Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes—Hydrazones with Antileishmanial and Antibacterial Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrazones Bearing the Vanillin Frame
2.1.1. Synthesis and Characterization
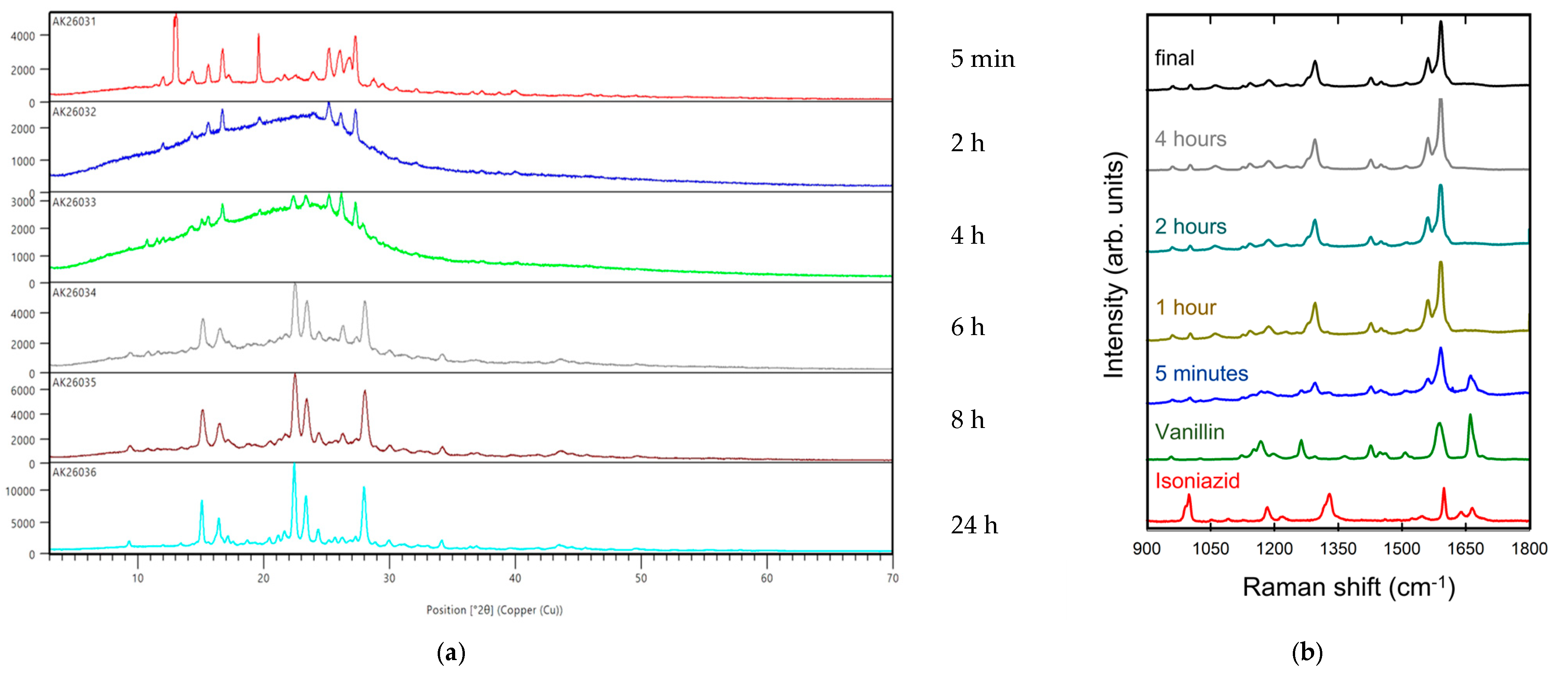
2.1.2. Aging
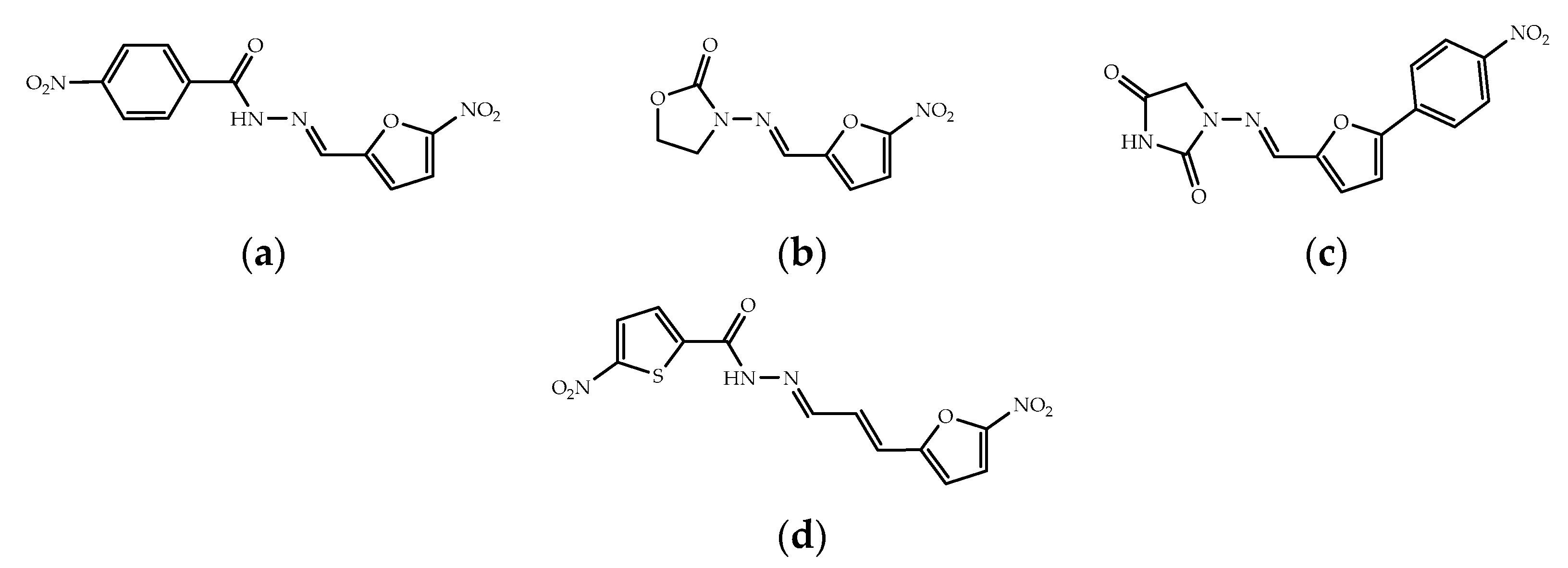
2.2. Hydrazones Bearing the 5-Nitrofuran-2-acrylaldehyde Frame
Synthesis and Characterization
2.3. Hydrazones Bearing the 5-(4-Nitrophenyl)-2-furaldehyde Frame
2.3.1. Synthesis and Characterization
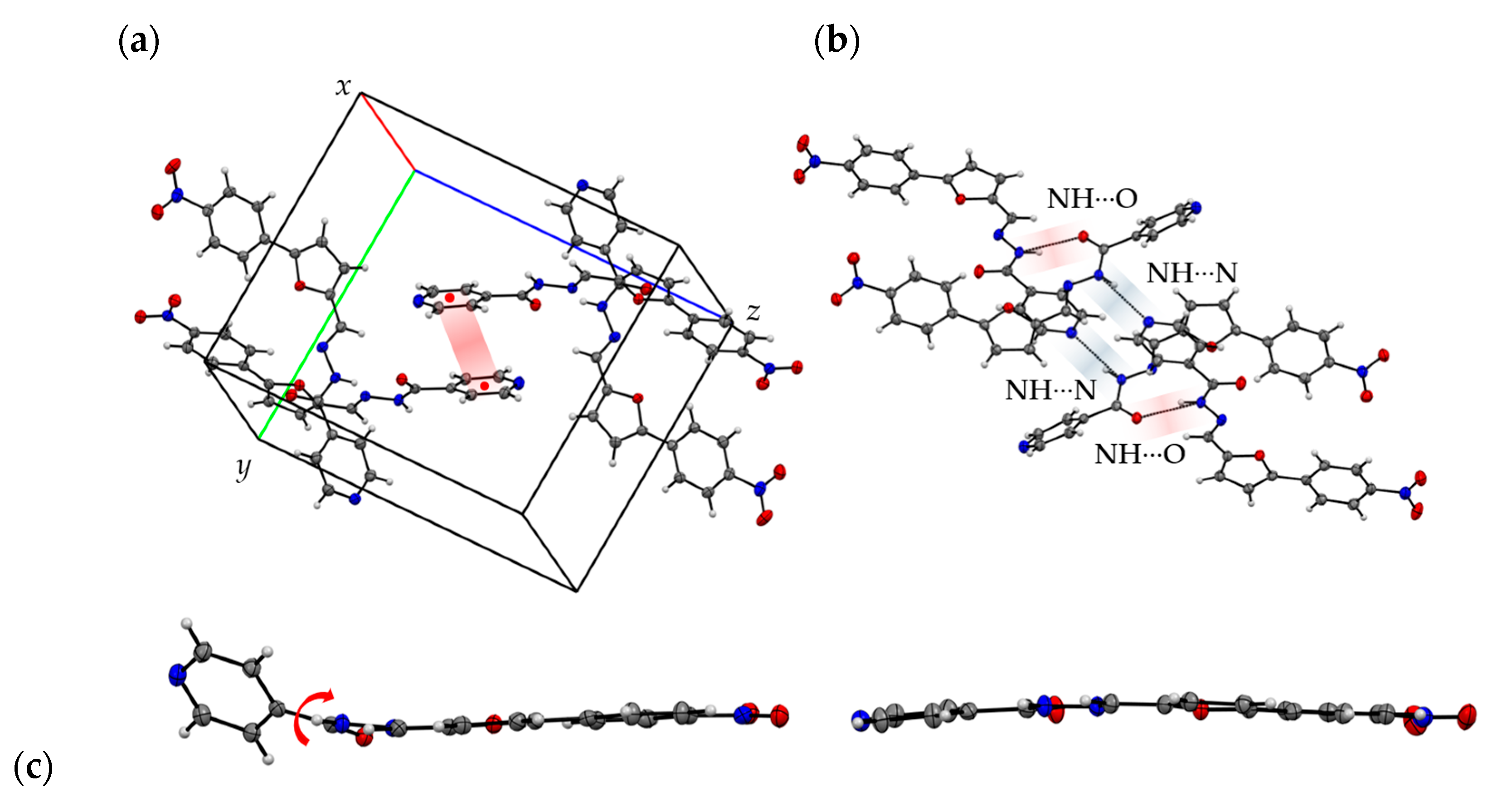
2.3.2. Compound 8: X-ray Structure
3. Biological Activities
3.1. Cytotoxicity
3.2. Activities against Leishmania donovani
3.3. Activities against Mycobacterium tuberculosis H37Rv
3.4. Activities against other Microorganisms
4. Conclusions
5. Materials and Methods
5.1. Reagents Used
5.2. NMR Analysis
5.3. Powder X-ray Diffraction (XRD) Analysis
5.4. X-ray Single Crystal
5.5. RAMAN Analysis
5.6. Fourier Transform Infrared Spectrometry (FTIR) Analysis
5.7. Mass Spectrometry Analysis
5.8. Biological Tests
5.8.1. Evaluation of Compound Cytotoxicity on RAW 264.7
5.8.2. Evaluation of the Antileishmanial Activity
5.8.3. MIC Determination for Mycobacterium tuberculosis H37Rv
5.8.4. MIC Determination for Antimicrobial Activities on S. aureus and E. coli
5.8.5. MIC Determination for Antimicrobial Activities on K. pneumoniae, A. baumannii, P. aeruginosa, and Enterococcus spp.
5.9. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Houngue, M.T.A.K.; N’bouke, M.; Atchade, B.; Doco, R.C.; Kuevi, U.A.; Kpotin, G.A.; Kpoviessi, S.D.S.; Atohoun, Y.G.S.; Badawi, M.; Mensah, J.-B. Quantum Chemical Studies of Some Hydrazone Derivatives. Comput. Chem. 2018, 06, 1–14. [Google Scholar] [CrossRef]
- Salah, B.A.; Kandil, A.T.; Abd El-Nasser, M.G. Synthesis, Characterization, Computational and Biological Activity of Novel Hydrazone Complexes. J. Radiat. Res. Appl. Sci. 2019, 12, 383–392. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Rehman, M.A.; Anwar, F.; Zain-Ul-Aabidin, H.; Akhtar, M.N.; Khan, M.U.; Braga, A.A.C.; Assiri, M.A.; Imran, M. An Experimental and Computational Exploration on the Electronic, Spectroscopic, and Reactivity Properties of Novel Halo-Functionalized Hydrazones. ACS Omega 2020, 5, 18907–18918. [Google Scholar] [CrossRef]
- Palanimuthu, D.; Wu, Z.; Jansson, P.J.; Braidy, N.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Novel Chelators Based on Adamantane-Derived Semicarbazones and Hydrazones That Target Multiple Hallmarks of Alzheimer’s Disease. Dalton Trans. 2018, 47, 7190–7205. [Google Scholar] [CrossRef]
- Anjum, R.; Palanimuthu, D.; Kalinowski, D.S.; Lewis, W.; Park, K.C.; Kovacevic, Z.; Khan, I.U.; Richardson, D.R. Synthesis, Characterization, and in Vitro Anticancer Activity of Copper and Zinc Bis(Thiosemicarbazone) Complexes. Inorg. Chem. 2019, 58, 13709–13723. [Google Scholar] [CrossRef]
- Liu, R.; Cui, J.; Ding, T.; Liu, Y.; Liang, H. Research Progress on the Biological Activities of Metal Complexes Bearing Polycyclic Aromatic Hydrazones. Molecules 2022, 27, 8393. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Hydrazide–Hydrazones as Potential Antimicrobial Agents: Overview of the Literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, J.; Milkowski, S. The Use of Hydrazones for Biomedical Applications. SLAS Technol. 2019, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, E.; Kargar, H.; Rad, N.P. A Study on Antitubercular and Antimicrobial Activity of Isoniazid Derivative. Zahedan J. Res. Med. Sci. 2015, 17. [Google Scholar] [CrossRef]
- Gil-Longo, J.; Laguna, M.D.L.R.; Verde, I.; Castro, M.E.; Orallo, F.; Fontenla, J.A.; Calleja, J.M.; Ravina, E.; Teran, C. Pyridazine Derivatives. XI: Antihypertensive Activity of 3-Hydrazinocycloheptyl[1,2-c]Pyridazine and Its Hydrazone Derivatives. J. Pharm. Sci. 1993, 82, 286–290. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone Comprising Compounds as Promising Anti-Infective Agents: Chemistry and Structure-Property Relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Singh, V.; Saharan, B.S. Design, Synthesis, Conformational and Molecular Docking Study of Some Novel Acyl Hydrazone Based Molecular Hybrids as Antimalarial and Antimicrobial Agents. Chem. Cent. J. 2017, 11, 115. [Google Scholar] [CrossRef]
- Vargas, E.; Echeverri, F.; Upegui, Y.; Robledo, S.; Quiñones, W. Hydrazone Derivatives Enhance Antileishmanial Activity of Thiochroman-4-Ones. Molecules 2017, 23, 70. [Google Scholar] [CrossRef]
- Ali, R.; Marella, A.; Alam, T.; Naz, R.; Saha, R.; Tanwar, O.; Hooda, J. Review of Biological Activities of Hydrazones. Indones. J. Pharm. 2012, 23, 193–202. [Google Scholar]
- Potůčková, E.; Hrušková, K.; Bureš, J.; Kovaříková, P.; Špirková, I.A.; Pravdíková, K.; Kolbabová, L.; Hergeselová, T.; Hašková, P.; Jansová, H.; et al. Structure-Activity Relationships of Novel Salicylaldehyde Isonicotinoyl Hydrazone (SIH) Analogs: Iron Chelation, Anti-Oxidant and Cytotoxic Properties. PLoS ONE 2014, 9, e112059. [Google Scholar] [CrossRef]
- Świątek, P.; Saczko, J.; Rembiałkowska, N.; Kulbacka, J. Synthesis of New Hydrazone Derivatives and Evaluation of Their Efficacy as Proliferation Inhibitors in Human Cancer Cells. Med. Chem. 2019, 15, 903–910. [Google Scholar] [CrossRef]
- Amato, J.; Miglietta, G.; Morigi, R.; Iaccarino, N.; Locatelli, A.; Leoni, A.; Novellino, E.; Pagano, B.; Capranico, G.; Randazzo, A. Monohydrazone Based G-Quadruplex Selective Ligands Induce DNA Damage and Genome Instability in Human Cancer Cells. J. Med. Chem. 2020, 63, 3090–3103. [Google Scholar] [CrossRef] [PubMed]
- Amariucai-Mantu, D.; Mangalagiu, V.; Bejan, I.; Aricu, A.; Mangalagiu, I.I. Hybrid azine derivatives: A useful approach for antimicrobial therapy. Pharmaceutics 2022, 14, 2026. [Google Scholar] [CrossRef]
- Younis, A.; Awad, G. Utilization of Ultrasonic as an Approach of Green Chemistry for Synthesis of Hydrazones and Bishydrazones as Potential Antimicrobial Agents. Egypt. J. Chem. 2019, 63, 599–610. [Google Scholar] [CrossRef]
- Ješelnik, M.; Varma, R.S.; Polanc, S.; Kočevar, M. Solid-State Synthesis of Heterocyclic Hydrazones Using Microwaves under Catalyst-Free ConditionsPresented, in Part, at the 5th Electronic Conference on Synthetic Organic Chemistry, (ECSOC-5), 1–30 September 2001, E0014, Http://Www.Mdpi.Org/Ecsoc-5.Htm. Green Chem. 2002, 4, 35–38. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mohammadpoor-Baltork, I.; Bigdeli, M. A Convenient and Mild Procedure for the Synthesis of Hydrazones and Semicarbazones from Aldehydes or Ketones under Solvent-Free Conditions. J. Chem. Res. 1999, 9, 570–571. [Google Scholar] [CrossRef]
- Kaupp, G.; Schmeyers, J.; Boy, J. Iminium Salts in Solid-State Syntheses Giving 100% Yield. J. Für Prakt. Chem. 2000, 342, 269–280. [Google Scholar] [CrossRef]
- Nun, P.; Martin, C.; Martinez, J.; Lamaty, F. Solvent-Free Synthesis of Hydrazones and Their Subsequent N-Alkylation in a Ball-Mill. Tetrahedron 2011, 67, 8187–8194. [Google Scholar] [CrossRef]
- Colacino, E.; Porcheddu, A.; Halasz, I.; Charnay, C.; Delogu, F.; Guerra, R.; Fullenwarth, J. Mechanochemistry for “No Solvent, No Base” Preparation of Hydantoin-Based Active Pharmaceutical Ingredients: Nitrofurantoin and Dantrolene. Green Chem. 2018, 20, 2973–2977. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Baron, M.; Chamayou, A.; André-Barrès, C.; Guidetti, B.; Baltas, M. Solvent-Free Mechanochemical Route for Green Synthesis of Pharmaceutically Attractive Phenol-Hydrazones. RSC Adv. 2014, 4, 56736–56742. [Google Scholar] [CrossRef]
- Oliveira, P.; Guidetti, B.; Chamayou, A.; André-Barrès, C.; Madacki, J.; Korduláková, J.; Mori, G.; Orena, B.; Chiarelli, L.; Pasca, M.; et al. Mechanochemical Synthesis and Biological Evaluation of Novel Isoniazid Derivatives with Potent Antitubercular Activity. Molecules 2017, 22, 1457. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, L.; André-Barrès, C.; Guidetti, B.; Chamayou, A.; Menendez, C.; Baron, M.; Calvet, R.; Baltas, M. Study of the Two Steps and One-Pot Two-Step Mechanochemical Synthesis of Annulated 1,2,4-Triazoles. ACS Sustain. Chem. Eng. 2020, 8, 3114–3125. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N. Development of new antituberculosis drugs among of 1,3- and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. Int. Ed. 2019, 68, 2172–2189. [Google Scholar] [CrossRef]
- Elsaman, T.; Mohamed, M.S.; Mohamed, M.A. Current Development of 5-Nitrofuran-2-yl Derivatives as Antitubercular Agents. Bioorganic Chem. 2019, 88, 102969. [Google Scholar] [CrossRef]
- Le, V.V.H.; Rakonjac, J. Nitrofurans: Revival of an “Old” Drug Class in the Fight against Antibiotic Resistance. PLOS Pathog. 2021, 17, e1009663. [Google Scholar] [CrossRef]
- Kargar, H.; Moghimi, A.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Munawar, K.S. New Oxovanadium and Dioxomolybdenum Complexes as Catalysts for Sulfoxidation: Experimental and Theoretical Investigations of E and Z Isomers of ONO Tridentate Schiff Base Ligand. J. Sulfur. Chem. 2022, 43, 22–36. [Google Scholar] [CrossRef]
- Huskić, I.; Lennox, C.B.; Friščić, T. Accelerated Ageing Reactions: Towards Simpler, Solvent-Free, Low Energy Chemistry. Green Chem. 2020, 22, 5881–5901. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; et al. In Vitro Activity and Mechanism of Action against the Protozoan Parasite Trypanosoma Cruzi of 5-Nitrofuryl Containing Thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef]
- Vieites, M.; Otero, L.; Santos, D.; Olea-Azar, C.; Norambuena, E.; Aguirre, G.; Cerecetto, H.; González, M.; Kemmerling, U.; Morello, A.; et al. Platinum-Based Complexes of Bioactive 3-(5-Nitrofuryl)Acroleine Thiosemicarbazones Showing Anti-Trypanosoma Cruzi Activity. J. Inorg. Biochem. 2009, 103, 411–418. [Google Scholar] [CrossRef]
- Foscolos, A.-S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Kellici, T.F.; Mavromoustakos, T.; Taylor, M.C.; Kelly, J.M. New Hydrazones of 5-Nitro-2-Furaldehyde with Adamantanealkanohydrazides: Synthesis and in Vitro Trypanocidal Activity. MedChemComm 2016, 7, 1229–1236. [Google Scholar] [CrossRef]
- Harwood, L.M.; Claridge, T.D.W. Introduction to Organic Spectroscopy; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-184907-7. [Google Scholar]
- Nikitin, K.; O’Gara, R. Mechanisms and Beyond: Elucidation of Fluxional Dynamics by Exchange NMR Spectroscopy. Chem.–Eur. J. 2019, 25, 4551–4589. [Google Scholar] [CrossRef]
- Crawford, D.E.; Porcheddu, A.; McCalmont, A.S.; Delogu, F.; James, S.L.; Colacino, E. Solvent-Free, Continuous Synthesis of Hydrazone-Based Active Pharmaceutical Ingredients by Twin-Screw Extrusion. ACS Sustain. Chem. Eng. 2020, 8, 12230–12238. [Google Scholar] [CrossRef]
- Sović, I.; Lukin, S.; Meštrović, E.; Halasz, I.; Porcheddu, A.; Delogu, F.; Ricci, P.C.; Caron, F.; Perilli, T.; Dogan, A.; et al. Mechanochemical Preparation of Active Pharmaceutical Ingredients Monitored by In Situ Raman Spectroscopy. ACS Omega 2020, 5, 28663–28672. [Google Scholar] [CrossRef] [PubMed]
- Bolognino, I.; Giangregorio, N.; Tonazzi, A.; Martínez, A.L.; Altomare, C.D.; Loza, M.I.; Sablone, S.; Cellamare, S.; Catto, M. Synthesis and Biological Evaluation of Dantrolene-Like Hydrazide and Hydrazone Analogues as Multitarget Agents for Neurodegenerative Diseases. ChemMedChem 2021, 16, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro; Version 1.171.33.66; Oxford Diffraction Ltd.: Oxford, UK, 2010.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pomel, S.; Cojean, S.; Pons, V.; Cintrat, J.-C.; Nguyen, L.; Vacus, J.; Pruvost, A.; Barbier, J.; Gillet, D.; Loiseau, P.M. An Adamantamine Derivative as a Drug Candidate for the Treatment of Visceral Leishmaniasis. J. Antimicrob. Chemother. 2021, 76, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Martin, A. A New Rapid and Simple Colorimetric Method to Detect Pyrazinamide Resistance in Mycobacterium Tuberculosis Using Nicotinamide. J. Antimicrob. Chemother. 2006, 58, 327–331. [Google Scholar] [CrossRef] [PubMed]
- CLSI. CLSI Document M100-S21; Performance Standards for Antimicrobial Susceptibility Testing, Twenty-First Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]


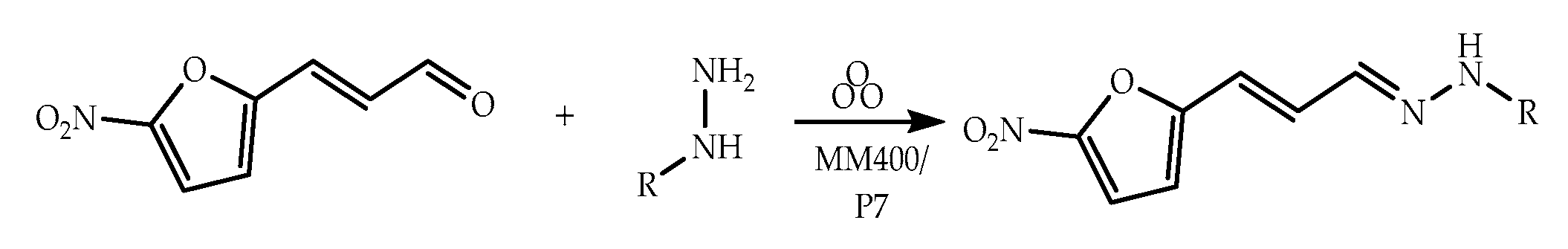

| Hydrazines (R1R2N–NH2) | MM400 | P7 | ||
|---|---|---|---|---|
| Time, Cycles × Min | Ald. Conversion (Yield), % | Time, Cycles × Min | Ald. Conversion (Yield), % | |
 | 3 × 10 | 1:1′ = 90:10 70 | 2 × 30 1 | >99 (99) |
| 2 × 30 | 1:1′ = 90:10 90 | |||
| 3 × 30 | 1:1′ = 90:10 >99 (99) | |||
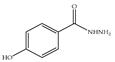 | 3 × 30 | 2 >84 | 3 × 30 2 | >99 (99) |
| Hydrazines (R1R2N–NH2) | MM400 | P7 | ||
|---|---|---|---|---|
| Time, Cycles × Min | Ald. Conversion (Yield), % | Time, Cycles × Min | Ald. Conversion (Yield), % | |
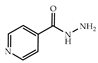 | 3 × 30 | 3 78 (70) | 6 × 30 | >99 (98) |
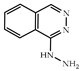 | 3 × 30 | 41 >99 (99) | 3 × 30 | >99 (99) |
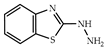 | 3 × 30 | 5 >99 (99) | 3 × 30 | >99 (99) |
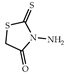 | 3 × 30 | 6:6′ = 90:10 75 (63) | 6 × 30 | >99 (97) |
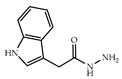 | 3 × 30 | 7:7′ = 52:48 >99 (99) | 6 × 30 | >99 (99) |
| Hydrazines (R1R2N–NH2) | MM400 | P7 | ||
|---|---|---|---|---|
| Time, Cycles × Min | Ald. Conversion (Yield), % | Time, Cycles × Min | Ald. Conversion (Yield), % | |
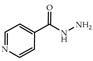 | 3 × 30 | 8 >98 (98) | 3 × 30 | >99 (99) |
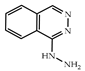 | 3 × 30 | 91 99 (85) | 3 × 30 | >99 (91) |
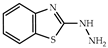 | 3 × 30 | 10 95 (84) | 3 × 30 | >99 (92) |
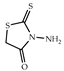 | 3 × 30 | 11 8 | 3 × 30 | 37 |
| 6 × 30 | 97 (93) | |||
 | 3 × 30 | 12:12′ = 50:50 95 (70) | 6 × 30 | >99 (98) |
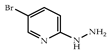 | 3 × 30 | 13:13′ = 82:18 99 (95) | 3 × 30 | >99 (95) |
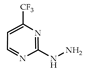 | 3 × 30 | 14:14′ = 78:22 60 (47) | 6 × 30 | >99 (90) |
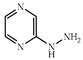 | 3 × 30 | 15:15′ = 70:30 80 (60) | 6 × 30 | >97 (95) |
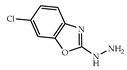 | 3 × 30 | 16 90 (54) | 6 × 30 | >99 (95) |
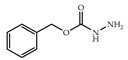 | 3 × 30 | 17:17′ = 84:16 40 (35) | 6 × 30 | >98 (97) |
| Formula, fw, g/mol | C17H12N4O4, 336.31 g/mol | ||||
|---|---|---|---|---|---|
| temperature, K | 100(2) | 150(2) | 200(2) | 250(2) | 300(2) |
| crystal size, mm | 0.1 × 0.05 × 0.02 | 0.1 × 0.05 × 0.02 | 0.1 × 0.05 × 0.02 | 0.1 × 0.05× 0.02 | 0.1 × 0.05 × 0.02 |
| crystal color | orange | orange | orange | orange | orange |
| crystal system | triclinic | triclinic | triclinic | triclinic | triclinic |
| space group, Z | P, 4 | P, 4 | P, 4 | P, 4 | P, 4 |
| a, Å | 8.6900(1) | 8.7108(2) | 8.7423(1) | 8.7685(1) | 8.7970(1) |
| b, Å | 12.7764(1) | 12.8057(2) | 12.8394(1) | 12.8688(1) | 12.9010(2) |
| c, Å | 14.5517(2) | 14.5707(3) | 14.5736(2) | 14.5959(1) | 14.6224(2) |
| α, ° | 94.261(1) | 94.255(2) | 94.210(1) | 94.170(1) | 108.031(2) |
| β, ° | 107.297(1) | 107.188(2) | 107.000(1) | 106.818(1) | 100.416(2) |
| γ, ° | 98.283(1) | 98.309(2) | 98.305(2) | 98.309(1) | 107.389(2) |
| V, Å3 | 1514.70(3) | 1524.65(6) | 1536.34(3) | 1548.56(3) | 1562.09(4) |
| ρ, g/cm3 | 1.475 | 1.465 | 1.454 | 1.460 | 1.447 |
| µ, mm–1 | 0.910 | 0.904 | 0.897 | 0.890 | 0.883 |
| F (000) | 696 | 696 | 696 | 696 | 696 |
| θ max, ° | 77.528 | 77.241 | 77.549 | 77.497 | 77.556 |
| limiting indices | −10⇒h⇒8 | −11⇒h⇒8 | −11⇒h⇒8 | −11⇒h⇒8 | −11 ⇒ h⇒9 |
| −16⇒k⇒16 | −16⇒k⇒16 | −16⇒k⇒16 | −16⇒k⇒16 | −16⇒ k⇒16 | |
| −16⇒l⇒18 | −16⇒l⇒18 | −16⇒l⇒18 | −16⇒l⇒18 | −16⇒l⇒18 | |
| reflns collected | 6124 | 6151 | 6221 | 6260 | 6303 |
| Rint | 0.0240 | 0.0238 | 0.0241 | 0.0237 | 0.0363 |
| data/parameters | 5467/451 | 5373/451 | 5295/451 | 5178/451 | 4974/451 |
| GOF on F2 | 1.083 | 1.082 | 1.072 | 1.054 | 1.076 |
| R1 [I > 2σ(I)] | 0.0365 | 0.0369 | 0.0380 | 0.0381 | 0.0393 |
| R1 (all data) | 0.0411 | 0.0423 | 0.0452 | 0.0467 | 0.0537 |
| wR2 (all data) | 0.1005 | 0.1013 | 0.1026 | 0.1018 | 0.1031 |
| completeness to theta max | 95% | 95.1% | 95.1% | 95% | 94.7% |
| lrgst diff peak, e/Å3 | 0.23 | 0.20 | 0.21 | 0.20 | 0.17 |
| lrgst diff hole, e/Å3 | −0.31 | −0.27 | −0.30 | −0.28 | −0.24 |
| T (K) | π-π Stacking (Centroid- Centroid Distance, Å) | π-π Stacking (Centroid- Centroid Angle, °) | N8A-O25 Hydrogen Bond Distance (Å) | H8A-O25 Hydrogen Bond Distance (Å) | N8-N13 Hydrogen Bond Distance (Å) | H8-N13 Hydrogen Bond Distance (Å) | Torsion Angles N8-C9-C10-C15 (°) |
|---|---|---|---|---|---|---|---|
| 100 | 3.817 | 67.76 | 2.8661(14) | 2.0374 | 2.9019(16) | 2.1483 | 38.31(17) |
| 150 | 3.835 | 68.15 | 2.8727(14) | 2.0444 | 2.9169(16) | 2.1596 | 38.19(17) |
| 200 | 3.852 | 68.63 | 2.8781(14) | 2.0507 | 2.9253(16) | 2.1713 | 37.94(18) |
| 250 | 3.874 | 69.1 | 2.8855(14) | 2.0583 | 2.9333(16) | 2.1818 | 37.74(17) |
| 300 | 3.897 | 39.73 | 2.8935(15) | 2.0657 | 2.9451(17) | 2.1962 | 37.34(19) |
| Compound | L. donovani Axenic Amastigote | L. donovani Intramacrophage Amastigote | SI * (Selectivity Index) | M. tuberculosis H37Rv | S. aureus ATCC25923 and (ATCC29213) | E. coli ATCC25922 |
|---|---|---|---|---|---|---|
| IC50 ± SD (µM) | IC50 ± SD (µM) | MIC (µM) | MIC (µM) | MIC (µM) | ||
| 1 | 24.9 ± 2.9 | 40.2 ± 3.1 | >2.5 | 0.6 | >944 | >944 |
| 2 | 2.1 ± 0.1 | 5.9 ± 0.9 | >17 | >100 | >894 | >894 |
| 3 | 0.6 ± 0.1 | 0.5 ± 0.1 | >200 | 1.0 | 1.75 (7) | 28 |
| 4 | 1.9 ± 0.3 | 0.6 ± 0.1 | >166 | 129 | >828 | >828 |
| 5 | 2.3 ± 0.3 | 12.4 ± 1.2 | >8 | 1.0 | >814 | >814 |
| 6 | 0.7 ± 0.1 | 0.8 ± 0.1 | >125 | 4.2 | 13 (7) | >861 |
| 7 | 0.4 ± 0.1 | 0.8 ± 0.2 | >142 | 7.4 | 0.7 (3) | >757 (47) |
| 8 | 16.2 ± 1.2 | 36.2 ± 1.4 | >2 | 0.9 | >761 | >761 |
| 9 | 0.2 ± 0.1 | 0.3 ± 0.1 | >333 | >100 | >711 | >711 |
| 10 | 0.7 ± 0.1 | 5.8 ± 1.0 | >2 | >100 | >703 | >703 |
| 11 | 5.2 ± 0.3 | >100 | ND | >100 | >737 | >737 |
| 12 | 2.7 ± 0.7 | 20.5 ± 2.1 | >5 | 52 | >659 | >659 |
| 13 | 54.3 ± 4.5 | >100 | ND | >100 | >661 | >661 |
| 14 | 2.9 ± 0.2 | 11.9 ± 1.1 | >8 | >100 | >669 | >669 |
| 15 | 9.9 ± 0.8 | 23.3 ± 3.1 | >4 | >100 | >678 | >678 |
| 16 | >100 | >100 | ND | >100 | >828 | >828 |
| 17 | 12.2 ± 1.6 | 32.4 ± 2.8 | >3 | >100 | >701 | >701 |
| Reference | 1.5 ± 0.3 a | 2.2 ± 0.3 | 9 a | 0.4 b; 0.4 c | 3 (5) d | 0.75 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusterynska, A.; Bijani, C.; Paliwoda, D.; Vendier, L.; Bourdon, V.; Imbert, N.; Cojean, S.; Loiseau, P.M.; Recchia, D.; Scoffone, V.C.; et al. Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes—Hydrazones with Antileishmanial and Antibacterial Activities. Molecules 2023, 28, 5284. https://doi.org/10.3390/molecules28135284
Kapusterynska A, Bijani C, Paliwoda D, Vendier L, Bourdon V, Imbert N, Cojean S, Loiseau PM, Recchia D, Scoffone VC, et al. Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes—Hydrazones with Antileishmanial and Antibacterial Activities. Molecules. 2023; 28(13):5284. https://doi.org/10.3390/molecules28135284
Chicago/Turabian StyleKapusterynska, Anna, Christian Bijani, Damian Paliwoda, Laure Vendier, Valérie Bourdon, Nicolas Imbert, Sandrine Cojean, Philippe Marie Loiseau, Deborah Recchia, Viola Camilla Scoffone, and et al. 2023. "Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes—Hydrazones with Antileishmanial and Antibacterial Activities" Molecules 28, no. 13: 5284. https://doi.org/10.3390/molecules28135284
APA StyleKapusterynska, A., Bijani, C., Paliwoda, D., Vendier, L., Bourdon, V., Imbert, N., Cojean, S., Loiseau, P. M., Recchia, D., Scoffone, V. C., Degiacomi, G., Akhir, A., Saxena, D., Chopra, S., Lubenets, V., & Baltas, M. (2023). Mechanochemical Studies on Coupling of Hydrazines and Hydrazine Amides with Phenolic and Furanyl Aldehydes—Hydrazones with Antileishmanial and Antibacterial Activities. Molecules, 28(13), 5284. https://doi.org/10.3390/molecules28135284









