardiac collagen deposition in experimental models and human disease. Transl Res. 2019, 209, 138–155. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.; Inserra, F.; Ferder, M.; Ferder, L. From mitochondria to disease: Role of the renin-angiotensin system. Am. J. Nephrol. 2007, 27, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sunggip, C.; Kitajima, N.; Nishida, M. Redox control of cardiovascular homeostasis by angiotensin II. Curr. Pharm. Des. 2013, 19, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension Is linked to vascular inflammation and oxidative stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 7634–7639. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Jayas, R.; Afshar, P.; Guberman, M.; Graham, M.; Gerstein, J.; Lieberman, B.; Nepon, H.; Margulets, V.; Dhingra, R.; et al. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic. Biol. Med. 2017, 112, 411–422. [Google Scholar] [CrossRef]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef]
- Tavares, M.R.; Pavan, I.C.; Amaral, C.L.; Meneguello, L.; Luchessi, A.D.; Simabuco, F.M. The S6K protein family in health and disease. Life Sci. 2015, 131, 1–10. [Google Scholar] [CrossRef]
- Aisa, Z.; Liao, G.C.; Shen, X.L.; Chen, J.; Li, L.; Jiang, S.B. Effect of autophagy on myocardial infarction and its mechanism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3705–3713. [Google Scholar]
- Yao, Q.; Ke, Z.Q.; Guo, S.; Yang, X.S.; Zhang, F.X.; Liu, X.F.; Chen, X.; Chen, H.G.; Ke, H.Y.; Liu, C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J. Mol. Cell. Cardiol. 2018, 124, 26–34. [Google Scholar] [CrossRef]
- Forte, M.; Bianchi, F.; Cotugno, M.; Marchitti, S.; De Falco, E.; Raffa, S.; Stanzione, R.; Di Nonno, F.; Chimenti, I.; Palmerio, S.; et al. Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy 2019, 12, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ma, B.; Han, X. The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. J. Mol. Endocrinol. 2016, 57, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Munson, M.J.; Ganley, I.G. MTOR, PIK3C3, and autophagy: Signaling the beginning from the end. Autophagy 2015, 11, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lv, Z.; Chen, Q.; Guo, M.; Wang, X.; Huang, F. Vascular endothelial growth factor over-expressed mesenchymal stem cells-conditioned media ameliorate palmitate-induced diabetic endothelial dysfunction through PI-3K/AKT/m-TOR/eNOS and p38/MAPK signaling pathway. Biomed. Pharmacother. 2018, 106, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Lee, K.Y.; Kim, J.R.; Choi, H.C. Interaction between mTOR pathway inhibition and autophagy induction attenuates adriamycin induced vascular smooth muscle cell senescence through decreased expression of p53/p21/p16. Exp. Gerontol. 2018, 109, 51–58. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Vasheghani, F.; Li, Y.H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.P.; et al. Cartilage specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1432–1440. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Lim, H.; Lim, Y.M.; Kim, K.H.; Jeon, Y.E.; Park, K.; Kim, J.; Hwang, H.Y.; Lee, D.J.; Pagire, H.; Kwon, H.J.; et al. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nat. Commun. 2018, 9, 1438. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, X.; Zhang, Q.J.; Zhu, M.; Liu, Z.P.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.A.; Sun, Y.; et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Xue, L.; Pan, Z.; Yin, Q.; Zhang, P.; Zhang, J.; Qi, W. Liraglutide promotes autophagy by regulating the AMPK/mTOR pathway in a rat remnant kidney model of chronic renal failure. Int. Urol. Nephrol. 2019, 51, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.C.; Zanella, F.; Omens, J.H.; Sheikh, F. Mechanotransduction in cardiac hypertrophy and failure. Circ. Res. 2015, 116, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Vitacolonna, A.; Bonzano, A.; Comoglio, P.; Crepaldi, T. ERK: A key player in the pathophysiology of cardiac hypertrophy. J. Mol. Sci. 2019, 20, 2164. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The transition from hypertension to heart failure: Contemporary update. JACC Heart Fail. 2017, 8, 543–551. [Google Scholar] [CrossRef]
- Hattori, A.; Kawamura, I.; Yamada, Y.; Kanamori, H.; Aoyama, T.; Ushikoshi, H.; Kawasaki, M.; Nishigaki, K.; Tamemura, G.; Minatoguchi, S. Elevated plasma GLP-1 levels and enhanced expression of cardiac GLP-1 receptors as markers of left ventricular systolic dysfunction: A cross-sectional study. BMJ 2013, 3, e003201. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Dhillon, S. Dapagliflozin: A review in type 2 diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–426. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandemark, C.; Nguyen, J.; Zhao, Z.-Q. Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update. Molecules 2023, 28, 1369. https://doi.org/10.3390/molecules28031369
Vandemark C, Nguyen J, Zhao Z-Q. Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update. Molecules. 2023; 28(3):1369. https://doi.org/10.3390/molecules28031369
Chicago/Turabian StyleVandemark, Collin, Jimmy Nguyen, and Zhi-Qing Zhao. 2023. "Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update" Molecules 28, no. 3: 1369. https://doi.org/10.3390/molecules28031369
APA StyleVandemark, C., Nguyen, J., & Zhao, Z.-Q. (2023). Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update. Molecules, 28(3), 1369. https://doi.org/10.3390/molecules28031369






