In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Grape Pomace
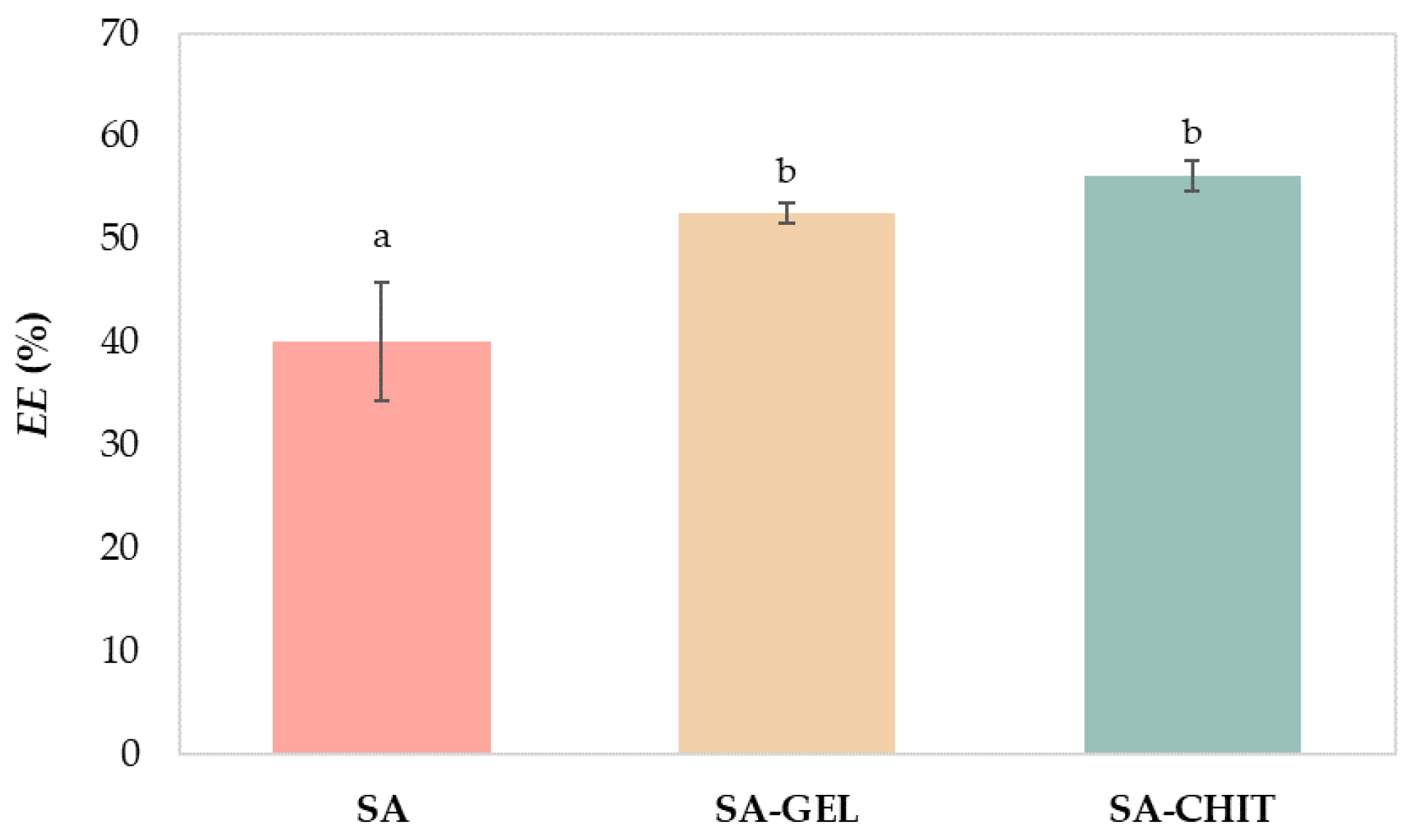
2.2. Encapsulation Efficiency of Grape Pomace Extract
2.3. Physicochemical Characterization of Microbeads
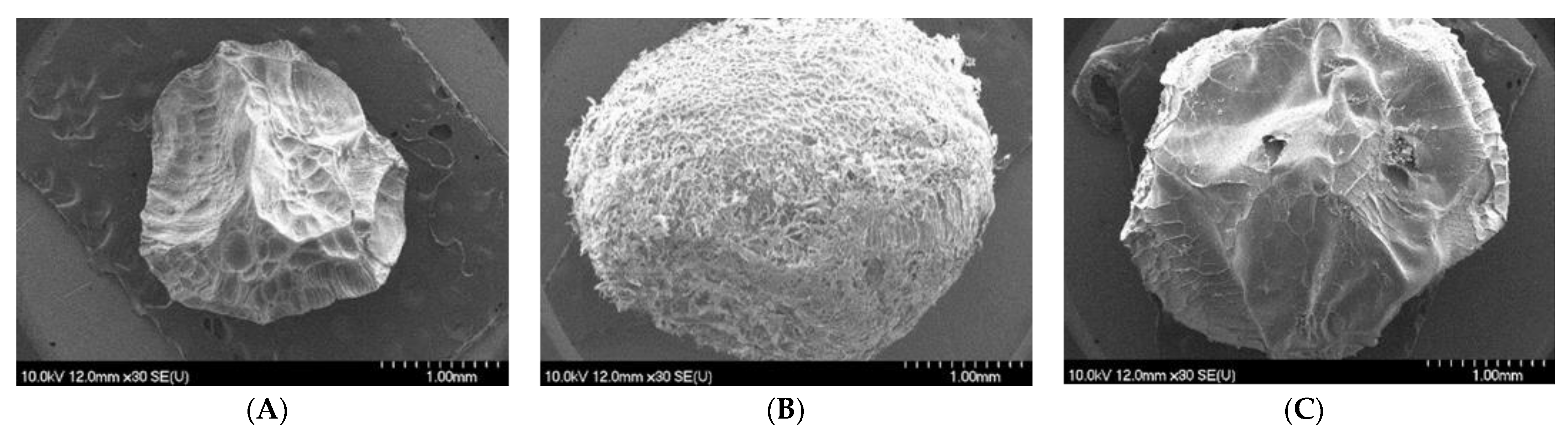
2.3.1. Geometric Parameters, Texture, and Morphology of Microbeads
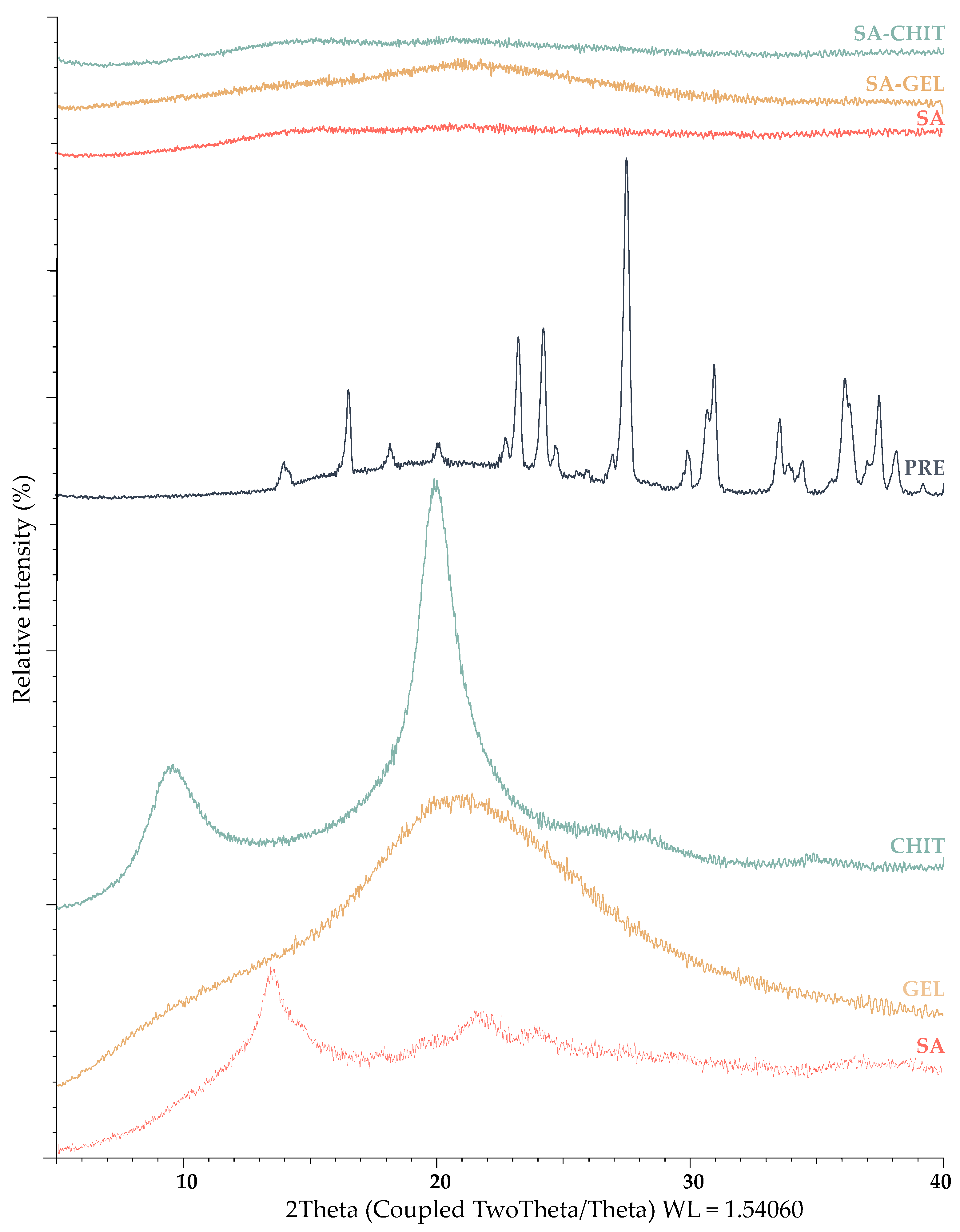
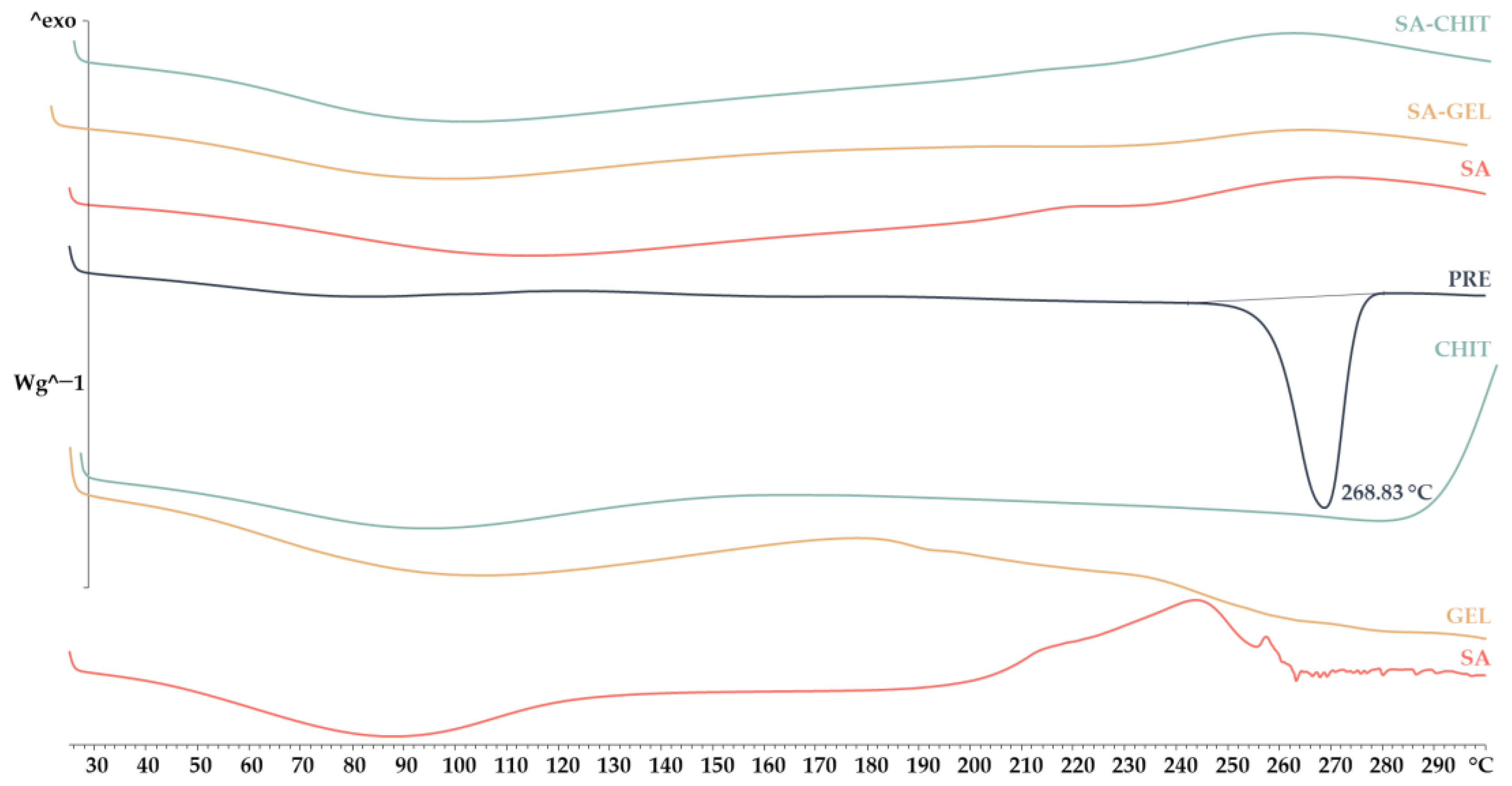
2.3.2. X-ray Powder Diffraction and Differential Scanning Calorimetry Analyses
2.4. Phenolic Content of Grape Pomace Extract before In Vitro Digestion
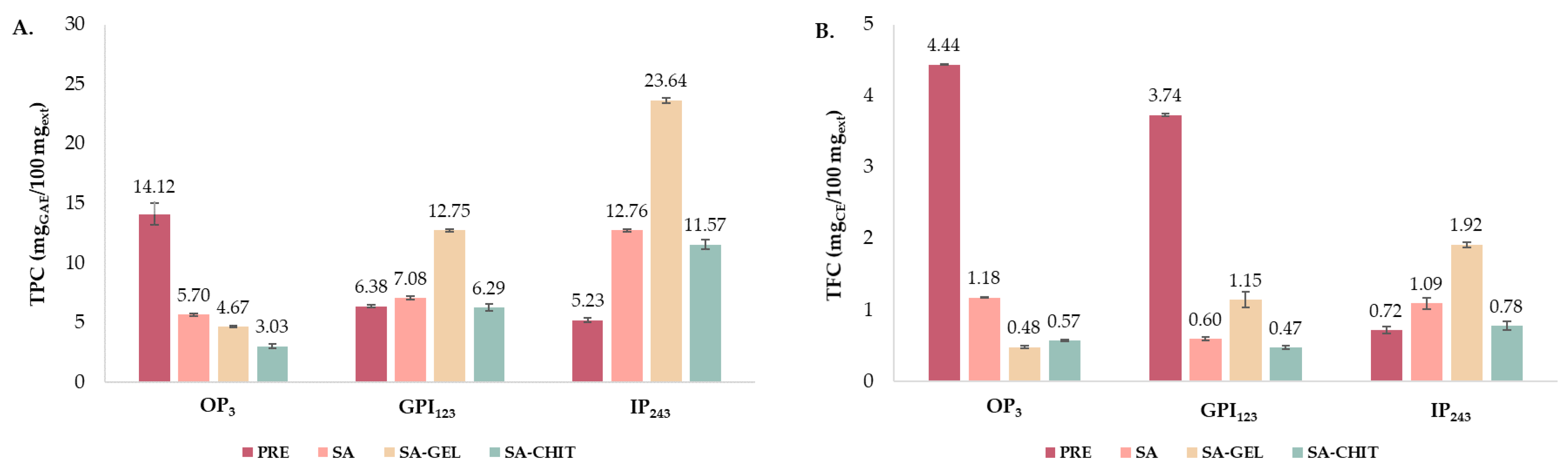
2.4.1. Total Phenolic Compounds
2.4.2. Individual Phenolic Compounds
2.5. In Vitro Simulated Digestion and Bioaccessibility Index of Phenolic Compounds
2.5.1. Total Phenolic Compounds
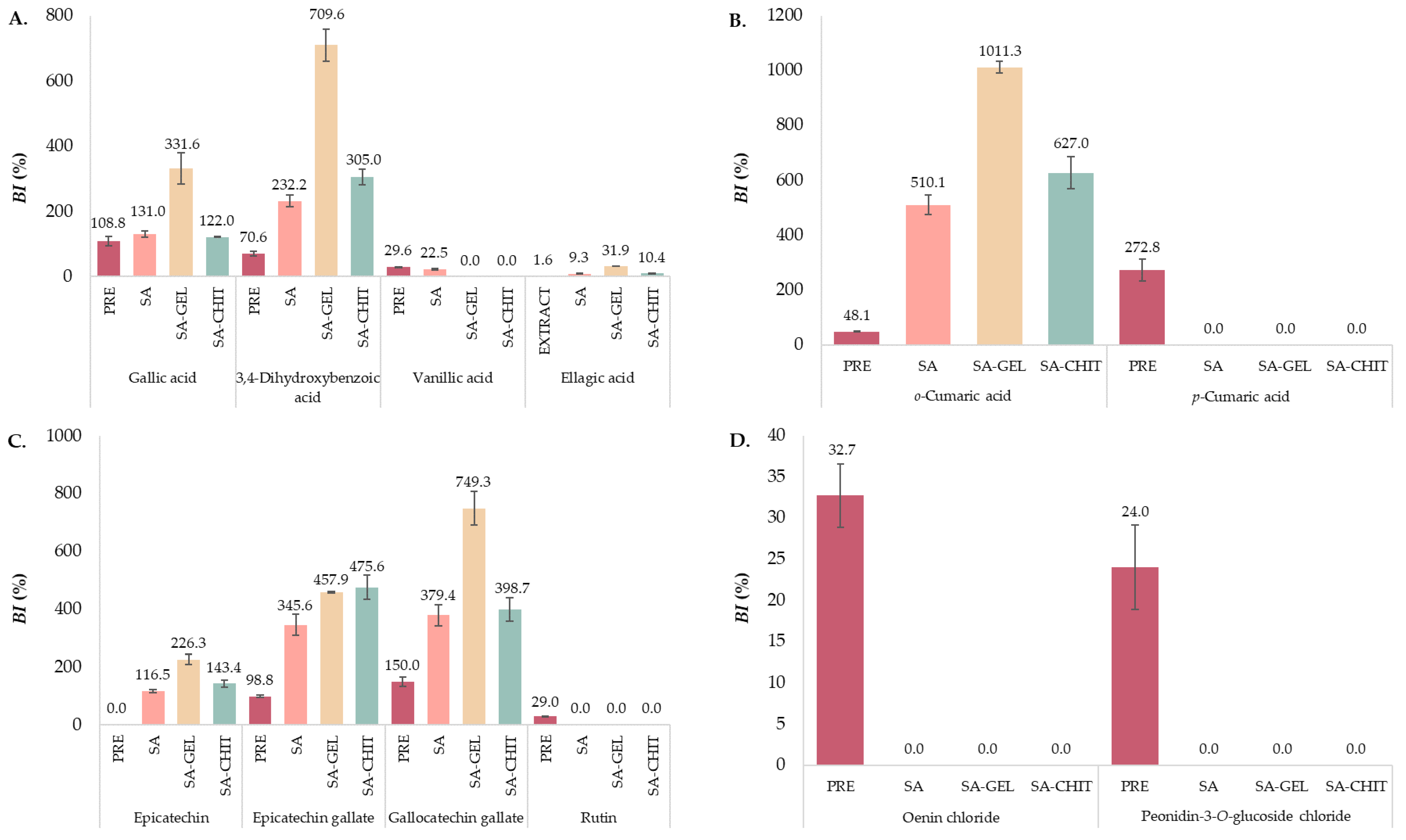
2.5.2. Individual Phenolic Compounds
Phenolic Acids
Flavan-3-ols
Flavonols
Anthocyanins
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Grape Pomace Sample
3.3. Chemical Composition of Grape Pomace
3.4. Grape Pomace Extract Preparation
3.5. Determination of Total Phenolic Compounds
3.6. Determination of Individual Phenolic Compounds
3.7. Encapsulation by the Ionic Gelation Method
3.8. Microbead Characterization
3.8.1. Geometric Parameters of Microbeads
3.8.2. Scanning Electron Microscopy (SEM)
3.8.3. Texture Analysis of Microbeads
3.8.4. X-ray Powder Diffraction (XPRD)
3.8.5. Differential Scanning Calorimetry (DSC)
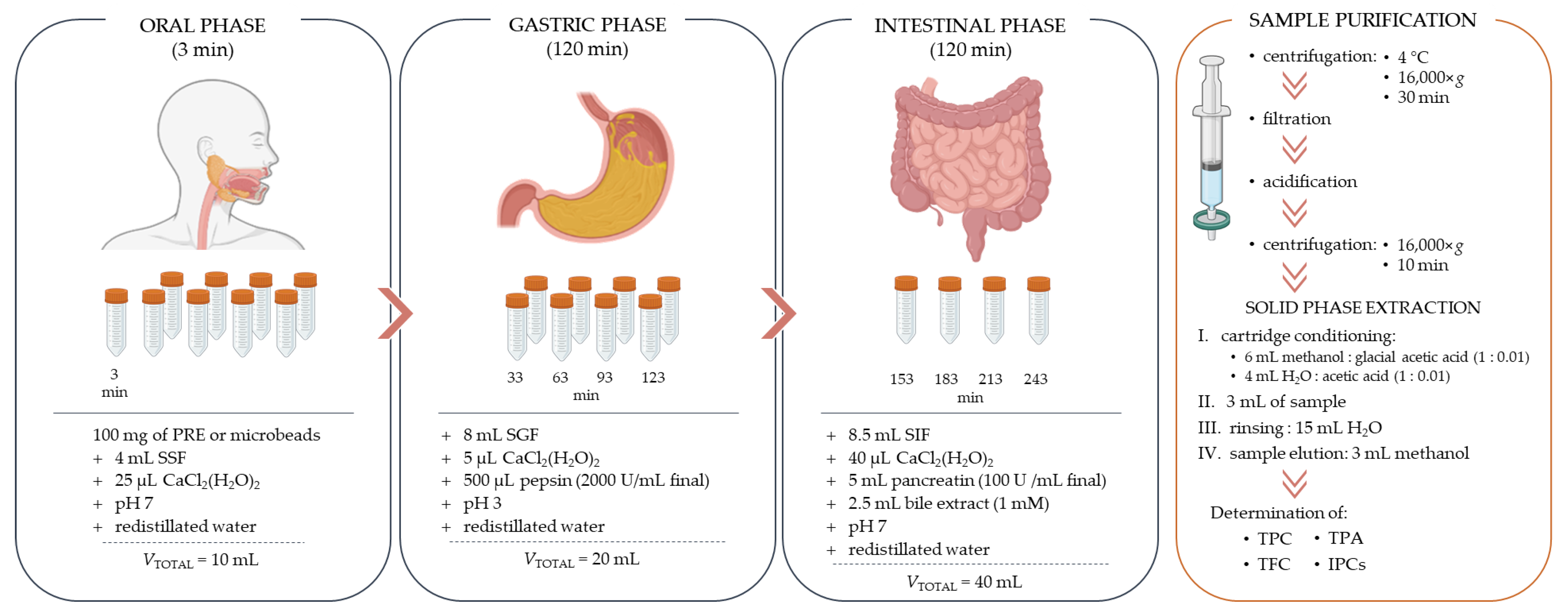
3.9. In Vitro Simulated Digestion and Bioaccessibility Index (BI)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šelo, G.; Planinić, M.; Tišma, M.; Martinović, J.; Perković, G.; Bucić-Kojić, A. Bioconversion of grape pomace with Rhizopus Oryzae under solid-state conditions: Changes in the chemical composition and profile of phenolic compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical characterization and evaluation of gastrointestinal in vitro behavior of alginate-based microbeads with encapsulated grape pomace extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Tišma, M.; Šelo, G.; Grgić, J.; Perković, G.; Planinić, M. Winery production residues as feedstocks within the biorefinery concept. Eng. Power Bull. Croat. Acad. Eng. 2022, 17, 11–17. [Google Scholar]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Dutra, R.L.T.; Dantas, A.M.; Marques, D.d.A.; Batista, J.D.F.; Meireles, B.R.L.d.A.; de Magalhães Cordeiro, Â.M.T.; Magnani, M.; Borges, G.d.S.C. Bioaccessibility and antioxidant activity of phenolic compounds in frozen pulps of brazilian exotic fruits exposed to simulated gastrointestinal conditions. Food Res. Int. 2017, 100, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Celep, E.; İnan, Y.; Akyüz, S.; Yesilada, E. The bioaccessible phenolic profile and antioxidant potential of Hypericum perfoliatum L. after simulated human digestion. Ind. Crops Prod. 2017, 109, 717–723. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galankis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 295–323. ISBN 978-0-12-813572-3. [Google Scholar]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Josef, E.; Zilberman, M.; Bianco-Peled, H. Composite alginate hydrogels: An innovative approach for the controlled release of hydrophobic drugs. Acta Biomater. 2010, 6, 4642–4649. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Bitton, R.; Ben-Yehuda, M.; Davidovich, M.; Balazs, Y.; Potin, P.; Delage, L.; Colin, C.; Bianco-Peled, H. Structure of algal-born phenolic polymeric adhesives. Macromol. Biosci. 2006, 6, 737–746. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Mi, F.-L.; Tan, Y.-C.; Liang, H.-F.; Sung, H.-W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S.; de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ryu, B.; Vo, T.-S.; Himaya, S.W.A.; Wijesekara, I.; Kim, S.-K. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ramezanzade, L.; Nikkhah, M. Nano-liposomal entrapment of bioactive peptidic fraction from fish gelatin hydrolysate. Int. J. Biol. Macromol. 2017, 105, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 2019, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from grape pomace an enzymatic extract with anti-inflammatory properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, C.; Gallardo, B.; Mantecón, Á.R.; del Álamo-Sanza, M.; Manso, T. Evaluation of grape pomace from red wine by-product as feed for sheep: Evaluation of grape pomace as feed for sheep. J. Sci. Food Agric. 2017, 97, 1885–1893. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Muchenje, V.; Nolte, J.E.; Dugan, M.E.R.; Mapiye, C. Grape pomace (Vitis vinifera L. cv. Pinotage) supplementation in lamb diets: Effects on growth performance, carcass and meat quality. Meat Sci. 2019, 147, 6–12. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Raffrenato, E.; Muchenje, V.; Musarurwa, H.T.; Mapiye, C. Varietal differences in nutrient, amino acid and mineral composition and in vitro rumen digestibility of grape (Vitis vinifera) pomace from the Cape Winelands vineyards in South Africa and impact of preservation techniques. Ind. Crops Prod. 2018, 118, 30–37. [Google Scholar] [CrossRef]
- Martínez Salgado, M.M.; Ortega Blu, R.; Janssens, M.; Fincheira, P. Grape pomace compost as a source of organic matter: Evolution of quality parameters to evaluate maturity and stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]
- Zhu, F.-M.; Du, B.; Li, J. Effect of ultrafine grinding on physicochemical and antioxidant properties of dietary fiber from wine grape pomace. Food Sci. Technol. Int. 2014, 20, 55–62. [Google Scholar] [CrossRef]
- Touriño, S.; Fuguet, E.; Vinardell, M.P.; Cascante, M.; Torres, J.L. Phenolic metabolites of grape antioxidant dietary fiber in rat urine. J. Agric. Food Chem. 2009, 57, 11418–11426. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Lizárraga, D.; Miranda, A.; Vinardell, M.P.; García-García, F.; Dopazo, J.; Torres, J.L.; Saura-Calixto, F.; Capellà, G.; Cascante, M. Grape antioxidant dietary fiber inhibits intestinal polyposis in Apc Min/+ mice: Relation to cell cycle and immune response. Carcinogenesis 2013, 34, 1881–1888. [Google Scholar] [CrossRef]
- Martín-Carrón, N.; Goñi, I.; Larrauri, J.A.; García-Alonso, A.; Saura-Calixto, F. Reduction in serum total and LDL cholesterol concentrations by a dietary fiber and polyphenol-rich grape product in hypercholesterolemic rats. Nutr. Res. 1999, 19, 1371–1381. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of insoluble fibers in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef]
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakić, V.; Nedović, V.; et al. Calcium-alginate-inulin microbeads as carriers for aqueous carqueja extract. J. Food Sci. 2016, 81, E65–E75. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.d.R.; Abud-Archila, M. Spray drying encapsulation of a native plant extract rich in phenolic compounds with combinations of maltodextrin and non-conventional wall materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, H.; Zannou, O.; Koca, I.; Alamri, A.S.; Galanakis, C.M. Optimization and encapsulation of phenolic compounds from the tea of maize husk using maltodextrin and different drying techniques. J. Food Process. Preserv. 2022, 46, e16858. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Stoica, R.; Pop, S.F.; Ion, R.M. Evaluation of natural polyphenols entrapped in calcium alginate beads prepared by the ionotropic gelation method. J. Optoelectron. Adv. Mater. 2013, 15, 893–898. [Google Scholar]
- Deladino, L.; Anbinder, P.S.; Navarro, A.S.; Martino, M.N. Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2008, 71, 126–134. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of encapsulates and their relations with encapsulation efficiency and controlled release of bioactive constituents: A review. Comp. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Mutalik, S.P.; Pandey, A.; Mutalik, S. Nanoarchitectronics: A versatile tool for deciphering nanoparticle interaction with cellular proteins, nucleic acids and phospholipids at biological interfaces. Int. J. Biol. Macromol. 2020, 151, 136–158. [Google Scholar] [CrossRef]
- Costa, C.; Anselmo, H.; Ferro, R.; Matos, A.S.; Casimiro, T.; Aguiar-Ricardo, A. Dry dosage forms of add-value bioactive phenolic compounds by supercritical CO2-assisted spray-drying. Molecules 2022, 27, 2001. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In vitro drug release, permeability, and structural test of ciprofloxacin-loaded nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In vitro and in vivo evaluation of the antioxidant and prooxidant activity of phenolic compounds obtained from grape (Vitis vinifera) pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, A.; Srivastava, S.; Rawat, A.K.S.; Khan, A.R. HPTLC-densitometric determination and kinetic studies on antioxidant potential of monomeric phenolic acids (MPAs) from Bergenia species. RSC Adv. 2014, 4, 52647–52657. [Google Scholar] [CrossRef]
- Ramachandran, V.; Raja, B. Protective effects of syringic acid against acetaminophen-induced hepatic damage in albino rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic acid extracted from Herba Dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid. Based Complement. Altern. Med. 2012, 2012, 426537. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.-Y. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.; Aras, A.B.; Topaloğlu, N.; Özkan, A.; Şen, H.M.; Kalkan, Y.; Okuyucu, A.; Akbal, A.; Gökmen, F.; Coşar, M. The protective effect of syringic acid on ischemia injury in rat brain. Turk. J. Med. Sci. 2015, 45, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yunhai, L.; Jianguo, F.; Ting, L.; Wenqing, W.; Aihua, L. Anti-endotoxic effects of syringic acid of Radix Isatidis. J. Huazhong Univ. Sci. 2003, 23, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, C.; Li, W.; Adu-Frimpong, M.; Wang, Q.; Yu, J.; Xu, X. Preparation and characterization of syringic acid–loaded TPGS liposome with enhanced oral bioavailability and in vivo antioxidant efficiency. AAPS PharmSciTech 2019, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Kowczyk-Sadowy, M.; Świsłocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H- and 13C-NMR), theoretical and microbiological study of trans o-coumaric acid and alkali metal o-coumarates. Molecules 2015, 20, 3146–3169. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Oniszczuk, A. LC-ESI-MS/MS analysis and extraction method of phenolic acids from gluten-free precooked buckwheat pasta. Food Anal. Methods 2016, 9, 3063–3068. [Google Scholar] [CrossRef]
- Anderson, J.W. Whole grains and coronary heart disease: The whole kernel of truth. Am. J. Clin. Nutr. 2004, 80, 1459–1460. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C. Dietary intake of antioxidant nutrients and the risk of incident alzheimer disease in a biracial community study. J. Am. Med. Assoc. 2002, 287, 3230. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Heptinstall, S.; May, J.; Fox, S.; Kwik-Uribe, C.; Zhao, L. Cocoa flavanols and platelet and leukocyte function: Recent in vitro and ex vivo studies in healthy adults. J. Cardiovasc. Pharmacol. 2006, 47, S197–S205. [Google Scholar] [CrossRef]
- Xu, Z.; Du, P.; Meiser, P.; Jacob, C. Proanthocyanidins: Oligomeric structures with unique biochemical properties and great therapeutic promise. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Beecher, G.R. Proanthocyanidins: Biological activities associated with human health. Pharm. Biol. 2004, 42, 2–20. [Google Scholar] [CrossRef]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef]
- Shen, D.; Feng, Y.; Zhang, X.; Gong, L.; Liu, J.; Li, Y.; Liao, H. Antiosteoporosis studies of 20 medicine food homology plants containing quercetin, rutin, and kaempferol: TCM characteristics, in vivo and in vitro activities, potential mechanisms, and food functions. Evid.-Based Complement. Altern. Med. 2022, 2022, 5902293. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape pomace valorization by extraction of phenolic polymeric pigments: A review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals: Anthocyanin-containing extracts as inhibitors of pancreatic lipase. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting cardiovascular diseases by flavonols: An update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.d.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from nature: A phytochemistry and biological activity review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Aguilera-Chávez, S.L.; Gallardo-Velázquez, T.; Meza-Márquez, O.G.; Osorio-Revilla, G. Spray drying and spout-fluid bed drying microencapsulation of mexican plum fruit (Spondias purpurea L.) extract and its effect on in vitro gastrointestinal bioaccessibility. Appl. Sci. 2022, 12, 2213. [Google Scholar] [CrossRef]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: Effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J. Food Eng. 2018, 223, 91–98. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable concentrations of delphinidin and its metabolite, gallic acid, induce antioxidant protection associated with increased intracellular glutathione in cultured endothelial cells. Oxid. Med. Cell. Longev. 2017, 2017, 9260701. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Rodrigo-García, J.; Martínez-Ruiz, N.; Cárdenas-Robles, A.; Mendoza-Díaz, S.; Álvarez-Parrilla, E.; González-Aguilar, G.; de la Rosa, L.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- de Morais, J.S.; Sant’Ana, A.S.; Dantas, A.M.; Silva, B.S.; Lima, M.S.; Borges, G.C.; Magnani, M. Antioxidant activity and bioaccessibility of phenolic compounds in white, red, blue, purple, yellow and orange edible flowers through a simulated intestinal barrier. Food Res. Int. 2020, 131, 109046. [Google Scholar] [CrossRef]
- de Lima Oliveira, P.M.; Dantas, A.M.; dos Santos Morais, A.R.; Gibbert, L.; Krüger, C.C.H.; dos Santos Lima, M.; Magnani, M.; da Silva Campelo Borges, G. Juá fruit (Ziziphus joazeiro) from Caatinga: A source of dietary fiber and bioaccessible flavanols. Food Res. Int. 2020, 129, 108745. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Hribar, U.; Ulrih, N.P. The metabolism of anthocyanins. Curr. Drug. Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of phenolic acid and enzyme production from corn silage biologically treated by Trametes versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef] [PubMed]
- ImageJ User Guide IJ 1.46r. Available online: https://imagej.nih.gov/ij/docs/guide/146.html (accessed on 22 March 2023).
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: An in vitro digestion study with a standardized static model. LWT-Food Sci. Technol. 2017, 77, 475–481. [Google Scholar] [CrossRef]


| Component | Content (Mean Value ± SD) |
|---|---|
| Dry matter (%) | 91.59 ± 0.02 |
| Crude proteins (%db) | 8.72 ± 0.26 |
| Free fats (%db) | 8.54 ± 0.37 |
| Glucose (mg/gdb) | 4.38 ± 0.13 |
| Arabinose (mg/gdb) | 1.50 ± 0.19 |
| Sucrose (mg/gdb) | 9.87 ± 0.17 |
| Fructose (mg/gdb) | 4.94 ± 0.04 |
| Ash (%db) | 7.16 ± 0.15 |
| NDF (%db) | 47.24 ± 0.56 |
| ADF (%db) | 37.60 ± 0.36 |
| ADL (lignin) (%db) | 23.77 ± 0.26 |
| Hemicellulose (%db) | 9.64 ± 0.55 |
| Cellulose (%db) | 13.82 ± 0.62 |
| TOC (mg/gdb) | 59.22 ± 0.49 |
| TOCLE (mg/gdb) | 53.32 ± 1.13 |
| TN (mg/gdb) | 0.96 ± 0.03 |
| Sample | Size Parameters | Shape Parameters | Texture | |||||
|---|---|---|---|---|---|---|---|---|
| Area (mm2) | Perimeter (mm) | FeretMAX (mm) | FeretMIN (mm) | Circularity (-) | Roundness (-) | Solidity (-) | Hardness (N) | |
| SA | 6.62 ± 1.72 a | 10.25 ± 1.26 a | 3.39 ± 0.38 a | 2.68 ± 0.36 a | 0.79 ± 0.06 b | 0.80 ± 0.08 a | 0.96 ± 0.02 b | 1.36 ± 0.66 b |
| SA-GEL | 11.18 ± 0.96 c | 13.79 ± 0.86 c | 4.24 ± 0.27 b | 3.53 ± 0.18 c | 0.74 ± 0.07 a | 0.86 ± 0.08 b | 0.97 ± 0.01 c | 0.49 ± 0.20 a |
| SA-CHIT | 7.63 ± 2.71 b | 11.37 ± 2.37 b | 3.51 ± 0.64 a | 2.90 ± 0.49 b | 0.72 ± 0.07 a | 0.86 ± 0.06 b | 0.94 ± 0.02 a | 0.56 ± 0.26 a |
| Component | Sample | Before Digestion | Oral Phase | Gastric Phase | Intestinal Phase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | OP3 | GP33 | GP63 | GP93 | GP123 | IP153 | IP183 | IP213 | IP243 | ||
| Phenolic acids (μg/100 mgext) | |||||||||||
| Gallic acid | PRE | 130.00 ± 10.98 | 2.09 ± 0.06 | - | - | - | 1.19 ±0.17 | - | - | - | 141.38 ± 6.37 |
| SA | 4.33 ± 1.49 | 10.78 ± 0.76 | 10.77 ± 0.62 | 9.66 ± 0.27 | 13.40 ± 0.27 | 260.87 ± 48.60 | 146.83 ± 8.58 | 229.33 ± 7.36 | 170.34 ± 2.39 | ||
| SA-GEL | 2.72 ± 0.80 | 2.24 ± 0.60 | 12.31 ± 0.84 | nd | 8.52 ± 0.46 | 906.35 ± 168.35 | 455.08 ± 7.73 | 459.47 ± 15.62 | 431.10 ± 25.41 | ||
| SA-CHIT | 1.00 ± 0.07 | 3.09 ± 0.17 | 12.44 ± 0.07 | 8.67 ± 0.30 | 8.94 ± 0.40 | 238.41 ± 5.11 | 181.35 ± 6.75 | 115.47 ± 7.02 | 158.57 ± 14.96 | ||
| 3,4-Dihydroxy-benzoic acid | PRE | 24.09 ± 1.97 | nd | - | - | - | nd | - | - | - | 17.01 ± 0.08 |
| SA | nd | nd | nd | nd | nd | 99.46 ± 2.40 | 81.70 ± 1.62 | 80.04 ± 1.63 | 55.94 ± 0.07 | ||
| SA-GEL | nd | nd | nd | nd | nd | 100.70 ± 4.98 | 76.46 ± 1.99 | 84.24 ± 0.61 | 75.93 ± 1.07 | ||
| SA-CHIT | nd | nd | nd | nd | nd | 129.42 ± 37.35 | 74.47 ± 0.55 | 40.28 ± 2.58 | 74.01 ± 0.20 | ||
| Syringic acid | PRE | 114.36 ± 3.02 | 6.92 ± 0.78 | - | - | - | nd | - | - | - | nd |
| SA | 5.98 ± 0.26 | 7.79 ± 0.51 | 4.46 ± 0.48 | 2.98 ± 0.00 | 3.79 ± 0.14 | nd | nd | nd | nd | ||
| SA-GEL | 5.97 ± 0.56 | 5.97 ± 0.13 | 4.53 ± 0.07 | 6.04 ± 0.61 | 5.68 ± 0.21 | nd | nd | nd | nd | ||
| SA-CHIT | 3.54 ± 0.12 | 3.30 ± 0.10 | 3.11 ± 0.85 | 2.58 ± 0.54 | 1.48 ± 0.34 | nd | nd | nd | nd | ||
| Vanillic acid | PRE | 14.83 ± 0.74 | nd | - | - | - | nd | - | - | - | 4.39 ± 0.00 |
| SA | nd | nd | nd | nd | nd | 3.87 ± 0.00 | 3.39 ± 0.20 | 3.56 ± 0.41 | 3.34 ± 0.21 | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Ellagic acid | PRE | 81.33 ± 1.23 | nd | - | - | - | nd | - | - | - | 1.28 ± 0.00 |
| SA | 1.72 ± 0.17 | 1.50 ± 1.03 | 1.21 ± 0.07 | 11.08 ± 0.24 | 10.63 ± 0.07 | 11.52 ± 0.14 | 8.84 ± 0.34 | 7.03 ± 0.27 | 7.59 ± 0.48 | ||
| SA-GEL | nd | nd | 4.33 ± 0.07 | 6.19 ± 0.61 | 7.74 ± 0.38 | 13.33 ± 0.94 | 12.48 ± 0.34 | 12.46 ± 1.29 | 11.52 ± 0.40 | ||
| SA-CHIT | nd | 1.59 ± 0.34 | 8.10 ± 0.00 | 10.62 ± 0.68 | 12.08 ± 0.77 | 13.30 ± 1.10 | 10.00 ± 0.41 | 9.32 ± 0.14 | 8.55 ± 0.88 | ||
| p-Hydroxybenzoic acid | PRE | 1.00 ± 0.04 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| o-Coumaric acid | PRE | 19.48 ± 0.58 | nd | - | - | - | nd | - | - | - | 9.37 ± 0.06 |
| SA | nd | 3.52 ± 1.00 | 5.19 ± 0.34 | 29.70 ± 0.14 | 28.23 ± 0.31 | 82.37 ± 5.20 | 94.99 ± 1.49 | 87.12 ± 1.02 | 99.36 ± 4.17 | ||
| SA-GEL | nd | nd | 10.69 ± 0.61 | 13.83 ± 0.71 | 20.34 ± 0.48 | 66.87 ± 6.26 | 75.19 ± 3.91 | 81.98 ± 7.21 | 87.50 ± 0.73 | ||
| SA-CHIT | nd | 4.46 ± 0.31 | 21.11 ± 1.02 | 30.61 ± 1.83 | 36.68 ± 2.22 | 90.29 ± 6.32 | 104.17 ± 0.55 | 105.93 ± 2.99 | 123.03 ± 7.91 | ||
| p-Coumaric acid | PRE | 1.44 ± 0.04 | 0.43 ± 0.06 | - | - | - | 0.44 ± 0.00 | - | - | - | 3.93 ± 0.70 |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Caffeic acid | PRE | 1.04 ± 0.02 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Ferulic acid | PRE | 2.52 ± 0.25 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Stilbenes (μg/100 mgext) | |||||||||||
| Resveratrol | PRE | 8.82 ± 0.04 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| ε-viniferin | PRE | 13.72 ± 0.06 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Flavan-3-ols (μg/100 mgext) | |||||||||||
| Epicatechin | PRE | 547.27 ± 25.23 | 343.19 ± 7.06 | - | - | - | 324.16 ± 4.16 | - | - | - | nd |
| SA | 103.46 ± 3.19 | 135.12 ± 11.02 | 96.92 ± 5.59 | 140.82 ± 2.79 | 157.62 ± 9.34 | 573.86 ± 13.62 | 624.57 ± 6.62 | 685.58 ± 11.71 | 637.35 ± 3.56 | ||
| SA-GEL | 64.03 ± 1.00 | 84.71 ± 1.27 | 95.96 ± 3.64 | 126.00 ± 3.67 | 133.18 ± 1.68 | 561.73 ± 0.74 | 634.47 ± 20.60 | 581.18 ± 22.59 | 550.13 ± 18.30 | ||
| SA-CHIT | 72.78 ± 14.36 | 84.77 ± 2.28 | 117.12 ± 12.92 | 127.29 ± 3.08 | 104.42 ± 10.91 | 541.26 ± 38.72 | 742.62 ± 2.33 | 619.73 ± 6.12 | 790.51 ± 29.93 | ||
| Catechin | PRE | 527.59 ± 28.62 | 100.95 ± 2.01 | - | - | - | 139.84 ± 2.90 | - | - | - | nd |
| SA | 97.24 ± 0.12 | 108.54 ± 15.03 | 59.50 ± 7.27 | 61.06 ± 2.89 | 89.93 ± 5.11 | nd | nd | nd | nd | ||
| SA-GEL | 43.37 ± 2.35 | 37.47 ± 0.57 | 32.03 ± 1.29 | 49.26 ± 0.61 | 71.02 ± 1.24 | nd | nd | nd | nd | ||
| SA-CHIT | 72.07 ± 12.00 | 61.44 ± 3.31 | 54.76 ± 4.43 | 67.08 ± 0.03 | 44.18 ± 1.11 | nd | nd | nd | nd | ||
| Epicatechin gallate | PRE | 32.46 ± 1.65 | nd | - | - | - | nd | - | - | - | 32.06 ± 0.06 |
| SA | 1.78 ± 0.29 | 17.79 ± 0.17 | 28.90 ± 2.74 | 252.14 ± 1.70 | 253.28 ±.9.30 | 205.06 ± 8.69 | 152.85 ± 0.47 | 133.54 ± 7.29 | 112.17 ± 6.02 | ||
| SA-GEL | nd | 10.69 ± 0.17 | 63.98 ± 2.08 | 82.26 ± 6.63 | 118.13 ± 1.06 | 100.66 ± 5.05 | 93.06 ± 1.03 | 69.38 ± 1.63 | 66.02 ± 2.94 | ||
| SA-CHIT | nd | 34.80 ± 0.14 | 183.86 ± 21.13 | 278.40 ± 10.69 | 300.12 ± 3.77 | 261.80 ± 22.31 | 224.08 ± 0.14 | 183.36 ± 6.59 | 155.47 ± 6.08 | ||
| Gallocatechin gallate | PRE | 127.23 ± 9.54 | nd | - | - | - | nd | - | - | - | 190.83 ± 6.03 |
| SA | nd | nd | nd | nd | nd | 545.98 ± 29.50 | 535.41 ± 8.65 | 518.14 ± 3.47 | 482.73 ± 10.39 | ||
| SA-GEL | nd | nd | nd | nd | nd | 391.35 ± 0.47 | 417.09 ± 8.38 | 430.16 ± 15.58 | 423.44 ± 1.14 | ||
| SA-CHIT | 89.18 ± 3.50 | 169.71 ± 2.69 | 318.62 ± 32.48 | 320.13 ± 12.21 | 212.59 ± 2.05 | 516.89 ± 5.77 | 511.21 ± 5.01 | 489.47 ± 1.63 | 510.90 ± 13.45 | ||
| Procyanidin B1 | PRE | 317.42 ± 2.59 | 60.20 ± 0.10 | - | - | - | 72.36 ± 7.13 | - | - | - | nd |
| SA | 30.94 ± 1.54 | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Procyanidin B2 | PRE | 126.51 ± 23.32 | 111.71 ± 5.15 | - | - | - | 137.25 ± 3.75 | - | - | - | nd |
| SA | 34.94 ± 2.09 | 65.53 ± 0.62 | 33.29 ± 4.70 | 73.27 ± 1.46 | 146.07 ± 5.77 | 41.72 ± 3.70 | nd | nd | nd | ||
| SA-GEL | nd | nd | 12.88 ± 0.44 | 16.63 ± 0.64 | 27.06 ± 2.57 | nd | nd | nd | nd | ||
| SA-CHIT | 39.02 ± 8.96 | 33.09 ± 1.19 | 77.44 ± 7.33 | 126.02 ± 4.94 | 108.64 ± 4.75 | nd | nd | nd | nd | ||
| Flavonols (μg/100 mgext) | |||||||||||
| Quercetin | PRE | 146.04 ± 3.93 | 19.62 ± 0.67 | - | - | - | 15.89 ± 0.38 | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Rutin | PRE | 65.08 ± 5.10 | 46.81 ± 0.68 | - | - | - | 64.19 ± 0.00 | - | - | - | 18.86 ± 1.80 |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Kaempferol | PRE | 10.40 ± 1.00 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Anthocyanins (μg/100 mgext) | |||||||||||
| Oenin chloride | PRE | 794.37 ± 21.84 | 511.54 ± 1.17 | - | - | - | 733.77 ± 1.11 | - | - | - | 259.78 ± 23.24 |
| SA | 20.00 ± 3.19 | 102.91 ± 0.07 | 107.27 ± 0.07 | 139.91 ± 0.54 | 123.64 ± 1.92 | nd | nd | nd | nd | ||
| SA-GEL | 1.63 ± 0.07 | 70.75 ± 0.30 | 79.32 ± 0.68 | 86.93 ± 0.10 | 88.30 ± 3.71 | nd | nd | nd | nd | ||
| SA-CHIT | nd | 109.96 ± 1.36 | 121.79 ± 0.17 | 65.26 ± 0.17 | 90.66 ± 1.75 | nd | nd | nd | nd | ||
| Myrtillin chloride | PRE | 35.15 ± 0.00 | 7.98 ± 0.30 | - | - | - | 6.73 ± 0.01 | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Petunidin chloride | PRE | 7.77 ± 0.01 | 3.11 ± 0.17 | - | - | - | 3.62 ± 0.22 | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Peonidin-3-O-glucoside chloride | PRE | 77.75 ± 7.28 | 45.42 ± 0.90 | - | - | - | 61.59 ± 1.32 | - | - | - | 18.64 ± 2.23 |
| SA | 1.16 ± 0.67 | 8.28 ± 0.65 | 10.04 ± 0.07 | 11.51 ± 0.31 | 9.61 ± 0.27 | nd | nd | nd | nd | ||
| SA-GEL | nd | 5.21 ± 0.34 | 6.00 ± 0.03 | 5.88 ± 0.71 | 6.00 ± 0.38 | nd | nd | nd | nd | ||
| SA-CHIT | nd | 6.58 ± 0.65 | 7.21 ± 0.10 | 3.04 ± 0.10 | 6.38 ± 0.54 | nd | nd | nd | nd | ||
| Kuromanin chloride | PRE | 4.88 ± 0.03 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Callistephin chloride | PRE | 2.27 ± 0.03 | nd | - | - | - | nd | - | - | - | nd |
| SA | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-GEL | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| SA-CHIT | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| Analysis | Equation | Marks | |
|---|---|---|---|
| Dry matter | (1) | w—dry matter content (%), m1—sample mass before drying (g), m2—sample mass after drying (g) | |
| Free fats | (2) | mE—mass of the extract containing free fats obtained after Soxhlet extraction (g), mS—sample mass used for Soxhlet extraction (g) | |
| Crude proteins | (3) | N—nitrogen content (%), a—volume of 0.01 M sodium hydroxide used for titration of the blank sample (mL), b—volume of 0.01 M sodium hydroxide used for titration of the tested sample (mL), f—factor of 0.01 M sodium hydroxide used (f = 1), c—amount of sample (g) | |
| (4) | |||
| Ash | (5) | m1—empty crucible mass (g), m2—mass of crucible with sample (g), m3—mass of crucible after combustion (g) | |
| Individual sugars | (6) | P—area under the peak, C—mass concentration of individual sugar (mg/L), G—glucose, A—arabinose, S—sucrose, F—fructose | |
| (7) | |||
| (8) | |||
| (9) | |||
| Fibers | (10) | NDF—neutral detergent fibers (%), ADF—acid detergent fibers (%), m1—residue after drying (g), mS—sample mass (g), ADL (lignin)—acid detergent lignin (%), m1—residue after drying (g), m2—residue after combustion (g), mS—sample mass (g) | |
| (11) | |||
| (12) | |||
| (13) | |||
| Total organic carbon | (14) | TOC—total organic carbon (%), TC—total carbon (%), IC—inorganic carbon (%) |
| Constituents | Simulated Salivary Fluid | Simulated Gastric Fluid | Simulated Intestinal Fluid |
|---|---|---|---|
| (SSF) | (SGF) | (SIF) | |
| KCl (mM) | 15.1 | 6.9 | 6.8 |
| KH2PO4 (mM) | 3.7 | 0.9 | 0.8 |
| NaHCO3 (mM) | 13.6 | 25 | 85 |
| NaCl (mM) | - | 47.2 | 38.4 |
| MgCl2(H2O)6 (mM) | 0.15 | 0.1 | 0.33 |
| (NH4)2CO3 (mM) | 0.06 | 0.5 | - |
| CaCl2(H2O)2 (mM) | 1.5 | 0.15 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. https://doi.org/10.3390/molecules28135285
Martinović J, Lukinac J, Jukić M, Ambrus R, Planinić M, Šelo G, Klarić A-M, Perković G, Bucić-Kojić A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules. 2023; 28(13):5285. https://doi.org/10.3390/molecules28135285
Chicago/Turabian StyleMartinović, Josipa, Jasmina Lukinac, Marko Jukić, Rita Ambrus, Mirela Planinić, Gordana Šelo, Ana-Marija Klarić, Gabriela Perković, and Ana Bucić-Kojić. 2023. "In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation" Molecules 28, no. 13: 5285. https://doi.org/10.3390/molecules28135285
APA StyleMartinović, J., Lukinac, J., Jukić, M., Ambrus, R., Planinić, M., Šelo, G., Klarić, A.-M., Perković, G., & Bucić-Kojić, A. (2023). In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules, 28(13), 5285. https://doi.org/10.3390/molecules28135285