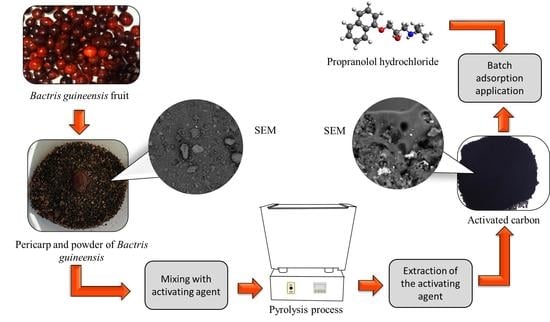
The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption
Abstract
1. Introduction
2. Results and Discussion
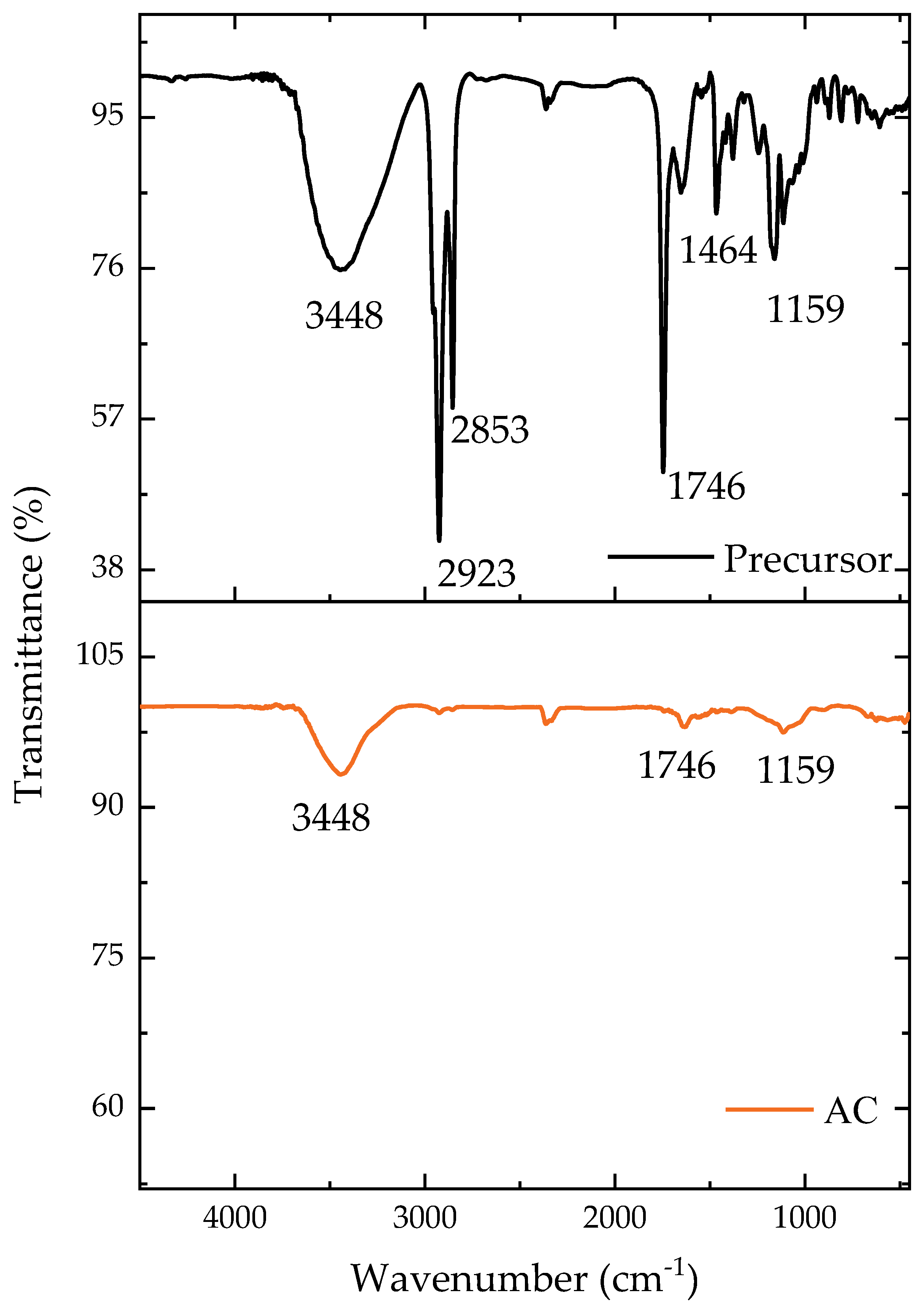
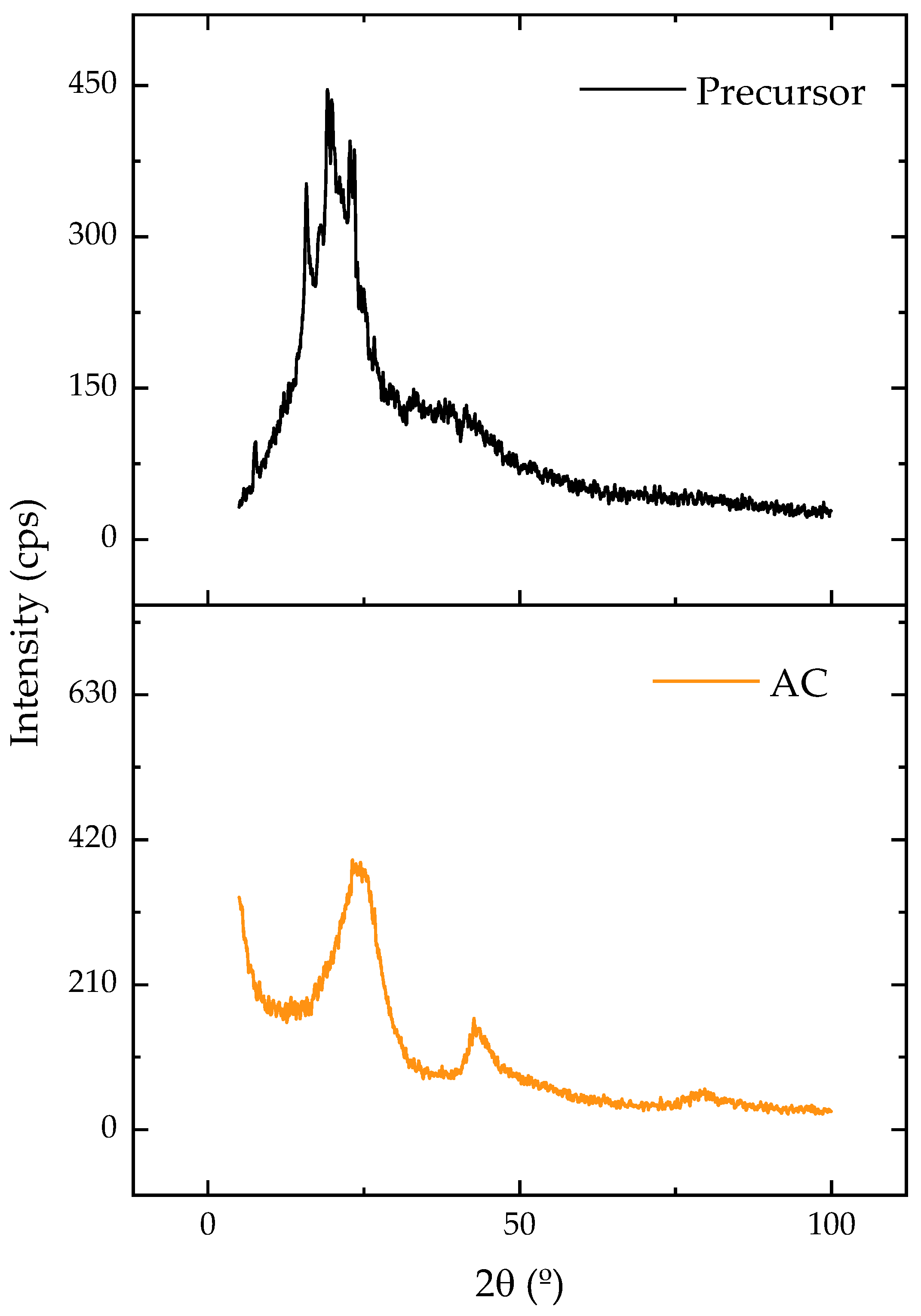
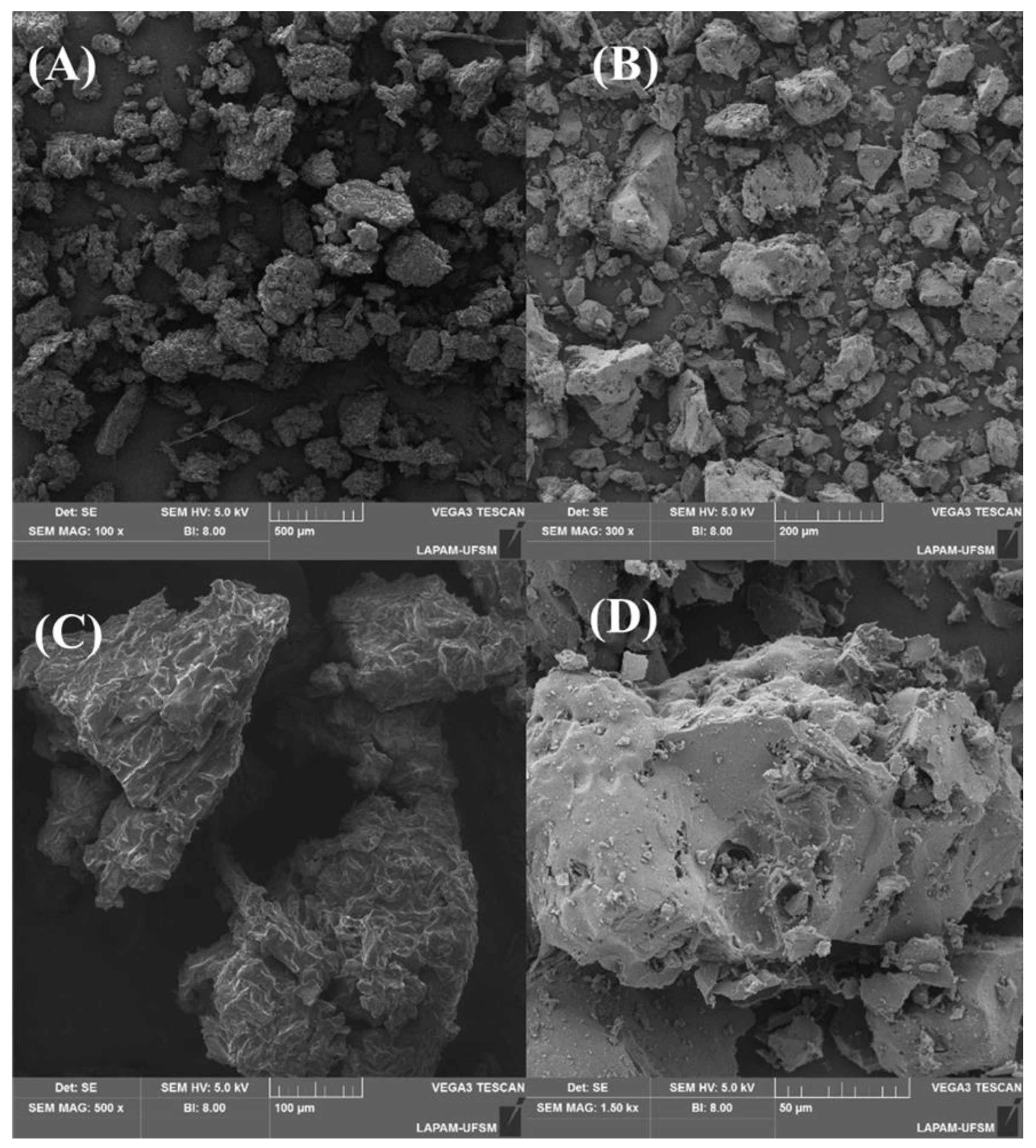
2.1. Characterization Results
2.2. Adsorbent Dosage and Solution pH Optimization
2.3. Isothermal Experiments and Estimative of the Adsorption’s Thermodynamic Proprieties
2.4. Propranolol Adsorption Kinetics
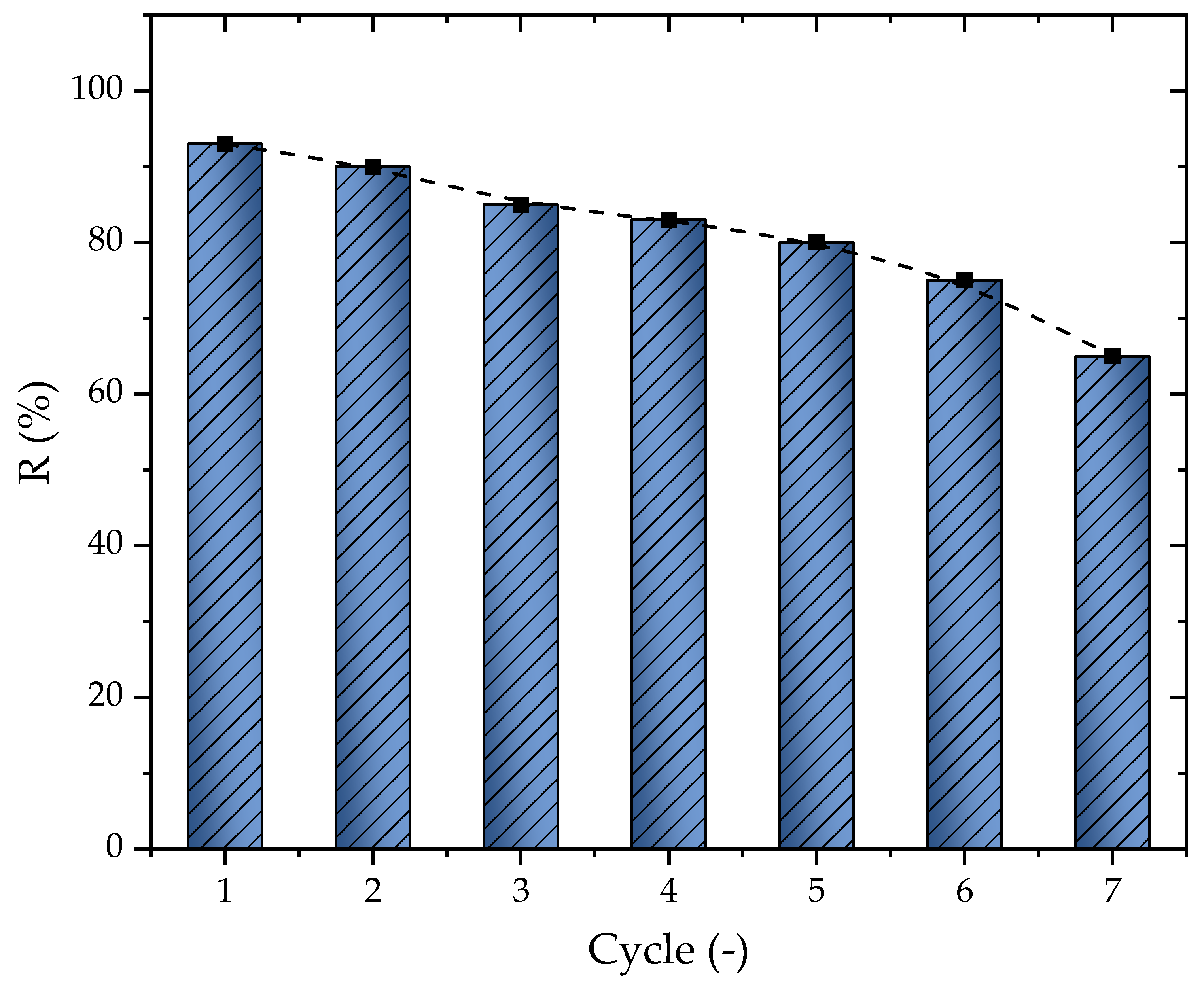
2.5. Adsorption Regeneration Results
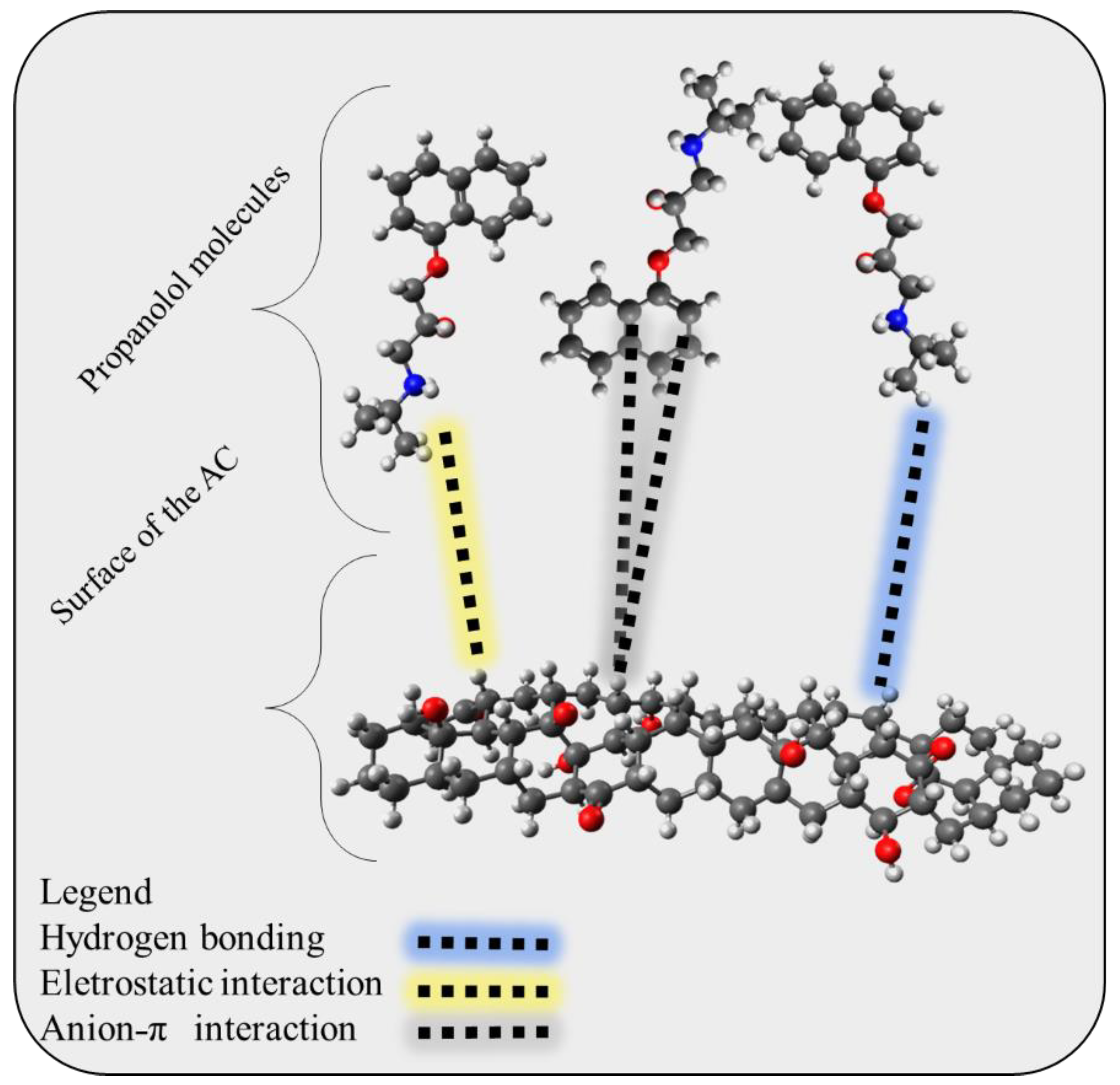
2.6. Possible Adsorption Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation Techniques
3.3. Preparation of Precursor Material and Activated Carbon
3.4. Propranolol Adsorption Experiments
3.5. Adsorption Regeneration
3.6. Adsorption Isotherm and Thermodynamics
3.7. Adsorption Kinetics
3.8. Estimation of Looped Parameters and Solution of the Differential Equation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- do Nascimento, D.C.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Propranolol Hydrochloride from Aqueous Solutions onto Thermally Treated Bentonite Clay: A Complete Batch System Evaluation. J. Mol. Liq. 2021, 337, 116442. [Google Scholar] [CrossRef]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Pescara, I.C.; Albuquerque, A.F.; Umbuzeiro, G.A.; Jardim, W.F. Ten Years-Snapshot of the Occurrence of Emerging Contaminants in Drinking, Surface and Ground Waters and Wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Terzić, S.; Senta, I.; Ahel, M.; Gros, M.; Petrović, M.; Barcelo, D.; Müller, J.; Knepper, T.; Martí, I.; Ventura, F.; et al. Occurrence and Fate of Emerging Wastewater Contaminants in Western Balkan Region. Sci. Total Environ. 2008, 399, 66–77. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced Biological Activated Carbon Filter for Removing Pharmaceutically Active Compounds from Treated Wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Menz, J.; Toolaram, A.P.; Rastogi, T.; Leder, C.; Olsson, O.; Kümmerer, K.; Schneider, M. Transformation Products in the Water Cycle and the Unsolved Problem of Their Proactive Assessment: A Combined in Vitro/in Silico Approach. Environ. Int. 2017, 98, 171–180. [Google Scholar] [CrossRef]
- Vieira, Y.; Ceretta, M.B.; Foletto, E.L.; Wolski, E.A.; Silvestri, S. Application of a Novel RGO-CuFeS2 Composite Catalyst Conjugated to Microwave Irradiation for Ultra-Fast Real Textile Wastewater Treatment. J. Water Process Eng. 2020, 36, 101397. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of Pharmaceuticals in Water Using Fe3O4 Coated Polymer Clay Composite. Microporous Mesoporous Mater. 2016, 232, 273–280. [Google Scholar] [CrossRef]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the Environmental Transport of Human Pharmaceuticals to Streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef]
- Rastogi, T.; Leder, C.; Kümmerer, K. Re-Designing of Existing Pharmaceuticals for Environmental Biodegradability: A Tiered Approach with β-Blocker Propranolol as an Example. Environ. Sci. Technol. 2015, 49, 11756–11763. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-Blockers in the Environment: Part II. Ecotoxicity Study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of Hospital Effluents to the Load of Pharmaceuticals in Urban Wastewaters: Identification of Ecologically Relevant Pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved Removal of Estrogenic and Pharmaceutical Compounds in Sewage Effluent by Full Scale Granular Activated Carbon: Impact on Receiving River Water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, Control and Fate of Contaminants of Emerging Concern in Environmental Compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Zepon Tarpani, R.R.; Azapagic, A. Life Cycle Environmental Impacts of Advanced Wastewater Treatment Techniques for Removal of Pharmaceuticals and Personal Care Products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Gao, N.Y.; Yin, D.Q.; Tian, F.X.; Zheng, Q. feng Oxidation of the Β-Blocker Propranolol by UV/Persulfate: Effect, Mechanism and Toxicity Investigation. Chemosphere 2018, 201, 50–58. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Georgin, J.; Stracke, D.; Franco, P.; Sher, F. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Da Boit Martinello, K.; Lima, E.C.; Silva, L.F.O. A Review of the Toxicology Presence and Removal of Ketoprofen through Adsorption Technology. J. Environ. Chem. Eng. 2022, 10, 107798. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef] [PubMed]
- Henrique, D.C.D.C.; Henrique, D.C.D.C.; Solano, J.R.S.; Barbosa, V.T.; Silva, A.O.S.; Dornelas, C.B.; Duarte, J.L.S.; Meili, L. Calcined Mytella falcata Shells as a Source for CaAl/LDH Production: Synthesis and Characterization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128752. [Google Scholar] [CrossRef]
- Manzar, M.S.; Ahmad, T.; Zubair, M.; Ullah, N.; Alqahtani, H.A.; da Gama, B.M.V.; Georgin, J.; Nasir, M.; Mu’azu, N.D.; Al Ghamdi, J.M.; et al. Comparative Adsorption of Tetracycline onto Unmodified and NaOH-Modified Silicomanganese Fumes: Kinetic and Process Modeling. Chem. Eng. Res. Des. 2023, 192, 521–533. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Ramos, C.G.; Netto, M.S.; Ojeda, N.J.; Vega, N.A.; Meili, L.; Lima, E.C.; Naushad, M. The Production of Activated Biochar Using Calophyllum inophyllum Waste Biomass and Use as an Adsorbent for Removal of Diuron from the Water in Batch and Fixed Bed Column. Environ. Sci. Pollut. Res. 2023, 30, 52498–52513. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Gama, B.M.V.; Fernandes, D.P.; Sepúlveda, P.; Silva, L.F.O.; Meili, L. Effective Adsorption of Harmful Herbicide Diuron onto Novel Activated Carbon from Hovenia dulcis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129900. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Lima, E.C.; Silva, L.F.O. Advances Made in Removing Paraquat Herbicide by Adsorption Technology: A Review. J. Water Process Eng. 2022, 49, 102988. [Google Scholar] [CrossRef]
- Brieva-Oviedo, E.; Maia, A.C.D.; Núñez-Avellaneda, L.A. Pollination of Bactris guineensis (Arecaceae), a Potential Economically Exploitable Fruit Palm from the Colombian Caribbean. Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 269, 151628. [Google Scholar] [CrossRef]
- Osorio, C.; Carriazo, J.G.; Almanza, O. Antioxidant Activity of Corozo (Bactris guineensis) Fruit by Electron Paramagnetic Resonance (EPR) Spectroscopy. Eur. Food Res. Technol. 2011, 233, 103–108. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Vallejo, W.; Romero, E.; Villareal, M.; Padilla, M.; Hazbun, N.; Muñoz-Acevedo, A.; Schott, E.; Zarate, X. TiO2 Thin Films Sensitization with Natural Dyes Extracted from Bactris guineensis for Photocatalytic Applications: Experimental and DFT Study. J. Saudi Chem. Soc. 2020, 24, 407–416. [Google Scholar] [CrossRef]
- Sequeda-Castañeda, L.G.; Barrera-Bugallo, A.R.; Celis, C.; Iglesias, J.; Morales, L. Evaluation of Antioxidant and Cytotoxic Activity of Extracts from Fruits in Fibroblastoma HT1080 Cell Lines: Four Fruits with Commercial Potential in Colombia. Emirates J. Food Agric. 2016, 28, 143–151. [Google Scholar] [CrossRef]
- Üner, O.; Bayrak, Y. The Effect of Carbonization Temperature, Carbonization Time and Impregnation Ratio on the Properties of Activated Carbon Produced from Arundo Donax. Microporous Mesoporous Mater. 2018, 268, 225–234. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Netto, M.S.; da Boit Martinello, K.; Silva, L.F.O. Preparation of Activated Carbons from Fruit Residues for the Removal of Naproxen (NPX): Analytical Interpretation via Statistical Physical Model. J. Mol. Liq. 2022, 356, 119021. [Google Scholar] [CrossRef]
- Ramirez, R.; Schnorr, C.E.; Georgin, J.; Netto, M.S.; Franco, D.S.P.; Carissimi, E.; Wolff, D.; Silva, L.F.O.; Dotto, G.L. Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters. Molecules 2022, 27, 7781. [Google Scholar] [CrossRef]
- Georgin, J.; Pinto, D.; Franco, D.S.P.; Netto, M.S.; Lazarotto, J.S.; Allasia, D.G.; Tassi, R.; Silva, L.F.O.; Dotto, G.L. Improved Adsorption of the Toxic Herbicide Diuron Using Activated Carbon Obtained from Residual Cassava Biomass. Molecules 2022, 27, 7574. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Alagbe, E.E.; Odika, B.; Babalola, R.; Oladimeji, T.E.; Abatan, O.G.; Yusuf, E.O. Preparation and Characterization of Activated Carbon from Plantain Peel and Coconut Shell Using Biological Activators. J. Phys. Conf. Ser. 2019, 1378, 032035. [Google Scholar] [CrossRef]
- Wu, H.; Gong, L.; Zhang, X.; He, F.; Li, Z. Bifunctional Porous Polyethyleneimine-Grafted Lignin Microspheres for Efficient Adsorption of 2,4-Dichlorophenoxyacetic Acid over a Wide PH Range and Controlled Release. Chem. Eng. J. 2021, 411, 128539. [Google Scholar] [CrossRef]
- Cruz González, G.; Julcour, C.; Chaumat, H.; Jáuregui-Haza, U.; Delmas, H. Degradation of 2,4-Dichlorophenoxyacetic Acid by Photolysis and Photo-Fenton Oxidation. J. Environ. Chem. Eng. 2018, 6, 874–882. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Effect of Activation Temperature on the Textural and Chemical Properties of Potassium Hydroxide Activated Carbon Prepared from Pistachio-Nut Shell. J. Colloid Interface Sci. 2004, 274, 594–601. [Google Scholar] [CrossRef]
- Spessato, L.; Bedin, K.C.; Cazetta, A.L.; Souza, I.P.A.F.; Duarte, V.A.; Crespo, L.H.S.; Silva, M.C.; Pontes, R.M.; Almeida, V.C. KOH-Super Activated Carbon from Biomass Waste: Insights into the Paracetamol Adsorption Mechanism and Thermal Regeneration Cycles. J. Hazard. Mater. 2019, 371, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.P. Preparation and Characterization of Activated Carbon from Date Stones by Physical Activation with Steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of Pyrolysis Conditions on the Properties of Activated Carbons Prepared from Pistachio-Nut Shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Taamalli, S.; Louis, F.; El, A.; Badawi, M.; Georgin, J.; Franco, D.S.P.; Silva, L.F.O. Enhanced Adsorption of Ketoprofen and 2,4-Dichlorophenoxyactic Acid on Physalis Peruviana Fruit Residue Functionalized with H2SO4: Adsorption Properties and Statistical Physics Modeling Adri A. Chem. Eng. J. 2022, 445, 136773. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Application of Araçá Fruit Husks (Psidium cattleianum) in the Preparation of Activated Carbon with FeCl3 for Atrazine Herbicide Adsorption. Chem. Eng. Res. Des. 2022, 180, 67–78. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.P.; Netto, M.S.; Manzar, M.S.; Zubair, M.; Meili, L.; Piccilli, D.G.A.A.; Silva, L.F.O.O. Adsorption of the First-Line COVID Treatment Analgesic onto Activated Carbon from Residual Pods of Erythrina Speciosa. Environ. Manag. 2022, 2019, 795–808. [Google Scholar] [CrossRef]
- Vieira, Y.; Silveira, J.P.; Dotto, G.L.; Knani, S.; Vieillard, J.; Georgin, J.; Franco, D.S.P.; Lima, E.C. Mechanistic Insights and Steric Interpretations through Statistical Physics Modelling and Density Functional Theory Calculations for the Adsorption of the Pesticides Atrazine and Diuron by Hovenia dulcis biochar. J. Mol. Liq. 2022, 367, 120418. [Google Scholar] [CrossRef]
- Sellaoui, L.; Bouzidi, M.; Franco, D.S.P.; Alshammari, A.S.; Gandouzi, M.; Georgin, J.; Mohamed, N.B.H.; Erto, A.; Badawi, M. Exploitation of Bauhinia forficata Residual Fruit Powder for the Adsorption of Cationic Dyes. Chem. Eng. J. 2023, 456, 141033. [Google Scholar] [CrossRef]
- Hernandes, P.T.; Oliveira, M.L.S.S.; Georgin, J.; Franco, D.S.P.P.; Allasia, D.; Dotto, G.L. Adsorptive Decontamination of Wastewater Containing Methylene Blue Dye Using Golden Trumpet Tree Bark (Handroanthus albus). Environ. Sci. Pollut. Res. 2019, 26, 31924–31933. [Google Scholar] [CrossRef]
- Grassi, P.; Drumm, F.C.; Georgin, J.; Franco, D.S.P.; Dotto, G.L.; Foletto, E.L.; Jahn, S.L. Application of Cordia Trichotoma Sawdust as an Effective Biosorbent for Removal of Crystal Violet from Aqueous Solution in Batch System and Fixed-Bed Column. Environ. Sci. Pollut. Res. 2021, 28, 6771–6783. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Dotto, G.L. Evaluation of Ocotea Puberula Bark Powder (OPBP) as an Effective Adsorbent to Uptake Crystal Violet from Colored Effluents: Alternative Kinetic Approaches. Environ. Sci. Pollut. Res. 2020, 27, 25727–25739. [Google Scholar] [CrossRef] [PubMed]
- Salomón, Y.L.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Pinto, D.; Oliveira, M.L.S.; Dotto, G.L. Adsorption of Atrazine Herbicide from Water by Diospyros Kaki Fruit Waste Activated Carbon. J. Mol. Liq. 2022, 347, 117990. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Alwarthan, A. Uptake of Propranolol on Ionic Liquid Iron Nanocomposite Adsorbent: Kinetic, Thermodynamics and Mechanism of Adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Del Mar Orta, M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of Propranolol onto Montmorillonite: Kinetic, Isotherm and PH Studies. Appl. Clay Sci. 2019, 173, 107–114. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI.* A System. J. Chem. Soc. 1960, 846, 3973–3993. [Google Scholar] [CrossRef]
- Bonilla-petriciolet, A.; Mendoza-Castillo, D.I.; Dotto, G.L.; Duran-Valle, C.J.; Aguascalientes, I.T. De Adsorption in Water Treatment. In Chemistry Molecular Sciences and Chemical Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–21. ISBN 9780124095472. [Google Scholar]
- Machida, M.; Aikawa, M.; Tatsumoto, H. Prediction of Simultaneous Adsorption of Cu(II) and Pb(II) onto Activated Carbon by Conventional Langmuir Type Equations. J. Hazard. Mater. 2005, 120, 271–275. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of Kinetic Langmuir Model. Part I: Integrated Kinetic Langmuir Equation (IKL): A New Complete Analytical Solution of the Langmuir Rate Equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yan, C.; Jiang, W.; Chang, C. The Langmuir Monolayer Adsorption Model of Organic Matter into Effective Pores in Activated Carbon. J. Colloid Interface Sci. 2013, 389, 213–219. [Google Scholar] [CrossRef]
- Westreich, P.; Selig, S.; Fortier, H.; Dahn, J.R. Two Distinct Langmuir Isotherms Describe the Adsorption of Certain Salts onto Activated Carbon over a Wide Concentration Range. Carbon N. Y. 2006, 44, 3145–3148. [Google Scholar] [CrossRef]
- Wang, F.; Ren, X.; Sun, H.; Ma, L.; Zhu, H.; Xu, J. Sorption of Polychlorinated Biphenyls onto Biochars Derived from Corn Straw and the Effect of Propranolol. Bioresour. Technol. 2016, 219, 458–465. [Google Scholar] [CrossRef]
- Paixão, G.R.; Camparotto, N.G.; de Brião, G.V.; de Oliveira, R.L.; Colmenares, J.C.; Prediger, P.; Vieira, M.G.A. Synthesis of Mesoporous P-doped Carbon and Its Application in Propranolol Drug Removal: Characterization, Kinetics and Isothermal Studies. Chem. Eng. Res. Des. 2022, 187, 225–239. [Google Scholar] [CrossRef]
- Feizi, F.; Sarmah, A.K.; Rangsivek, R.; Gobindlal, K. Adsorptive Removal of Propranolol under Fixed-Bed Column Using Magnetic Tyre Char: Effects of Wastewater Effluent Organic Matter and Ball Milling. Environ. Pollut. 2022, 305, 119283. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; del Orta, M.M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Removal of Priority and Emerging Pollutants from Aqueous Media by Adsorption onto Synthetic Organo-Funtionalized High-Charge Swelling Micas. Environ. Res. 2018, 164, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of Atenolol by Adsorption–Study of Kinetics and Equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Koltsakidou, A.; Nanaki, S.G.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of Beta-Blockers from Aqueous Media by Adsorption onto Graphene Oxide. Sci. Total Environ. 2015, 537, 411–420. [Google Scholar] [CrossRef]
- Remache, W.; Ramos, D.R.; Mammeri, L.; Boucheloukh, H.; Marín, Z.; Belaidi, S.; Sehili, T.; Santaballa, J.A.; Canle, M. An Efficient Green Photo-Fenton System for the Degradation of Organic Pollutants. Kinetics of Propranolol Removal from Different Water Matrices. J. Water Process Eng. 2022, 46, 102514. [Google Scholar] [CrossRef]
- Ribas, M.C.; Adebayo, M.A.; Prola, L.D.T.; Lima, E.C.; Cataluña, R.; Feris, L.A.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Calvete, T. Comparison of a Homemade Cocoa Shell Activated Carbon with Commercial Activated Carbon for the Removal of Reactive Violet 5 Dye from Aqueous Solutions. Chem. Eng. J. 2014, 248, 315–326. [Google Scholar] [CrossRef]
- He, S.; Chen, Q.; Chen, G.; Shi, G.; Ruan, C.; Feng, M.; Ma, Y.; Jin, X.; Liu, X.; Du, C.; et al. N-Doped Activated Carbon for High-Efficiency Ofloxacin Adsorption. Microporous Mesoporous Mater. 2022, 335, 111848. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic Modeling of Azo Dye Adsorption on Non-Living Cells of Nannochloropsis Oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Worch, E. Fixed-Bed Adsorption in Drinking Water Treatment: A Critical Review on Models and Parameter Estimation. J. Water Supply Res. Technol. 2008, 57, 171–183. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Preparation of Activated Carbon from the Residues of the Mushroom (Agaricus bisporus) Production Chain for the Adsorption of the 2,4-Dichlorophenoxyacetic Herbicide. J. Environ. Chem. Eng. 2021, 9, 106843. [Google Scholar] [CrossRef]
- Georgin, J.; de Salomón, Y.L.O.; Franco, D.S.P.P.; Netto, M.S.; Piccilli, D.G.A.; Perondi, D.; Silva, L.F.O.O.; Foletto, E.L.; Dotto, G.L.; Daniel, G.; et al. Development of Highly Porous Activated Carbon from Jacaranda mimosifolia Seed Pods for Remarkable Removal of Aqueous-Phase Ketoprofen. J. Environ. Chem. Eng. 2021, 9, 105676. [Google Scholar] [CrossRef]
- Dehmani, Y.; Franco, D.S.P.; Georgin, J.; Lamhasni, T.; Brahmi, Y.; Oukhrib, R.; Mustapha, B.; Moussout, H.; Ouallal, H.; Sadik, A. Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays. Water 2023, 15, 1881. [Google Scholar] [CrossRef]
- Moussout, H.; Dehmani, Y.; Franco, D.S.P.; Georgin, J. Towards an In-Depth Experimental and Theoretical Understanding of the Cadmium Uptake Mechanism on a Synthesized Chitin Biopolymer. J. Mol. Liq. 2023, 383, 122106. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Koble, R.A.; Corrigan, T.E. Adsorption isotherms for pure hydrocarbons. Ind. Eng. Chem. 1952, 44, 383–387. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic Parameters of Liquid–Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the Nonlinear and Linear Forms of the van’t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Shen, L. A General Rate Law Equation for Biosorption. Biochem. Eng. J. 2008, 38, 390–394. [Google Scholar] [CrossRef]
- Glueckauf, E. Theory of Chromatography. Part 10.—Formulæ for Diffusion into Spheres and Their Application to Chromatography. Trans. Faraday Soc. 1955, 51, 1540–1551. [Google Scholar] [CrossRef]


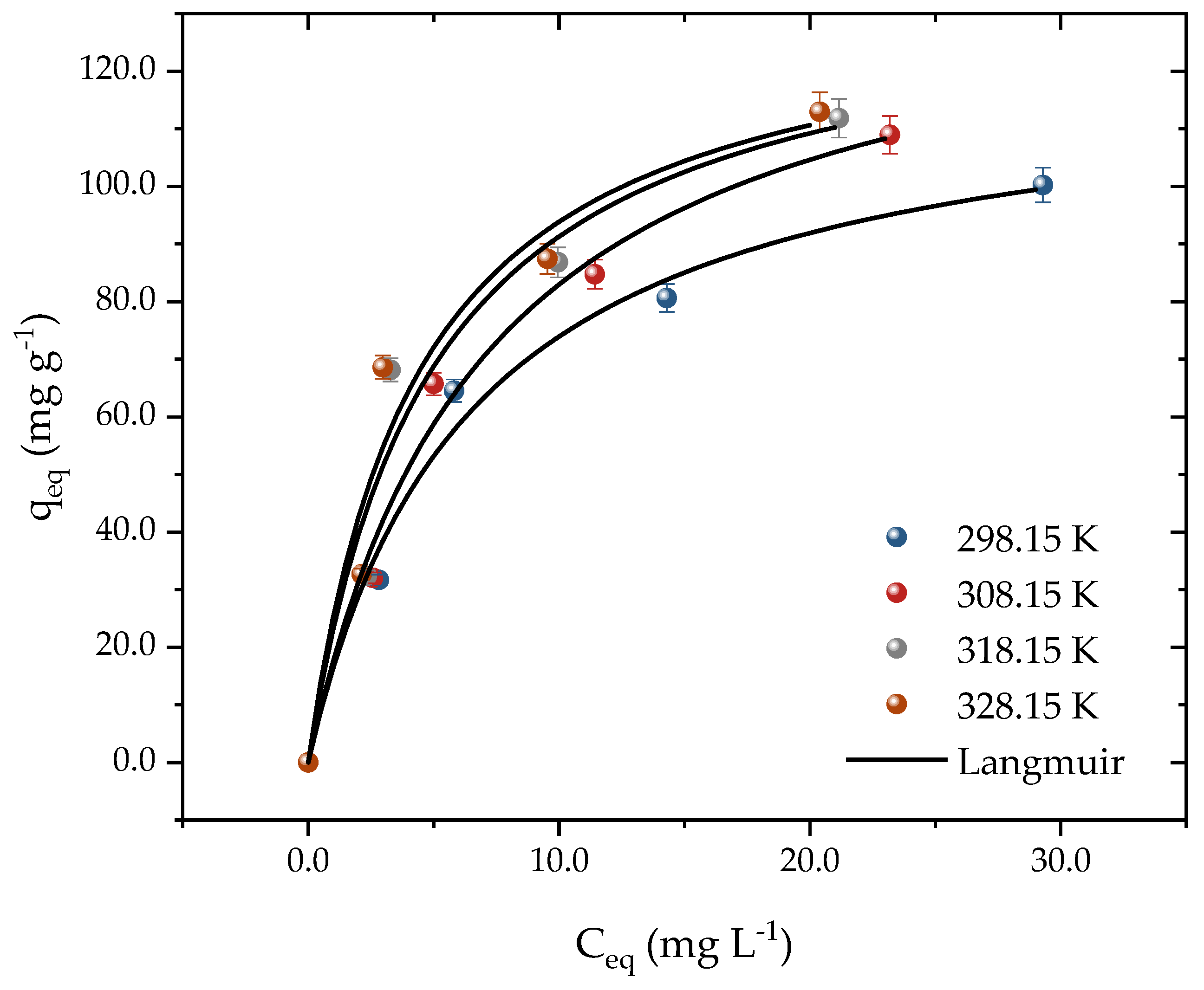
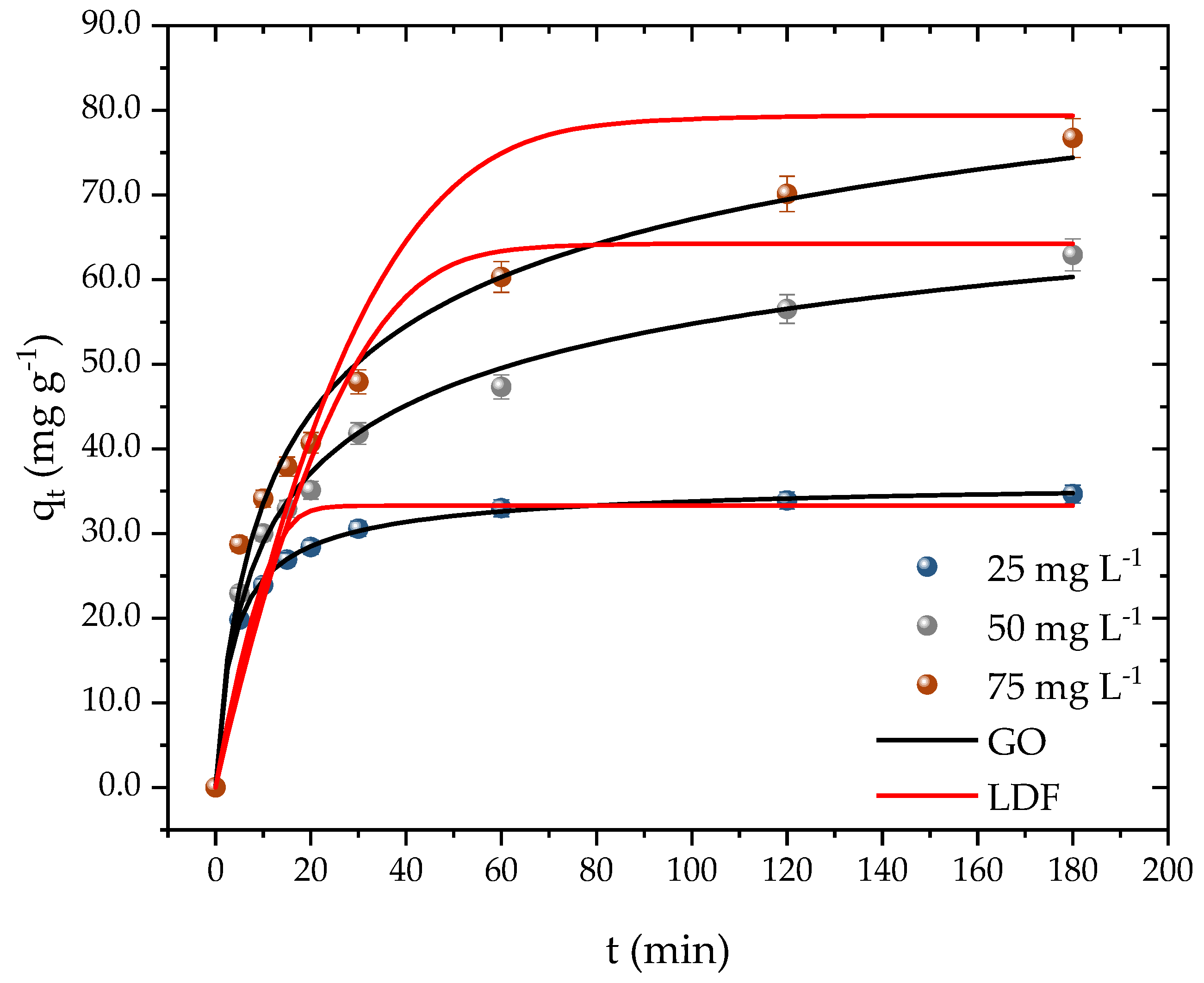
| Temperature (K) | ||||
|---|---|---|---|---|
| Model | 298.15 | 308.15 | 318.15 | 328.15 |
| Langmuir | ||||
| qmL (mg g−1) | 121.4 | 141.6 | 135.9 | 134.7 |
| KL (L mg−1) | 0.1556 | 0.1416 | 0.2044 | 0.2299 |
| R2 | 0.9867 | 0.9876 | 0.9571 | 0.9551 |
| R2adj | 0.9733 | 0.9753 | 0.9142 | 0.9102 |
| ARE (%) | 7.964 | 8.143 | 15.41 | 15.83 |
| MSR (mg g−1)2 | 28.28 | 30.51 | 112.0 | 119.4 |
| BIC | 18.16 | 18.54 | 25.04 | 25.36 |
| Freundlich | ||||
| KF ((mg g−1)(mg L−1)−1/nF) | 25.06 | 27.24 | 27.96 | 28.24 |
| 1/nF (dimensionless) | 0.4229 | 0.4501 | 0.4680 | 0.4744 |
| R2 | 0.9656 | 0.9723 | 0.9355 | 0.9293 |
| R2adj | 0.9312 | 0.9447 | 0.8710 | 0.8585 |
| ARE (%) | 12.31 | 12.85 | 16.67 | 16.27 |
| MSR (mg g−1)2 | 72.97 | 68.29 | 168.5 | 188.1 |
| BIC | 22.90 | 22.56 | 27.08 | 27.63 |
| Redlich-Peterson | ||||
| KRP (L g−1) | 18.89 | 20.04 | 28.18 | 33.19 |
| aRP (L mg−1) | 0.1556 | 0.1416 | 0.2142 | 0.2842 |
| nRP (dimensionless) | 1.000 | 1.000 | 0.9900 | 0.9556 |
| R2 | 0.9867 | 0.9876 | 0.9571 | 0.9553 |
| R2adj | 0.9467 | 0.9506 | 0.8285 | 0.8210 |
| ARE (%) | 7.964 | 8.143 | 15.40 | 15.74 |
| MSR (mg g−1)2 | 42.42 | 45.77 | 168.0 | 178.4 |
| BIC | 20.16 | 20.54 | 27.04 | 27.34 |
| Koble-Corrigan | ||||
| A (mg g−1) × (L mg−1)nKC | 12.41 | 14.60 | 20.92 | 26.91 |
| KKC (L mg−1)nKC | 0.1173 | 0.1199 | 0.1775 | 0.2191 |
| nKC (dimensionless) | 1.361 | 1.298 | 1.353 | 1.204 |
| qKC (mg g−1) | 105.8 | 121.7 | 117.9 | 122.8 |
| R2 | 0.9891 | 0.9895 | 0.9583 | 0.9555 |
| R2adj | 0.9563 | 0.9579 | 0.8330 | 0.8221 |
| ARE (%) | 6.397 | 6.914 | 15.24 | 15.79 |
| MSR (mg g−1)2 | 34.73 | 38.94 | 163.6 | 177.4 |
| BIC | 19.16 | 19.73 | 26.90 | 27.31 |
| qmax,exp (mg g−1) | 100.2 | 108.9 | 111.8 | 112.9 |
| T(K) | Ke × 10−4 | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) |
|---|---|---|---|---|
| 298.15 | 4.035 | −26.28 | 12.39 | 0.1290 |
| 308.15 | 3.672 | −26.92 | ||
| 318.15 | 5.301 | −28.77 | ||
| 328.15 | 5.962 | −29.99 |
| Model | C0 (mg L−1) | ||
|---|---|---|---|
| 25 | 50 | 75 | |
| Pseudo-first order | |||
| q1 (mg g−1) | 32.53 | 55.34 | 69.40 |
| k1 (min−1) | 0.1416 | 0.06094 | 0.05189 |
| R2 | 0.9697 | 0.9178 | 0.9182 |
| R2adj | 0.9204 | 0.7898 | 0.7909 |
| ARE (%) | 6.307 | 12.92 | 13.56 |
| MSR (mg g−1)2 | 4.033 | 33.10 | 50.82 |
| BIC | 14.29 | 33.23 | 37.09 |
| Pseudo-second order | |||
| q2 (mg g−1) | 35.09 | 62.20 | 78.27 |
| k2 × 103 (g mg−1 min−1) | 6.603 | 1.279 | 0.8580 |
| R2 | 0.9980 | 0.9690 | 0.9649 |
| R2adj | 0.9946 | 0.9186 | 0.9080 |
| ARE (%) | 1.585 | 8.001 | 9.085 |
| MSR (mg g−1)2 | 0.2686 | 12.48 | 21.82 |
| BIC | −10.09 | 24.46 | 29.48 |
| General order | |||
| qAv (mg g−1) | 36.90 | 113.8 | 140.4 |
| kAv (min−1) | 1.124 × 10−3 | 7.336 × 10−14 | 9.458 × 10−14 |
| nAv (dimensionless) | 2.530 | 6.849 | 6.508 |
| R2 | 0.9980 | 0.9690 | 0.9649 |
| R2adj | 0.9935 | 0.9023 | 0.8896 |
| ARE (%) | 0.9727 | 3.733 | 5.228 |
| MSR (mg g−1)2 | 0.1283 | 3.650 | 8.898 |
| BIC | −16.13 | 14.00 | 22.02 |
| LDF | |||
| qpred (mg g−1) | 33.29 | 64.23 | 79.34 |
| kLDF × 104 (s−1) | 5.151 | 3.737 | 3.930 |
| DS × 109 (cm2 s−1) | 5.238 | 3.800 | 3.996 |
| R2 | 0.9213 | 0.7882 | 0.8278 |
| R2adj | 0.9101 | 0.7580 | 0.8032 |
| ARE (%) | 10.26 | 25.47 | 29.62 |
| MSR (mg g−1)2 | 9.159 | 74.58 | 93.65 |
| BIC | 20.87 | 39.75 | 41.80 |
| qexp (mg g−1) | 34.66 | 62.92 | 76.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, D.S.P.; Georgin, J.; Ramos, C.G.; Eljaiek, S.M.; Badillo, D.R.; de Oliveira, A.H.P.; Allasia, D.; Meili, L. The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption. Molecules 2023, 28, 5232. https://doi.org/10.3390/molecules28135232
Franco DSP, Georgin J, Ramos CG, Eljaiek SM, Badillo DR, de Oliveira AHP, Allasia D, Meili L. The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption. Molecules. 2023; 28(13):5232. https://doi.org/10.3390/molecules28135232
Chicago/Turabian StyleFranco, Dison Stracke Pfingsten, Jordana Georgin, Claudete Gindri Ramos, Salma Martinez Eljaiek, Daniel Romero Badillo, Anelise Hoch Paschoalin de Oliveira, Daniel Allasia, and Lucas Meili. 2023. "The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption" Molecules 28, no. 13: 5232. https://doi.org/10.3390/molecules28135232
APA StyleFranco, D. S. P., Georgin, J., Ramos, C. G., Eljaiek, S. M., Badillo, D. R., de Oliveira, A. H. P., Allasia, D., & Meili, L. (2023). The Synthesis and Evaluation of Porous Carbon Material from Corozo Fruit (Bactris guineensis) for Efficient Propranolol Hydrochloride Adsorption. Molecules, 28(13), 5232. https://doi.org/10.3390/molecules28135232