Protein Metalation by Medicinal Gold Compounds: Identification of the Main Features of the Metalation Process through ESI MS Experiments
Abstract
1. Introduction
2. The Gold Compounds Chosen for the Interaction Studies
2.1. Auranofin
2.2. The Two Gold Carbenes, Au(NHC)Cl and [Au(NHC)2]PF6
2.3. Auoxo6
2.4. Aubipyc
3. Adduct Formation between Medicinal Gold Compounds and Model Proteins Disclosed by ESI MS Measurements
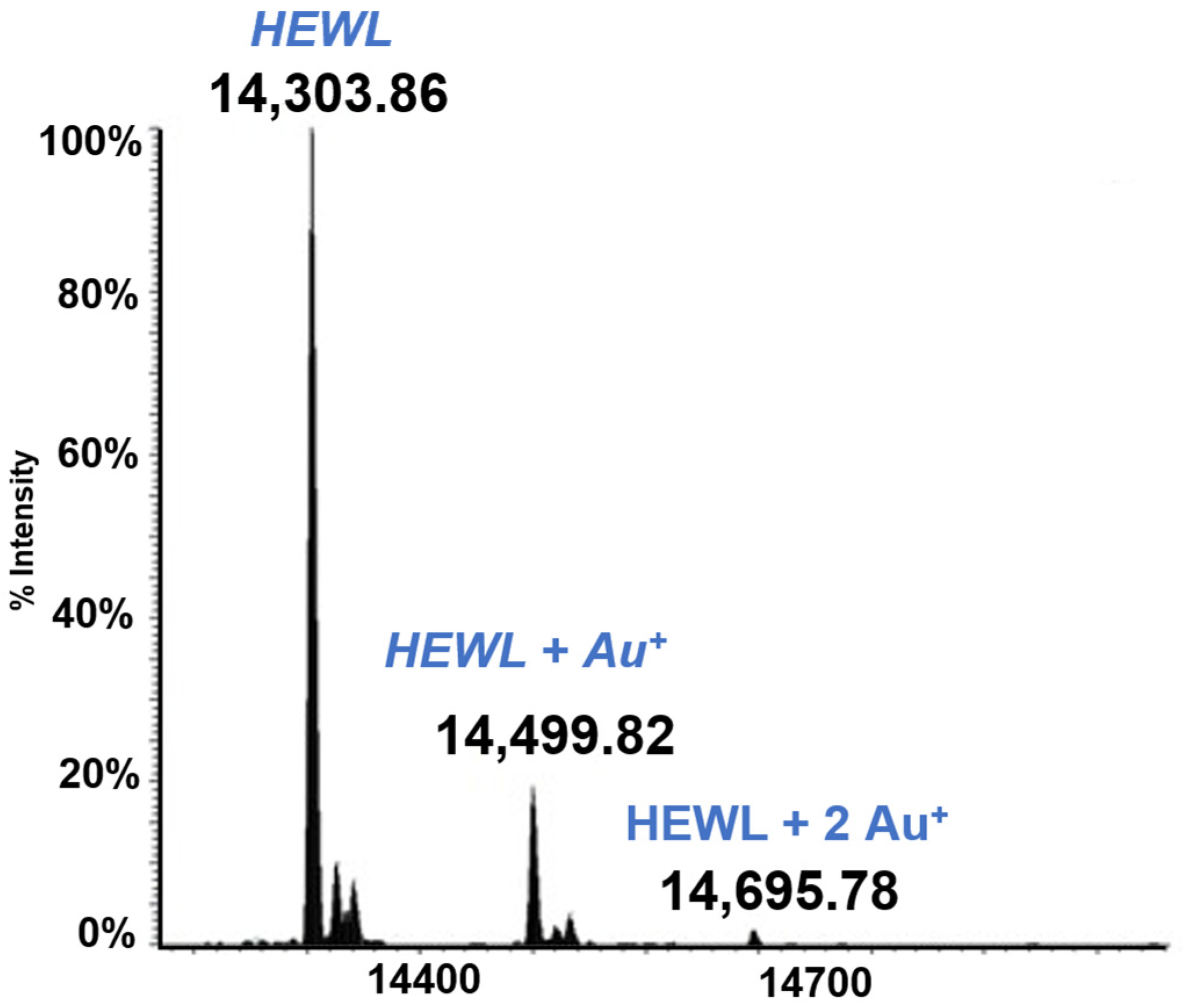
3.1. Lysozyme (HEWL)
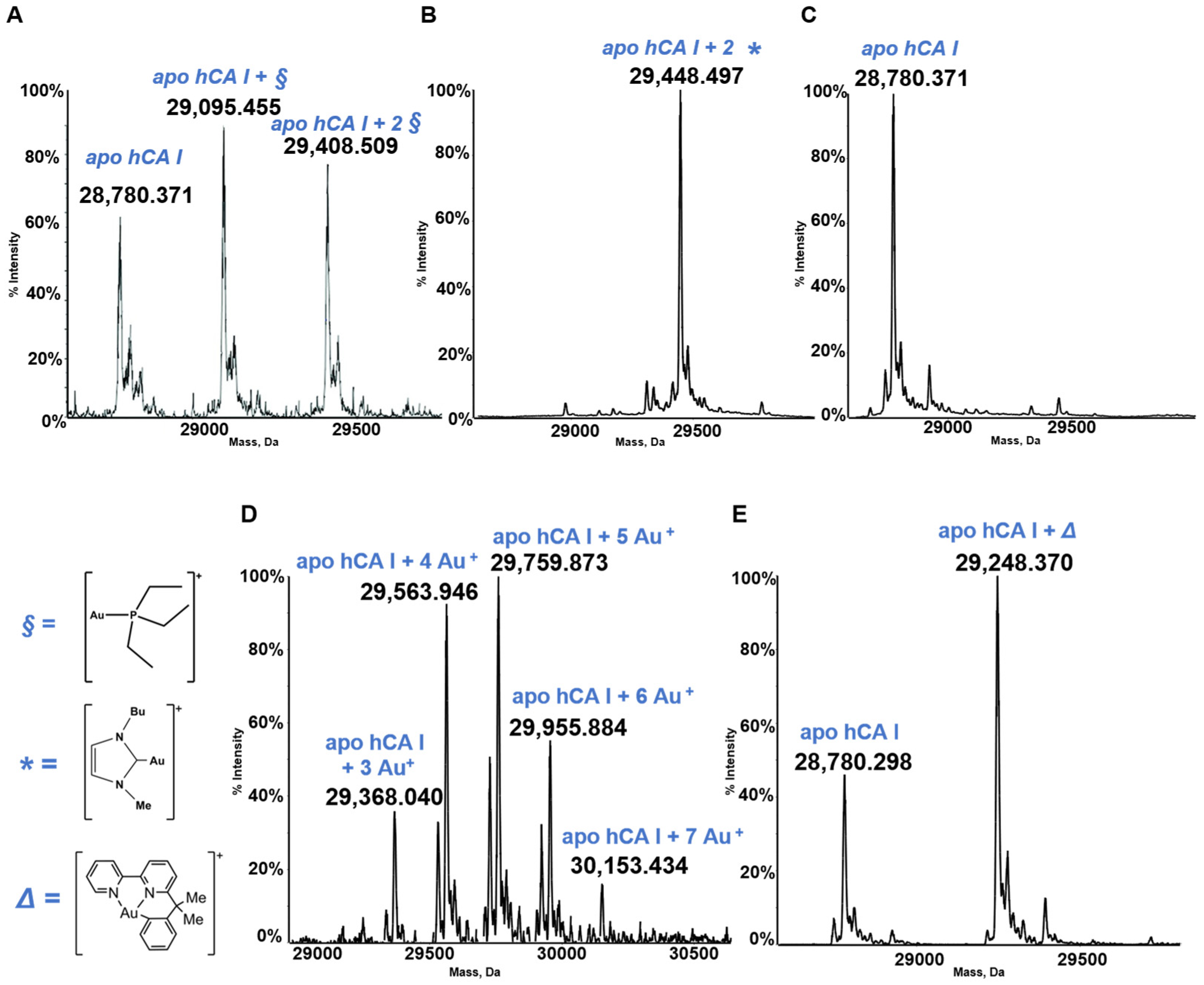
3.2. hCA I
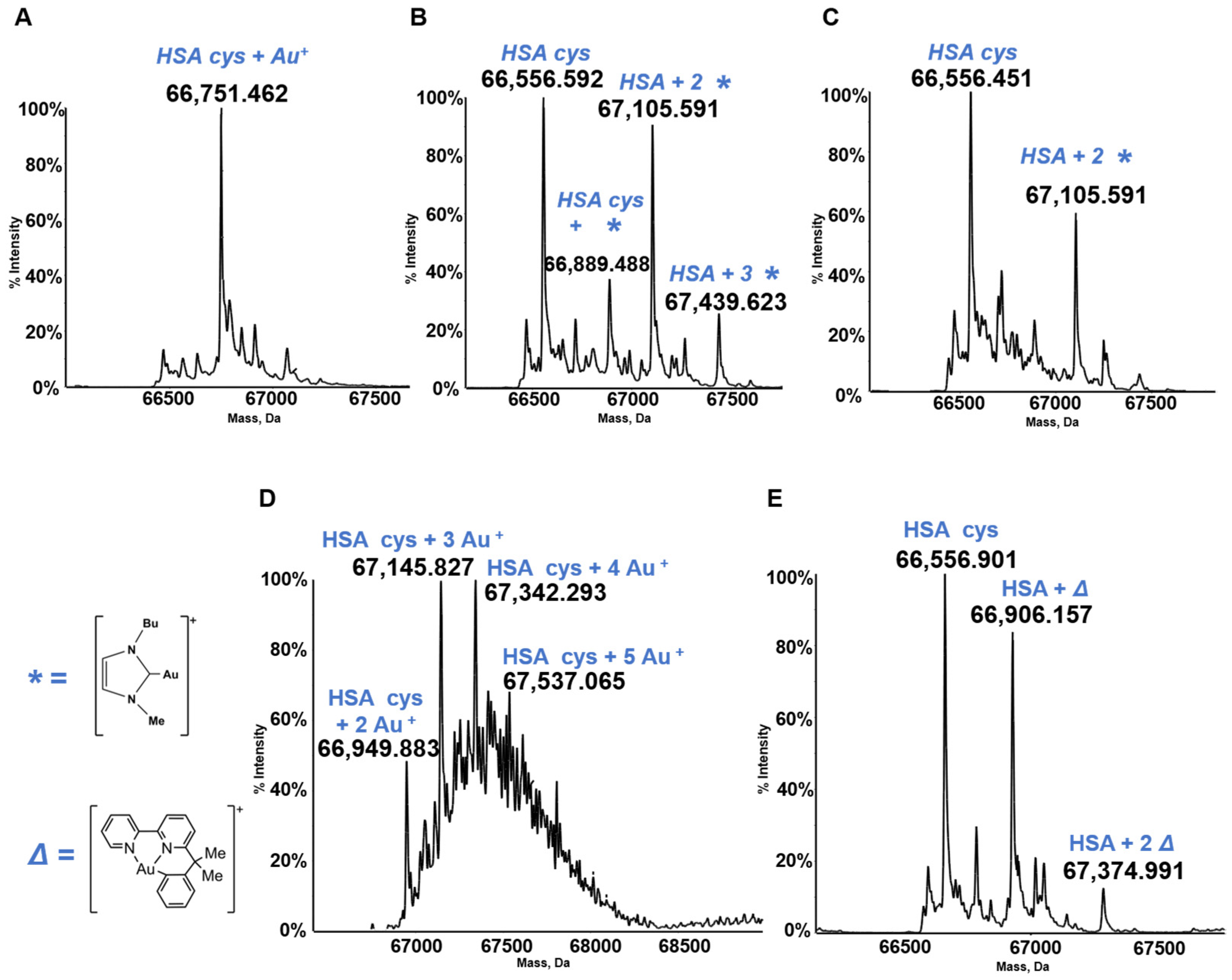
3.3. HSA
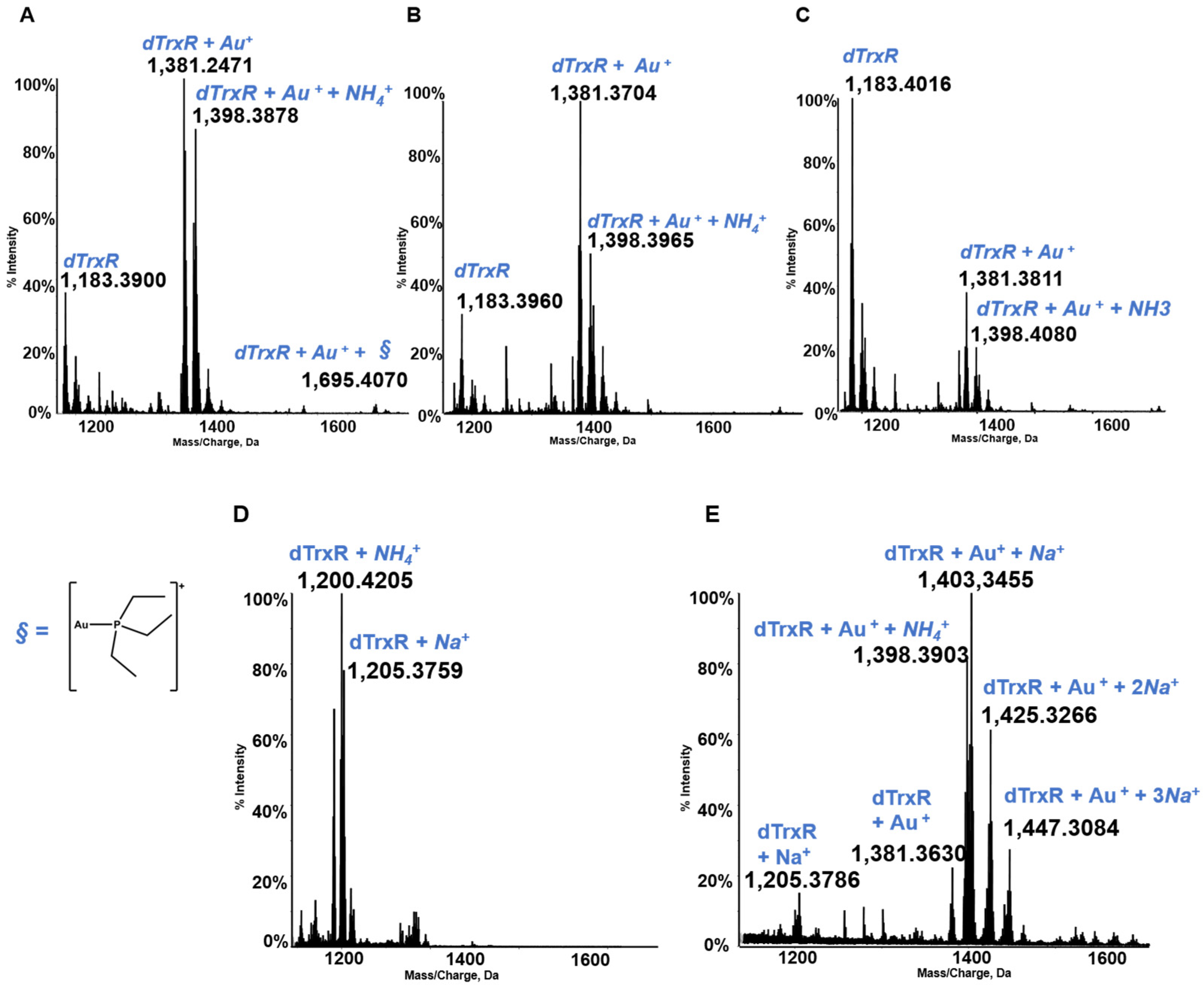
3.4. The TrxR Dodecapeptide
4. Discussion
- i.
- Gold compounds behave as prodrugs and must undergo chemical transformation in order to react with proteins.
- ii.
- The reactivity of gold compounds, its extent and selectivity are tightly controlled by the nature of the leaving group.
- iii.
- Upon reaction gold containing molecular fragments associate tightly to proteins through formation of strong coordinative bonds
- iv.
- Gold binding is highly selective for free cysteine (and selenocysteine) residues.
- v.
- Gold binding to specific protein residues may result in proteins’ loss of function.
- vi.
- In the case of gold(III) compounds, protein binding is often preceded by the reduction of gold(III) to gold(I)
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moreno-Alcántar, G.; Picchetti, P.; Casini, A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew. Chem. Int. Ed. 2023, 62, e202218000. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.; Ooi, K.; Tiekink, E. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef]
- Yue, S.; Luo, M.; Liu, H.; Wei, S. Recent Advances of Gold Compounds in Anticancer Immunity. Front. Chem. 2020, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent Development of Gold(I) and Gold(III) Complexes as Therapeutic Agents for Cancer Diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Reeder, E.; Parkin, S.; Awuah, S.G. Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents. Sci. Rep. 2019, 9, 12335. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent Advances in Gold–NHC Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) Complexes for Antitumor Applications: An Overview. Chem. Eur. J. 2018, 24, 11840–11851. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical Biology of Anticancer Gold(III) and Gold(I) Complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Herrera, R.P.; Gimeno, M.C. Anticancer Properties of Gold Complexes with Biologically Relevant Ligands. Pure Appl. Chem. 2019, 91, 247–269. [Google Scholar] [CrossRef]
- Possato, B.; Dalmolin, L.F.; Pereira, L.M.; Alves, J.Q.; Silva, R.T.C.; Gelamo, R.V.; Yatsuda, A.P.; Lopez, R.F.V.; De Albuquerque, S.; Leite, N.B.; et al. Gold(III) Complexes with Thiosemicarbazonate Ligands as Potential Anticancer Agents: Cytotoxicity and Interactions with Biomolecular Targets. Eur. J. Pharm. Sci. 2021, 162, 105834. [Google Scholar] [CrossRef]
- Tolbatov, I.; Coletti, C.; Marrone, A.; Re, N. Reactivity of Gold(I) Monocarbene Complexes with Protein Targets: A Theoretical Study. Int. J. Mol. Sci. 2019, 20, 820. [Google Scholar] [CrossRef]
- Lee, R.F.S.; Menin, L.; Patiny, L.; Ortiz, D.; Dyson, P.J. Versatile Tool for the Analysis of Metal–Protein Interactions Reveals the Promiscuity of Metallodrug–Protein Interactions. Anal. Chem. 2017, 89, 11985–11989. [Google Scholar] [CrossRef]
- Arsenijević, N.; Volarevic, V.; Milovanovic, M.; Bugarčić, Ž.D. Gold(III) Complexes, Cytotoxic Effects. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 922–927. ISBN 978-1-4614-1532-9. [Google Scholar]
- Fung, S.K.; Zou, T.; Cao, B.; Lee, P.-Y.; Fung, Y.M.E.; Hu, D.; Lok, C.-N.; Che, C.-M. Cyclometalated Gold(III) Complexes Containing N-Heterocyclic Carbene Ligands Engage Multiple Anti-Cancer Molecular Targets. Angew. Chem. Int. Ed. 2017, 56, 3892–3896. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, M.; Gross, M.L. Protein-Metal-Ion Interactions Studied by Mass Spectrometry-Based Footprinting with Isotope-Encoded Benzhydrazide. Anal. Chem. 2019, 91, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Merlino, A. Recent Advances in Protein Metalation: Structural Studies. Chem. Commun. 2021, 57, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, C.; Massai, L.; Scaletti, F.; Michelucci, E.; Maiore, L.; Cinellu, M.A.; Messori, L. Protein Metalation by Metal-Based Drugs: Reactions of Cytotoxic Gold Compounds with Cytochrome c and Lysozyme. J. Biol. Inorg. Chem. 2012, 17, 1293–1302. [Google Scholar] [CrossRef]
- Marzo, T.; Ferraro, G.; Merlino, A.; Messori, L. Protein Metalation by Inorganic Anticancer Drugs. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–17. ISBN 978-1-119-95143-8. [Google Scholar]
- Banerjee, S.; Mazumdar, S. Electrospray Ionization Mass Spectrometry: A Technique to Access the Information beyond the Molecular Weight of the Analyte. Int. J. Anal. Chem. 2012, 2012, 282574. [Google Scholar] [CrossRef]
- Farmer, T.B.; Caprioli, R.M. Electrospray Ionization Mass Spectrometry: Protein Structure. In Mass Spectrometry in Biomolecular Sciences; Caprioli, R.M., Malorni, A., Sindona, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 61–88. ISBN 978-94-010-6581-8. [Google Scholar]
- Zoppi, C.; Massai, L.; Cirri, D.; Gabbiani, C.; Pratesi, A.; Messori, L. Protein Metalation by Two Structurally Related Gold(I) Carbene Complexes: An ESI MS Study. Inorganica Chim. Acta 2021, 520, 120297. [Google Scholar] [CrossRef]
- Casini, A.; Kelter, G.; Gabbiani, C.; Cinellu, M.A.; Minghetti, G.; Fregona, D.; Fiebig, H.-H.; Messori, L. Chemistry, Antiproliferative Properties, Tumor Selectivity, and Molecular Mechanisms of Novel Gold(III) Compounds for Cancer Treatment: A Systematic Study. J. Biol. Inorg. Chem. 2009, 14, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Modesti, A.; Bini, L.; Puglia, M.; Landini, I.; Nobili, S.; Mini, E.; Cinellu, M.A.; Gabbiani, C.; Messori, L. Exploring the Biochemical Mechanisms of Cytotoxic Gold Compounds: A Proteomic Study. J. Biol. Inorg. Chem. 2010, 15, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marchetti, L.; Massai, L.; Scaletti, F.; Guerri, A.; Landini, I.; Nobili, S.; Perrone, G.; Mini, E.; Leoni, P.; et al. Chemistry and Biology of Two Novel Gold(I) Carbene Complexes as Prospective Anticancer Agents. Inorg. Chem. 2014, 53, 2396–2403. [Google Scholar] [CrossRef]
- Casini, A.; Cinellu, M.A.; Minghetti, G.; Gabbiani, C.; Coronnello, M.; Mini, E.; Messori, L. Structural and Solution Chemistry, Antiproliferative Effects, and DNA and Protein Binding Properties of a Series of Dinuclear Gold(III) Compounds with Bipyridyl Ligands. J. Med. Chem. 2006, 49, 5524–5531. [Google Scholar] [CrossRef] [PubMed]
- Marcon, G.; Carotti, S.; Coronnello, M.; Messori, L.; Mini, E.; Orioli, P.; Mazzei, T.; Cinellu, M.A.; Minghetti, G. Gold(III) Complexes with Bipyridyl Ligands: Solution Chemistry, Cytotoxicity, and DNA Binding Properties. J. Med. Chem. 2002, 45, 1672–1677. [Google Scholar] [CrossRef]
- Chaffman, M.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Auranofin: A Preliminary Review of Its Pharmacological Properties and Therapeutic Use in Rheumatoid Arthritis. Drugs 1984, 27, 378–424. [Google Scholar] [CrossRef]
- Kean, W.F.; Hart, L.; Buchanan, W.W. Auranofin. Rheumatology 1997, 36, 560–572. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Yamashita, M. Auranofin: Past to Present, and Repurposing. Int. Immunopharmacol. 2021, 101, 108272. [Google Scholar] [CrossRef] [PubMed]
- Abdalbari, F.H.; Telleria, C.M. The Gold Complex Auranofin: New Perspectives for Cancer Therapy. Discov. Oncol. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, C.; Messori, L.; Pratesi, A. ESI MS Studies Highlight the Selective Interaction of Auranofin with Protein Free Thiols. Dalton Trans. 2020, 49, 5906–5913. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Senzacqua, T.; Massai, L.; Gamberi, T.; Messori, L.; Turano, P. NMR Reveals the Metabolic Changes Induced by Auranofin in A2780 Cancer Cells: Evidence for Glutathione Dysregulation. Dalton Trans. 2021, 50, 6349–6355. [Google Scholar] [CrossRef]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer Metal-N-Heterocyclic Carbene Complexes of Gold, Platinum and Palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.K.; Nandy, A.; Bertolasi, V.; Bielawski, C.W.; Das Saha, K.; Dinda, J. Novel Gold(I)- and Gold(III)-N-Heterocyclic Carbene Complexes: Synthesis and Evaluation of Their Anticancer Properties. Organometallics 2014, 33, 2544–2548. [Google Scholar] [CrossRef]
- Magherini, F.; Fiaschi, T.; Valocchia, E.; Becatti, M.; Pratesi, A.; Marzo, T.; Massai, L.; Gabbiani, C.; Landini, I.; Nobili, S.; et al. Antiproliferative Effects of Two Gold(I)-N-Heterocyclic Carbene Complexes in A2780 Human Ovarian Cancer Cells: A Comparative Proteomic Study. Oncotarget 2018, 9, 28042–28068. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Messori, L.; Carpentieri, A.; Amoresano, A.; Melchiorre, C.; Fiaschi, T.; Modesti, A.; Gamberi, T.; Magherini, F. The Effects of Two Gold-N-Heterocyclic Carbene (NHC) Complexes in Ovarian Cancer Cells: A Redox Proteomic Study. Cancer Chemother. Pharmacol. 2022, 89, 809–823. [Google Scholar] [CrossRef]
- Cinellu, M.A.; Minghetti, G.; Pinna, M.V.; Stoccoro, S.; Zucca, A.; Manassero, M.; Sansoni, M. μ-Oxo and Alkoxo Complexes of Gold(III) with 6-Alkyl-2,2′-Bipyridines. Synthesis, Characterization and X-Ray Structures. J. Chem. Soc. Dalton Trans. 1998, 11, 1735–1742. [Google Scholar] [CrossRef]
- Gabbiani, C.; Casini, A.; Messori, L.; Guerri, A.; Cinellu, M.A.; Minghetti, G.; Corsini, M.; Rosani, C.; Zanello, P.; Arca, M. Structural Characterization, Solution Studies, and DFT Calculations on a Series of Binuclear Gold(III) Oxo Complexes: Relationships to Biological Properties. Inorg. Chem. 2008, 47, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, C.; Cinellu, M.A.; Maiore, L.; Massai, L.; Scaletti, F.; Messori, L. Chemistry and Biology of Three Representative Gold(III) Compounds as Prospective Anticancer Agents. Inorganica Chim. Acta 2012, 393, 115–124. [Google Scholar] [CrossRef]
- Kupiec, M.; Ziółkowski, R.; Massai, L.; Messori, L.; Pawlak, K. The Electrochemical Profiles of Auranofin and Aubipyc, Two Representative Medicinal Gold Compounds: A Comparative Study. J. Inorg. Biochem. 2019, 198, 110714. [Google Scholar] [CrossRef]
- Casini, A.; Hartinger, C.; Gabbiani, C.; Mini, E.; Dyson, P.J.; Keppler, B.K.; Messori, L. Gold(III) Compounds as Anticancer Agents: Relevance of Gold–Protein Interactions for Their Mechanism of Action. J. Inorg. Biochem. 2008, 102, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Casini, A. Mass Spectrometry as a Powerful Tool to Study Therapeutic Metallodrugs Speciation Mechanisms: Current Frontiers and Perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Messori, L.; Scaletti, F.; Massai, L.; Cinellu, M.A.; Gabbiani, C.; Vergara, A.; Merlino, A. The Mode of Action of Anticancer Gold-Based Drugs: A Structural Perspective. Chem. Commun. 2013, 49, 10100. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Zoppi, C.; Cirri, D.; Pratesi, A.; Messori, L. Reactions of Medicinal Gold(III) Compounds With Proteins and Peptides Explored by Electrospray Ionization Mass Spectrometry and Complementary Biophysical Methods. Front. Chem. 2020, 8, 581648. [Google Scholar] [CrossRef] [PubMed]
- Cirri, D.; Massai, L.; Giacomelli, C.; Trincavelli, M.L.; Guerri, A.; Gabbiani, C.; Messori, L.; Pratesi, A. Synthesis, Chemical Characterization, and Biological Evaluation of a Novel Auranofin Derivative as an Anticancer Agent. Dalton Trans. 2022, 51, 13527–13539. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Scalcon, V.; Bindoli, A.; Rigobello, M.P. Significance of the Mitochondrial Thioredoxin Reductase in Cancer Cells: An Update on Role, Targets and Inhibitors. Free Radic. Biol. Med. 2018, 127, 62–79. [Google Scholar] [CrossRef]
- Gromer, S.; Arscott, L.D.; Williams, C.H.; Schirmer, R.H.; Becker, K. Human Placenta Thioredoxin Reductase. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin Reductase: A Target for Gold Compounds Acting as Potential Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Rubbiani, R.; Schuh, E.; Meyer, A.; Lemke, J.; Wimberg, J.; Metzler-Nolte, N.; Meyer, F.; Mohr, F.; Ott, I. TrxR Inhibition and Antiproliferative Activities of Structurally Diverse Gold N-Heterocyclic Carbene Complexes. Med. Chem. Commun. 2013, 4, 942. [Google Scholar] [CrossRef]
- Pratesi, A.; Gabbiani, C.; Michelucci, E.; Ginanneschi, M.; Papini, A.M.; Rubbiani, R.; Ott, I.; Messori, L. Insights on the Mechanism of Thioredoxin Reductase Inhibition by Gold N-Heterocyclic Carbene Compounds Using the Synthetic Linear Selenocysteine Containing C-Terminal Peptide HTrxR(488-499): An ESI-MS Investigation. J. Inorg. Biochem. 2014, 136, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gusev, D.G. Donor Properties of a Series of Two-Electron Ligands. Organometallics 2009, 28, 763–770. [Google Scholar] [CrossRef]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-Targeted Chemotherapeutics: The Rational Design of Gold(I) N-Heterocyclic Carbene Complexes That Are Selectively Toxic to Cancer Cells and Target Protein Selenols in Preference to Thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Rubbiani, R.; Can, S.; Kitanovic, I.; Alborzinia, H.; Stefanopoulou, M.; Kokoschka, M.; Mönchgesang, S.; Sheldrick, W.S.; Wölfl, S.; Ott, I. Comparative in Vitro Evaluation of N-Heterocyclic Carbene Gold(I) Complexes of the Benzimidazolylidene Type. J. Med. Chem. 2011, 54, 8646–8657. [Google Scholar] [CrossRef] [PubMed]
- Berners-Price, S.J.; Filipovska, A. Gold Compounds as Therapeutic Agents for Human Diseases. Metallomics 2011, 3, 863. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geri, A.; Massai, L.; Messori, L. Protein Metalation by Medicinal Gold Compounds: Identification of the Main Features of the Metalation Process through ESI MS Experiments. Molecules 2023, 28, 5196. https://doi.org/10.3390/molecules28135196
Geri A, Massai L, Messori L. Protein Metalation by Medicinal Gold Compounds: Identification of the Main Features of the Metalation Process through ESI MS Experiments. Molecules. 2023; 28(13):5196. https://doi.org/10.3390/molecules28135196
Chicago/Turabian StyleGeri, Andrea, Lara Massai, and Luigi Messori. 2023. "Protein Metalation by Medicinal Gold Compounds: Identification of the Main Features of the Metalation Process through ESI MS Experiments" Molecules 28, no. 13: 5196. https://doi.org/10.3390/molecules28135196
APA StyleGeri, A., Massai, L., & Messori, L. (2023). Protein Metalation by Medicinal Gold Compounds: Identification of the Main Features of the Metalation Process through ESI MS Experiments. Molecules, 28(13), 5196. https://doi.org/10.3390/molecules28135196








