The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation
Abstract
1. Introduction
2. Results and Discussion
2.1. TGA, DTG, UL-94 HB, LOI Results
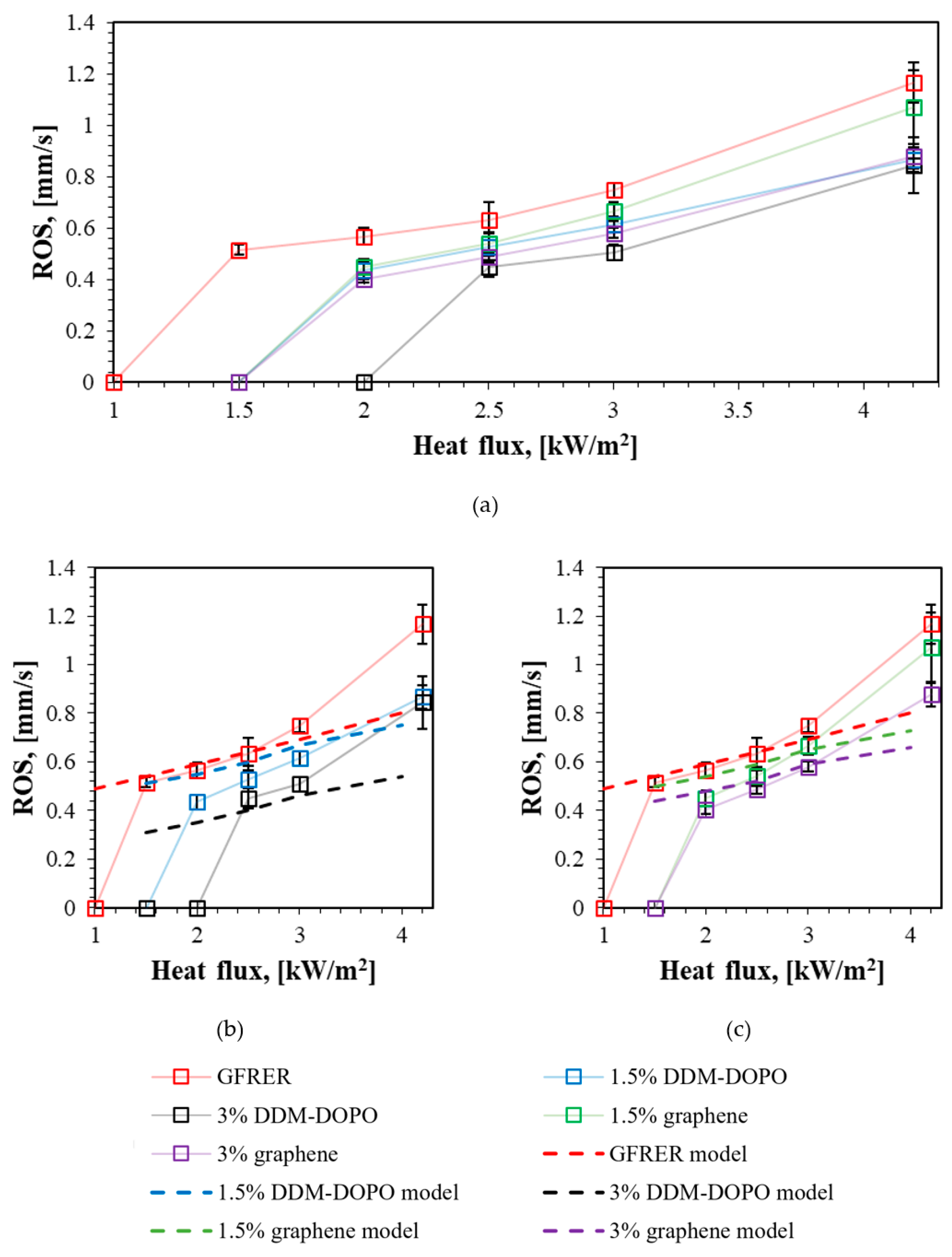
2.2. The Flame Spread Rate over the Samples Surface
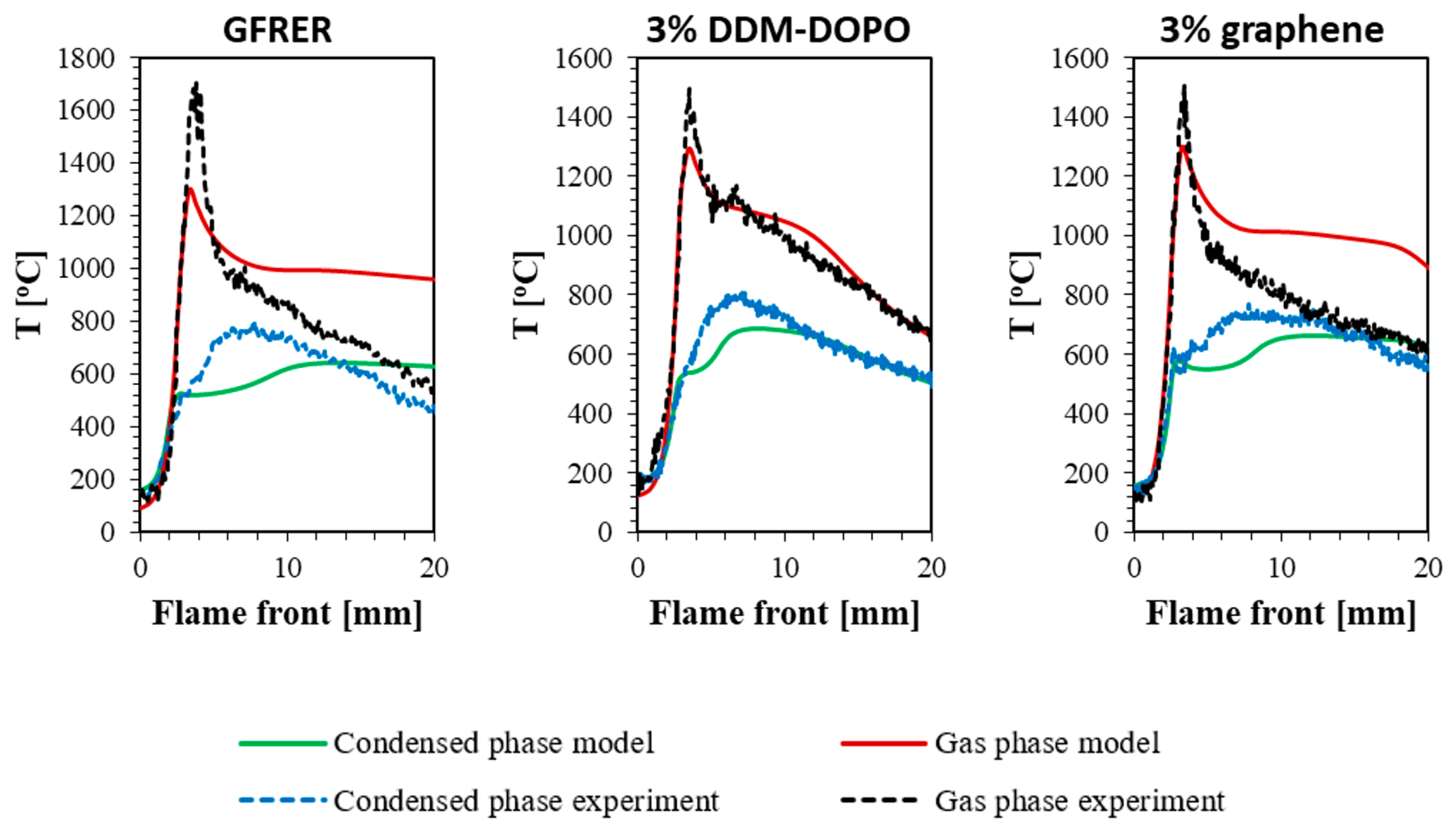
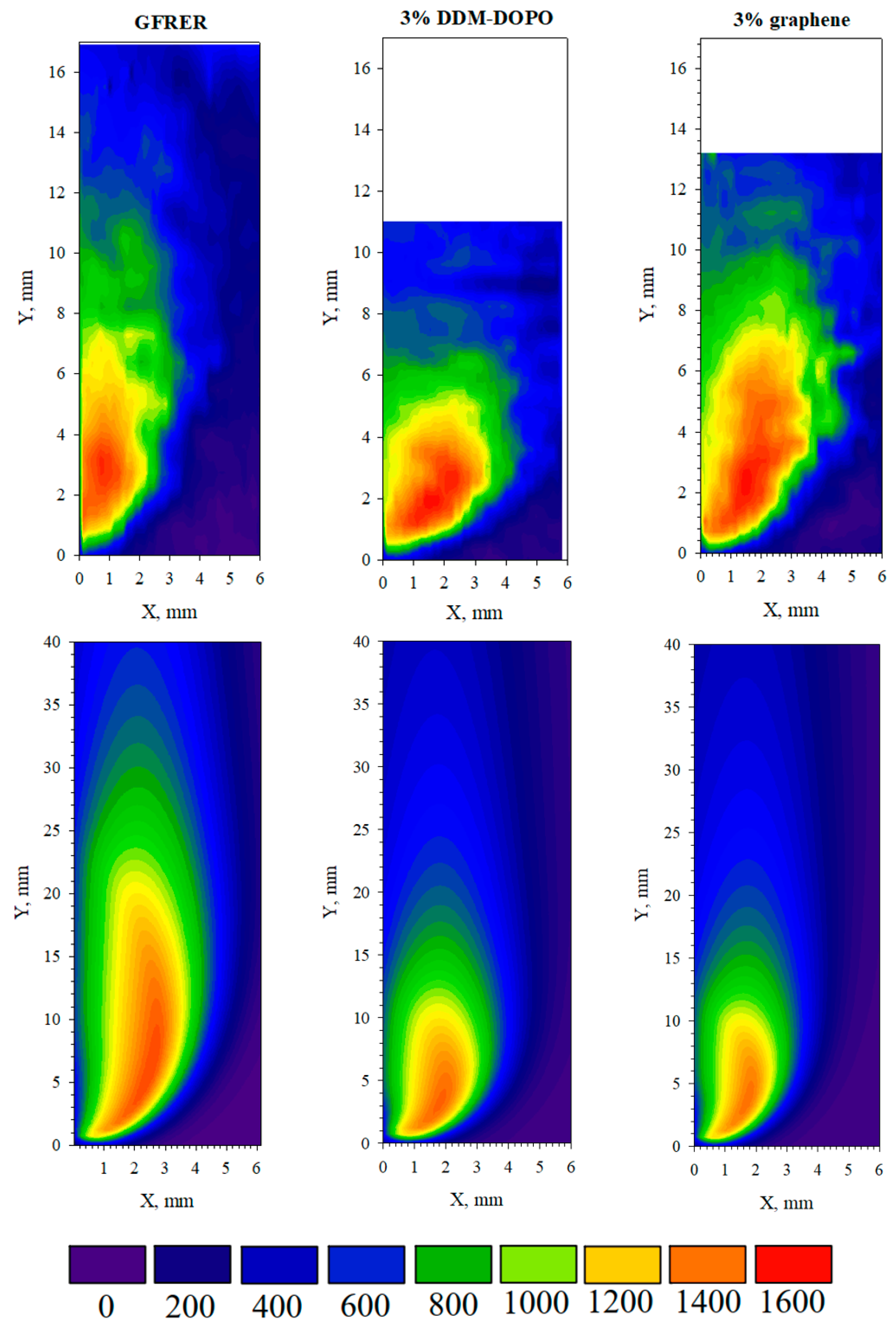
2.3. Thermal Flame Structure
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Epoxy Matrix
3.1.2. Additives
3.2. Thermal Degradation Analysis
3.3. Elemental Analysis
3.4. Flammability Tests
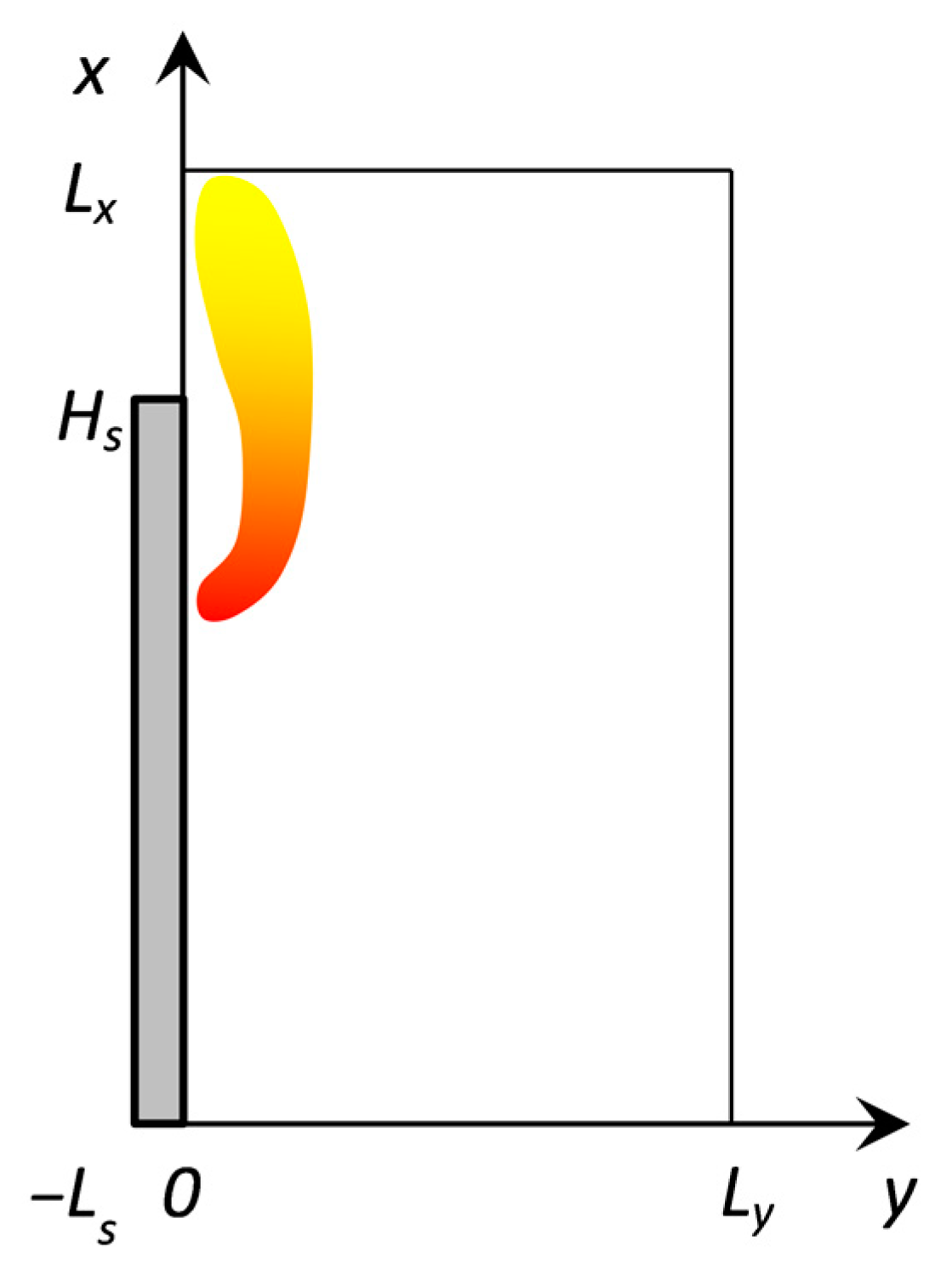
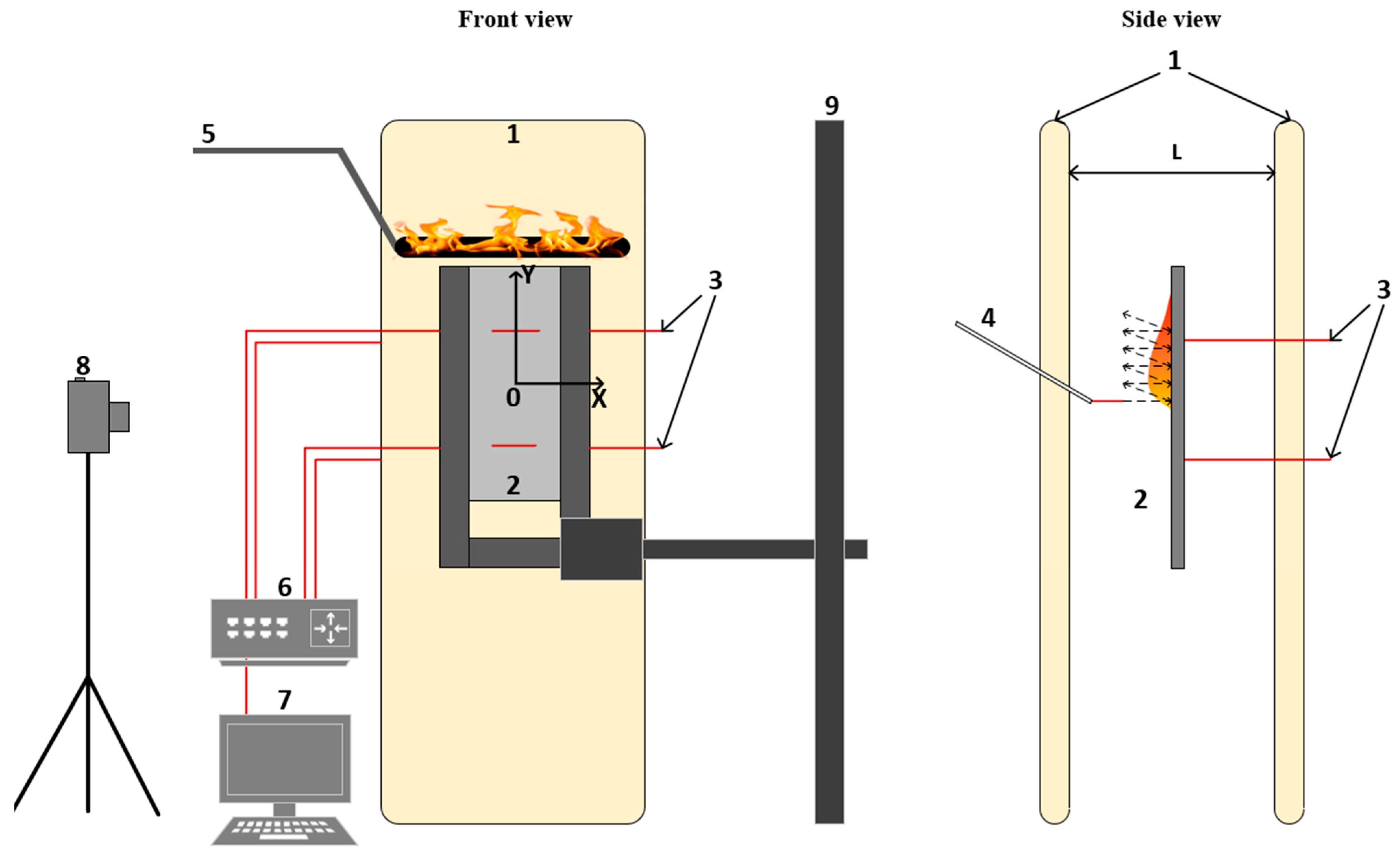
3.5. Description of the Experimental Setup for Downward Flame Propagation under the Action of Bilateral External Heating
3.6. Numerical Approach
| : | , , , , , ; |
| : | , , ; |
| : | , , , , , ; |
| , : | , , , ; |
| , , : , | ; |
| , : | , , , , , , ; |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visakh, P.M.; Yoshihiko, A. Flame Retardants: Polymer Blends, Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-03466-9. [Google Scholar]
- Shahari, S.; Fathullah, M.; Abdullah, M.M.A.B.; Shayfull, Z.; Mia, M.; Budi Darmawan, V.E. Recent Developments in Fire Retardant Glass Fibre Reinforced Epoxy Composite and Geopolymer as a Potential Fire-Retardant Material: A Review. Constr. Build. Mater. 2021, 277, 122246. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on Flammability of Biofibres and Biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef]
- Liu, H.; Xu, K.; Ai, H.; Zhang, L.; Chen, M. Preparation and Characterization of Phosphorus-Containing Mannich-Type Bases as Curing Agents for Epoxy Resin. Polym. Adv. Technol. 2009, 20, 753–758. [Google Scholar] [CrossRef]
- Zang, L.; Wagner, S.; Ciesielski, M.; Müller, P.; Döring, M. Novel Star-Shaped and Hyperbranched Phosphorus-Containing Flame Retardants in Epoxy Resins. Polym. Adv. Technol. 2011, 22, 1182–1191. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Wang, P.; Qiu, S.; Cai, W.; Hu, Y. Ultra-Low Phosphorus Loading to Achieve the Superior Flame Retardancy of Epoxy Resin. Polym. Degrad. Stab. 2018, 149, 119–128. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. Novel DOPO-Based Flame Retardants in High-Performance Carbon Fibre Epoxy Composites for Aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Gu, L.; Chen, G.; Yao, Y. Two Novel Phosphorus–Nitrogen-Containing Halogen-Free Flame Retardants of High Performance for Epoxy Resin. Polym. Degrad. Stab. 2014, 108, 68–75. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and Nitrogen-Containing Polyols: Synergistic Effect on the Thermal Property and Flame Retardancy of Rigid Polyurethane Foam Composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Liu, J.; Xin, F.; Chen, Y. The Flame Retardant Group-Synergistic-Effect of a Phosphaphenanthrene and Triazine Double-Group Compound in Epoxy Resin. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Cheng, L.; Wang, M. The Synergistic Effect of Maleimide and Phosphaphenanthrene Groups on a Reactive Flame-Retarded Epoxy Resin System. Polym. Degrad. Stab. 2015, 115, 63–69. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, G.; Wang, Y.; Hu, J. Preparation of Phosphorus-Containing Epoxy Emulsion and Flame Retardancy of Its Thermoset. High Perform. Polym. 2014, 26, 526–531. [Google Scholar] [CrossRef]
- Gaan, S.; Liang, S.; Mispreuve, H.; Perler, H.; Naescher, R.; Neisius, M. Flame Retardant Flexible Polyurethane Foams from Novel DOPO-Phosphonamidate Additives. Polym. Degrad. Stab. 2015, 113, 180–188. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a Novel P/N/S-Containing Flame Retardant and Its Application in Epoxy Resin: Thermal Property, Flame Retardance, and Pyrolysis Behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, X.; Zhang, S.; Wu, S.; Zhao, Q.; Hu, Z. Synthesis, Characterization, and Utilization of a Novel Phosphorus/Nitrogen-Containing Flame Retardant. Ind. Eng. Chem. Res. 2015, 54, 2974–2982. [Google Scholar] [CrossRef]
- Wang, P.; Fu, X.; Kan, Y.; Xin, W.; Hu, Y. Two High-Efficient DOPO-Based Phosphonamidate Flame Retardants for Transparent Epoxy Resin. High Perform. Polym. 2018, 31, 095400831876203. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The Effect of Graphene Presence in Flame Retarded Epoxy Resin Matrix on the Mechanical and Flammability Properties of Glass Fiber-Reinforced Composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How Can Graphene Reduce the Flammability of Polymer Nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Liu, S.; Yan, H.; Fang, Z.; Wang, H. Effect of Graphene Nanosheets on Morphology, Thermal Stability and Flame Retardancy of Epoxy Resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Q.-Y.; Cui, Y.; Wang, T.; Li, X.; Han, B.-H. Graphene–Terpyridine Complex Hybrid Porous Material for Carbon Dioxide Adsorption. Carbon 2014, 66, 592–598. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Liu, Y.; Wang, Q. Flame Retardant Polyoxymethylene with Aluminium Hydroxide/Melamine/Novolac Resin Synergistic System. Polym. Degrad. Stab. 2010, 95, 945–954. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Shaklein, A.; Trubachev, S.; Karpov, A.; Paletsky, A.; Chernov, A.; Sosnin, E.; Shmakov, A. The Influence of Flame Retardants on Combustion of Glass Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 3379. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Terashima, K.; Oiwa, R.; Tokoro, M.; Takahashi, S. Opposed-Flow Flame Spread over Carbon Fiber Reinforced Plastic under Variable Flow Velocity and Oxygen Concentration: The Effect of in-Plane Thermal Isotropy and Anisotropy. Proc. Combust. Inst. 2021, 38, 4857–4866. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Karpov, A.; Shaklein, A.; Paletsky, A.; Chernov, A.; Trubachev, S.; Glaznev, R.; Shmakov, A.; Barbot’ko, S. Experimental and Numerical Study of Downward Flame Spread over Glass-Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 911. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Oiwa, R.; Tokoro, M.; Takahashi, S. Buoyant-Flow Downward Flame Spread over Carbon Fiber Reinforced Plastic in Variable Oxygen Atmospheres. Combust. Flame 2021, 232, 111528. [Google Scholar] [CrossRef]
- Snegirev, A.; Kuznetsov, E.; Korobeinichev, O.; Shmakov, A.; Paletsky, A.; Shvartsberg, V.; Trubachev, S. Fully Coupled Three-Dimensional Simulation of Downward Flame Spread over Combustible Material. Polymers 2022, 14, 4136. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.A. Mechanisms of Fire Spread. Symp. (Int.) Combust. 1977, 16, 1281–1294. [Google Scholar] [CrossRef]
- Fernandez-pello, A.C. Upward Laminar Flame Spread under the Influence of Externally Applied Thermal Radiation. Combust. Sci. Technol. 1977, 17, 87–98. [Google Scholar] [CrossRef]
- Fernandez-Pello, A.C. Downward Flame Spread under the Influence of Externally Applied Thermal Radiation. Combust. Sci. Technol. 1977, 17, 1–9. [Google Scholar] [CrossRef]
- Hirano, T.; Sato, K. Effects of Radiation and Convection on Gas Velocity and Temperature Profiles of Flames Spreading over Paper. Symp. (Int.) Combust. 1975, 15, 233–241. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, B.; Zhang, X.; Yang, Y. A Comparative Study on Horizontal Flame Spread Behaviors of Thermoplastic Polymers with Different Melt Flow Indexes under External Radiation. Therm. Sci. Eng. Prog. 2022, 35, 101463. [Google Scholar] [CrossRef]
- Brehob, E.G.; Kulkarni, A.K. Experimental Measurements of Upward Flame Spread on a Vertical Wall with External Radiation. Fire Saf. J. 1998, 31, 181–200. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, H.; Yan, W.; An, W.; Jiang, L.; Sun, J. Horizontal Flame Spread Characteristics of Rigid Polyurethane and Molded Polystyrene Foams Under Externally Applied Radiation at Two Different Altitudes. Fire Technol 2015, 51, 1195–1216. [Google Scholar] [CrossRef]
- Vincent, C.; Corn, S.; Longuet, C.; Aprin, L.; Rambaud, G.; Ferry, L. Experimental and Numerical Thermo-Mechanical Analysis of the Influence of Thermoplastic Slabs Installation on the Assessment of Their Fire Hazard. Fire Saf. J. 2019, 108, 102850. [Google Scholar] [CrossRef]
- Bhattachariee, S.; King, M.D.; Takahashi, S.; Nagumo, T.; Wakai, K. Downward Flame Spread over Poly(Methyl)Methacrylate. Proc. Combust. Inst. 2000, 28, 2891–2897. [Google Scholar] [CrossRef]
- Trubachev, S.A.; Korobeinichev, O.P.; Karpov, A.I.; Shaklein, A.A.; Glaznev, R.K.; Gonchikzhapov, M.B.; Paletsky, A.A.; Tereshchenko, A.G.; Shmakov, A.G.; Bespalova, A.S.; et al. The Effect of Triphenyl Phosphate Inhibition on Flame Propagation over Cast PMMA Slabs. Proc. Combust. Inst. 2021, 38, 4635–4644. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of Thermal Decomposition of PMMA at Different Heating Rates and in a Wide Temperature Range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Gonchikzhapov, M.B.; Paletsky, A.A.; Tereshchenko, A.G.; Shundrina, I.K.; Kuibida, L.V.; Shmakov, A.G.; Hu, Y. Counterflow Flames of Ultrahigh-Molecular-Weight Polyethylene with and without Triphenylphosphate. Combust. Flame 2016, 169, 261–271. [Google Scholar] [CrossRef]
- Trubachev, S.A.; Korobeinichev, O.P.; Kostritsa, S.A.; Kobtsev, V.D.; Paletsky, A.A.; Kumar, A.; Smirnov, V.V. An Insight into the Gas-Phase Inhibition Mechanism of Polymers by Addition of Triphenyl Phosphate Flame Retardant. AIP Conf. Proc. 2020, 2304, 020019. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Trubachev, S.A.; Joshi, A.K.; Kumar, A.; Paletsky, A.A.; Tereshchenko, A.G.; Shmakov, A.G.; Glaznev, R.K.; Raghavan, V.; Mebel, A.M. Experimental and Numerical Studies of Downward Flame Spread over PMMA with and without Addition of Tri Phenyl Phosphate. Proc. Combust. Inst. 2021, 38, 4867–4875. [Google Scholar] [CrossRef]
- Ma, C.; Sánchez-Rodríguez, D.; Kamo, T. A Comprehensive Study on the Oxidative Pyrolysis of Epoxy Resin from Fiber/Epoxy Composites: Product Characteristics and Kinetics. J. Hazard. Mater. 2021, 412, 125329. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, M.-Q.; Yu, J.; Nie, S.-Q.; Zhang, D.-Q.; Qin, S.-H. Synergistic Flame-Retardant Effect of Epoxy Resin Combined with Phenethyl-Bridged DOPO Derivative and Graphene Nanosheets. Chin. J. Polym. Sci. 2019, 37, 79–88. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Kumaran, S.M.; Raghavan, V.; Trubachev, S.A.; Paletsky, A.A.; Shmakov, A.G.; Glaznev, R.K.; Chernov, A.A.; Tereshchenko, A.G.; Loboda, E.L.; et al. Investigation of the Impact of Pinus Silvestris Pine Needles Bed Parameters on the Spread of Ground Fire in Still Air. Combust. Sci. Technol. 2022, 1–23. [Google Scholar] [CrossRef]
- Singh, A.V.; Gollner, M.J. A Methodology for Estimation of Local Heat Fluxes in Steady Laminar Boundary Layer Diffusion Flames. Combust. Flame 2015, 162, 2214–2230. [Google Scholar] [CrossRef]


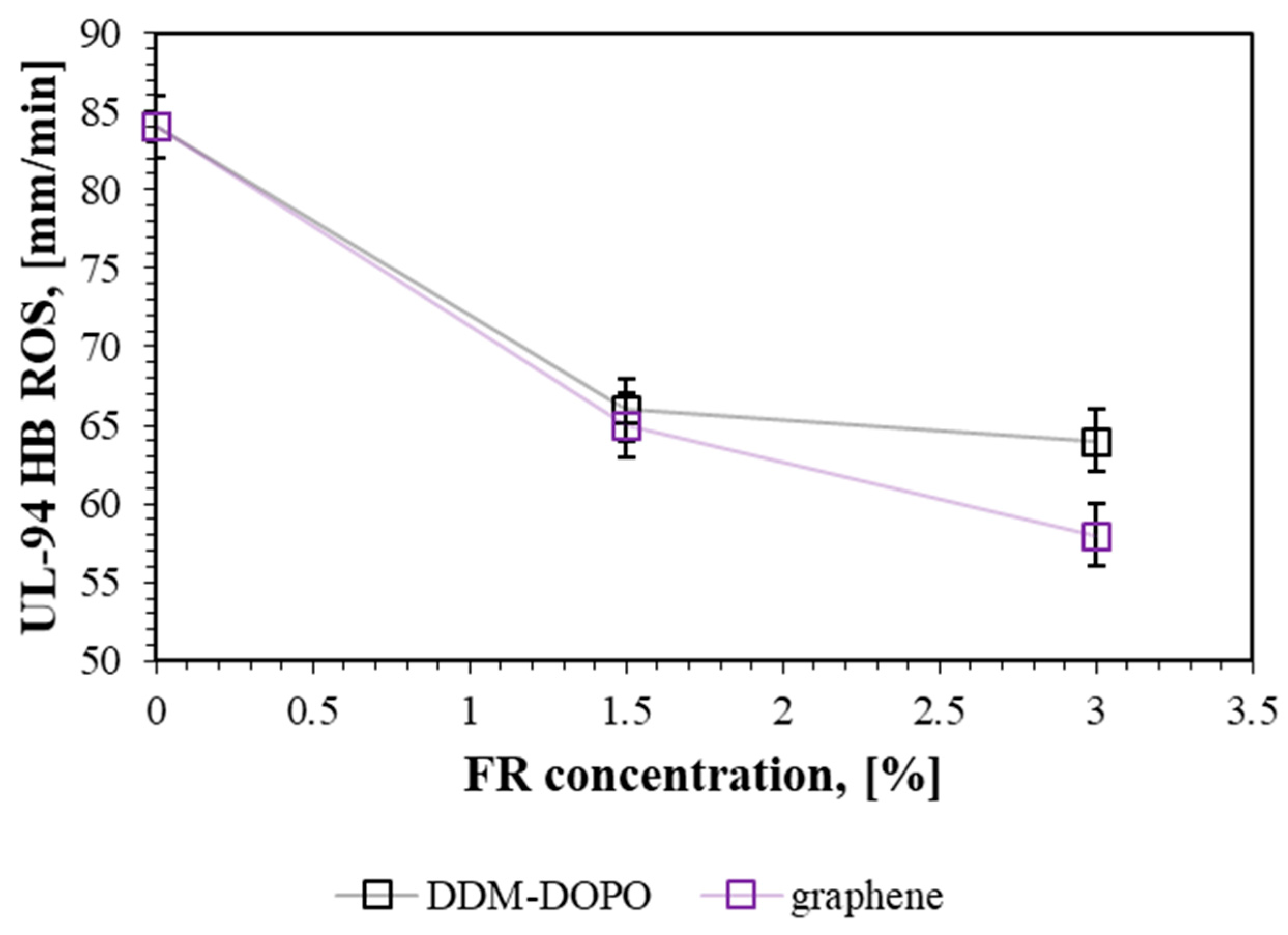

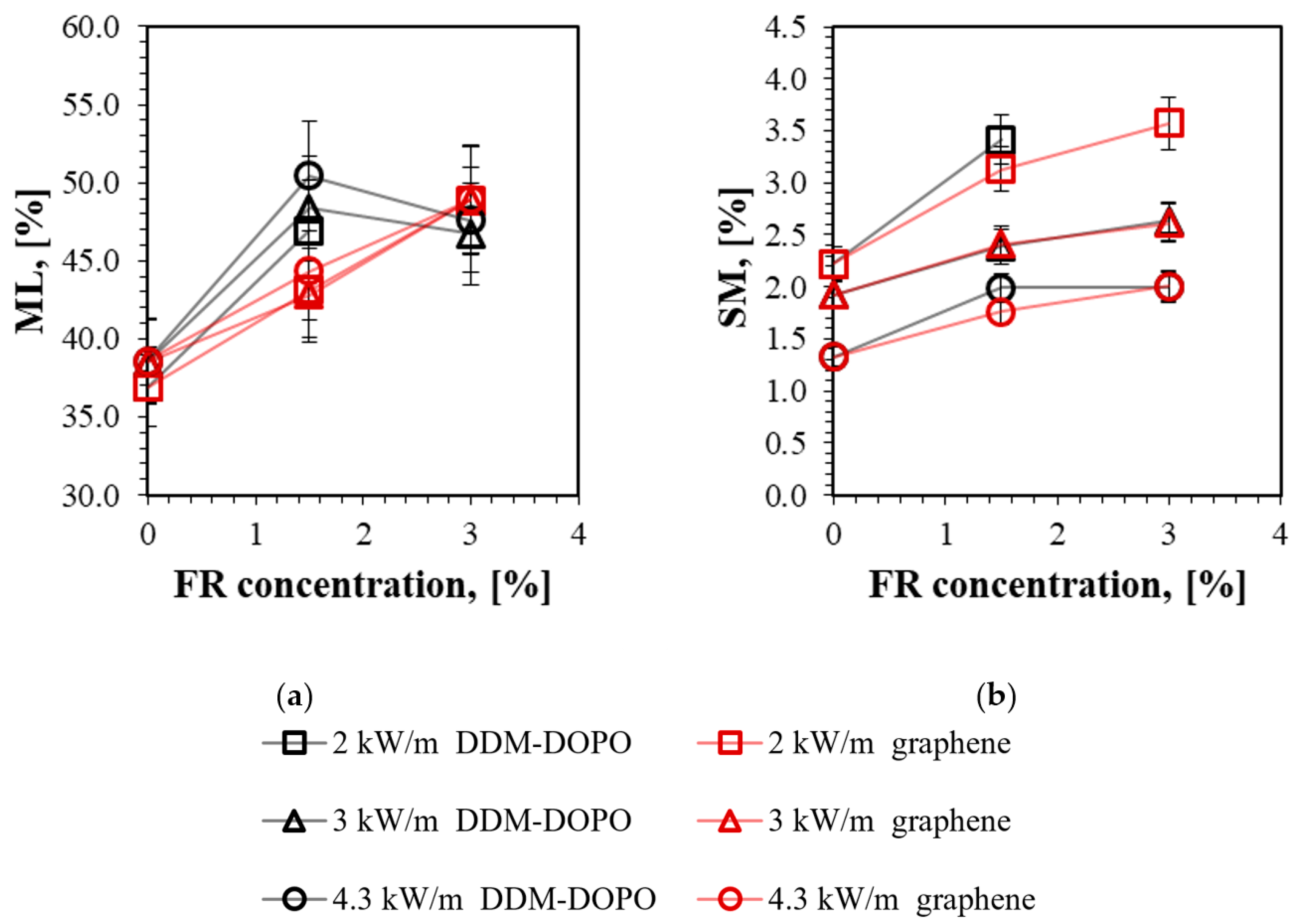
| Sample | UL-94 HB, with ROS mm/min | LOI, % Thin Samples | ML in % of the Initial Mass | SM in % of the Initial Mass | Tmax DTG in Inert, °C | Residue Mass in TGA, % |
|---|---|---|---|---|---|---|
| GFRER | 84 ± 2 1 | 21.0 | 18 | 12 | 442 ± 2 | 59.1 ± 1.5 |
| 1.5% DDM-DOPO | 66 ± 2 2 | 21.9 | 14 | 17 | 438 ± 2 | 56.5 ± 1.5 |
| 3% DDM-DOPO | 64 ± 2 2 | 21.9 | 13 | 20 | 444 ± 2 | 55.0 ± 1.5 |
| 1.5% graphene | 65 ± 2 2 | 21.7 | 13 | 18 | 442 ± 2 | 59.1 ± 1.5 |
| 3% graphene | 58 ± 2 2 | 21.6 | 11 | 23 | 448 ± 2 | 53.7 ± 0.5 |
| 6.5% DDM-DOPO | 23 ± 1 2 | 26.1 | - | - | - | - |
| 9.8% DDM-DOPO | 26 ± 1 2 | 26.4 | - | - | - | - |
| Heat Flux, kW/m2 | Samples | ||||
|---|---|---|---|---|---|
| GFRER | 1.5% DDM-DOPO | 3% DDM-DOPO | 1.5% Graphene | 3% Graphene | |
| 4.2 | 1.17 ± 0.08 | 0.87 ± 0.05 | 0.85 ± 0.1 | 1.07 ± 0.14 | 0.88 ± 0.05 |
| 3 | 0.75 ± 0.03 | 0.61 ± 0.03 | 0.51 ± 0.03 | 0.67 ± 0.04 | 0.58 ± 0.02 |
| 2.5 | 0.63 ± 0.07 | 0.53 ± 0.05 | 0.45 ± 0.04 | 0.54 ± 0.04 | 0.49 ± 0.02 |
| 2 | 0.57 ± 0.03 | 0.43 ± 0.03 | Self-extinguished | 0.45 ± 0.03 | 0.40 ± 0.02 |
| 1.5 | 0.52 ± 0.02 | Self-extinguished | Self-extinguished | Self-extinguished | Self-extinguished |
| Component | Composition, Mass Fraction | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Epoxy resin ED-22 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Curing agent #9 [24] | 5 | 5 | 5 | 5 | 5 | 13 | 13 |
| DDM-DOPO | - | 1.5 | 3 | - | - | 6.5 | 9.8 |
| Graphene | - | - | - | 1.5 | 3 | - | - |
| Ψ | kb, 1/s | Eb, kJ/mole | |
|---|---|---|---|
| GFRER | 0 | 109.93 | 157.3 |
| GFRER + DDM-DOPO | 29 | 108.49 | 140.4 |
| GFRER + graphene | 2/3 | 1011.68 | 183.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobeinichev, O.P.; Sosnin, E.A.; Shaklein, A.A.; Karpov, A.I.; Sagitov, A.R.; Trubachev, S.A.; Shmakov, A.G.; Paletsky, A.A.; Kulikov, I.V. The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation. Molecules 2023, 28, 5162. https://doi.org/10.3390/molecules28135162
Korobeinichev OP, Sosnin EA, Shaklein AA, Karpov AI, Sagitov AR, Trubachev SA, Shmakov AG, Paletsky AA, Kulikov IV. The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation. Molecules. 2023; 28(13):5162. https://doi.org/10.3390/molecules28135162
Chicago/Turabian StyleKorobeinichev, Oleg P., Egor A. Sosnin, Artem A. Shaklein, Alexander I. Karpov, Albert R. Sagitov, Stanislav A. Trubachev, Andrey G. Shmakov, Alexander A. Paletsky, and Ilya V. Kulikov. 2023. "The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation" Molecules 28, no. 13: 5162. https://doi.org/10.3390/molecules28135162
APA StyleKorobeinichev, O. P., Sosnin, E. A., Shaklein, A. A., Karpov, A. I., Sagitov, A. R., Trubachev, S. A., Shmakov, A. G., Paletsky, A. A., & Kulikov, I. V. (2023). The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation. Molecules, 28(13), 5162. https://doi.org/10.3390/molecules28135162








