Abstract
Titanium-manganites of LaLi2TiMnO6 and LaNa2TiMnO6 were synthesized by the methods of ceramic technology from the oxides of lanthanum, titanium (IV), manganese (III), and the carbonates of lithium and sodium. The types of their syngony and the parameters of their gratings were determined radiographically. The isobaric heat capacities of titanium-manganites were measured with experimental calorimetry in the range of 298.15–673 K. It was found that on the dependence curve of heat capacity versus temperature of C°p~f(T), for LaLi2TiMnO6 at 348 K and 598 K, and LaNa2TiMnO6 at 348 K, there are abnormal jumps in heat capacity, probably related to phase transitions of the second kind. Taking into account the temperatures of the phase transitions, the equations of the temperature dependence of the heat capacity of titanium-manganites were derived. Their standard entropies were calculated by the ion increments method. Temperature dependences of the thermodynamic functions of S°(T), H°(T)-H°(298.15), and Φxx(T) were calculated using the experimental data on heat capacities and the calculated values of the standard entropies. The standard heat capacities of the studied compounds were calculated by the independent methods of ion increments and Debye, the values of which were in satisfactory agreement with the experimental data. The standard enthalpy of the formation of LaLi2TiMnO6 and LaNa2TiMnO6 was calculated according to the methodology developed by the authors. The conducted electrophysical studies determined the nature of the second-order phase transition and the semiconductor features of their conductivity. Thus, all the above-mentioned data on the experimental and calculated studies of the temperature dependence of heat capacity, the thermodynamic functions to determine a standard enthalpy of formation of LaLi2TiMnO6 and LaNa2TiMnO6, and the investigation of their electrical properties are absolutely new, and they have no analogues.
1. Introduction
Ferroelectric materials are of interest in developing electrically controlled ultrahigh frequency devices [1]. They include manganites and compounds based on titanium dioxide and have unique physical and physical–chemical properties [2,3]. Manganites–perovskites, as representatives of strongly correlated systems, are currently the subject of intensive research; this is primarily due to the colossal magnetoresistance (CMS) observed in manganites. Such CMR values allow the use of manganites in the field of spin electronics: magnetic sensors, magnetoresistive reading heads, and magnetoresistive RAM [4]. Recently, manganites have been considered promising materials for creating magnetic refrigerators operating at room temperatures, which are compact, highly efficient, and environmentally safe [5].
It should be noted that the authors in [6] obtained substituted lanthanum–strontium manganites La0.7Sr0.3Mn0.9Me0.1O3 ± δ (Me = Ti, Cr, Fe, Cu) using standard ceramic and glycerin-nitrate technologies. Their crystal structure was studied by high-temperature X-ray powder diffraction, thermal expansion coefficients were calculated, and electrical conductivity was investigated. In [7], the structural, electrical, and magnetic properties of the La0.7Sr0.3Mn1-xTixO3 system, which is characterized by rhombohedral distortion of the structure, were considered. It was found that the substitution of manganese ions with titanium ions leads to a weakening of ferromagnetism and an increase in resistivity. The study [8], presents the results of a study of the electrochemical properties of perovskite-like solid solutions (La0.5+xSr0.5−x)1–yMn0.5Ti0.5O3−δ (x = 0–0.25, y = 0–0.03), synthesized by the citrate method, and studied as an oxide anode materials for solid oxide fuel cells. The authors in [9] present the structural, magnetic, and electrical properties of mixed Ti-MnSr(1−x)La(x)Ti(0.5)Mn(0.5)O3 (0 ≤ x ≤ 0.5). X-ray absorption spectroscopic measurements show that the addition of La3+ is compensated by a partial reduction of Mn4+ to Mn3+.
Along with manganites, semiconductor titanium oxides with transition metal impurities also attract attention as promising materials for their use in spin electronics and catalysis [10]. These include barium titanate, a traditional electro-ceramic material with properties of ferro-, ferroelectric, and paraelectric. It should be emphasized that the high values of the dielectric permittivity of ferroelectrics near the phase transition temperature allow them to be used in miniature capacitors [11]. The solid electrolytes of Li0.35La0.55TiO3-x wt.% LiF (LLTO–FX, x = 0.2, 4 and 6) were synthesized by the solid-phase reaction, as described in [12]; all samples formed a perovskite structure and the grain size gradually enlarged with increasing LiF content. The LLTO-F2 electrolyte showed high conductivity at a low activation energy of 0.26 eV; therefore, it is suitable to use in solid-state batteries. Titanates of Bi2Pr2Ti3O12 and Bi2Nd2Ti3O12 were obtained with solid-phase synthesis and sintered in air at temperatures of 1003–1323 K of the stoichiometric mixtures of Bi2O3, Nd2O3, Pr6O11, and TiO2. Their crystal structure was detected by X-ray diffraction. The high-temperature heat capacity was determined by differential scanning calorimetry. Based on the experimental data of Cp = f(T), the basic thermodynamic functions were calculated [13]. The heat capacities and thermodynamic characteristics of K2La2Ti3O10, K2Nd2Ti3O10, ErGaTi2O7, DyGaTi2O7, and EuGaTi2O7 were studied by the experimental and calculated methods described in [14,15,16]. The research of the thermodynamic properties of double and ternary substituted manganites of the compositions of LaMeIMn2O5, LnMeIIMn2O5.5, LnMeI3Mn2O6, and LnMeII3Mn4O12 (MeI-alkali, MeII-alkaline earth, Ln-rare-earth metals), was generalized in [17]. The above results demonstrate that the data available in the literature describe the thermodynamic properties of the individually substituted titanates and the individual manganites of the rare earth, alkali, and alkaline earth metals.
The study of the thermodynamic properties of substances is important for the directed synthesis of new compounds and materials. The thermodynamic functions in a wide temperature range are calculated during the study of heat capacity. Data on heat capacity allow the exploration of various ordering processes, determining the magnetic ferroelectric and superconductivity properties, etc. [18]. It should be stated that it is not possible to calculate the temperature dependences of enthalpy and entropy without heat capacity, i.e., the thermodynamic functions determining the direction of a chemical reaction such as Gibbs energy (ΔG) and the reduced thermodynamic potential (Φ**(T)) are calculated on their basis. However, the above data shows that there is no information in the literature about the synthesis and thermodynamic properties of combined double titanium-manganites of rare earth and alkali metals.
As a result of the above, the purpose of this study is a calorimetric investigation of heat capacity; calculations of the thermodynamic functions of the new titanium-manganites of LaLi2TiMnO6 and LaNa2TiMnO6; calculation of the fundamental thermodynamic constants of the studied compounds; the standard heat capacity, standard entropy, standard enthalpy of formation by the independent methods; and also the study of the temperature dependence of their electrophysical characteristics. This study is a continuation of our investigations; our results were summarized in [17]. The results obtained are of importance to predict the directed synthesis of the studied and analogous compounds, to analyze the heterogeneous equilibria according to II and III laws of thermodynamics involving titanium-manganites, and to discover their valuable physical and chemical properties. The new thermochemical constants of titanium-manganites are an initial data store to be included in fundamental reference books and information databanks.
2. Results and Discussion
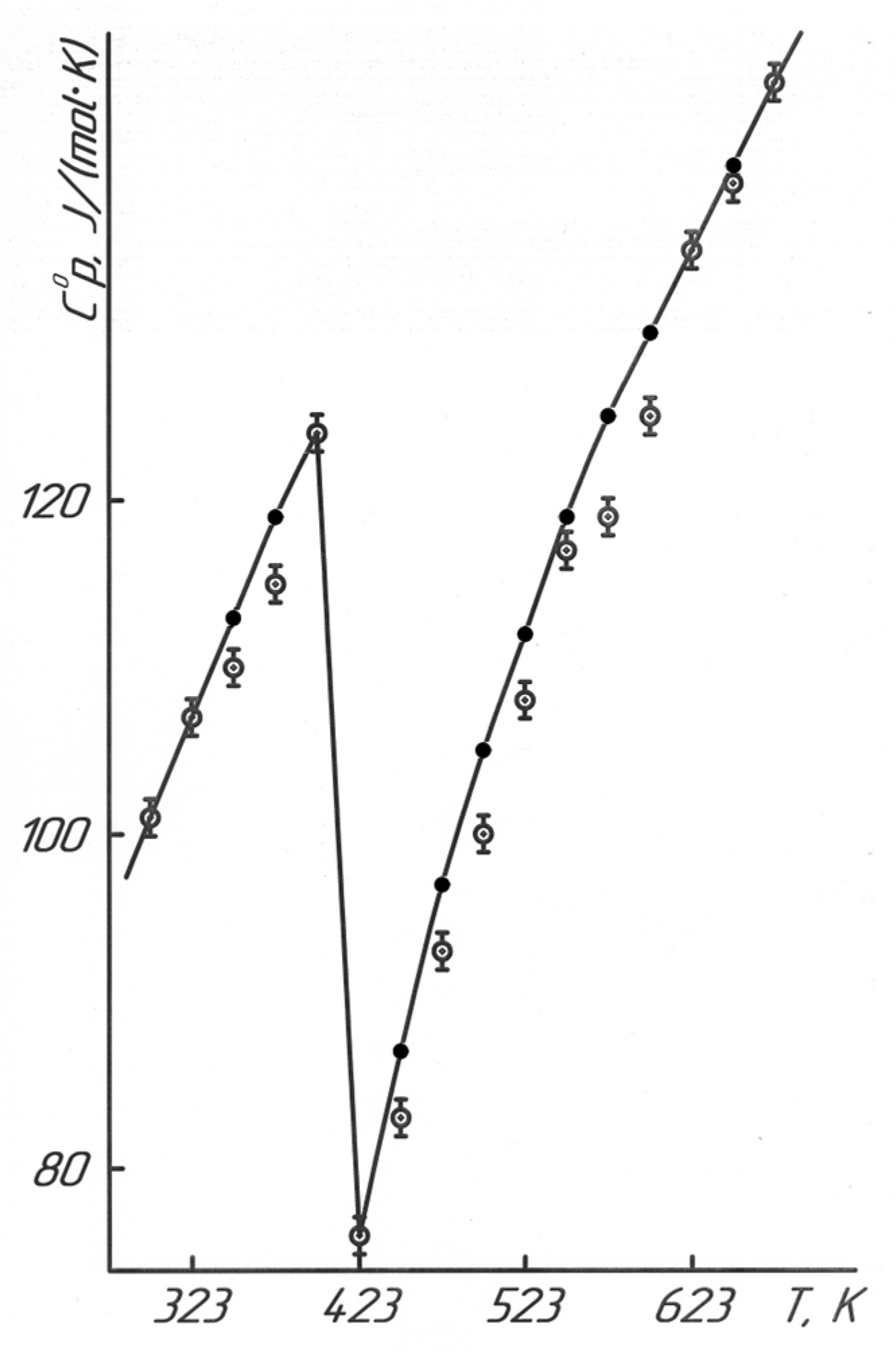
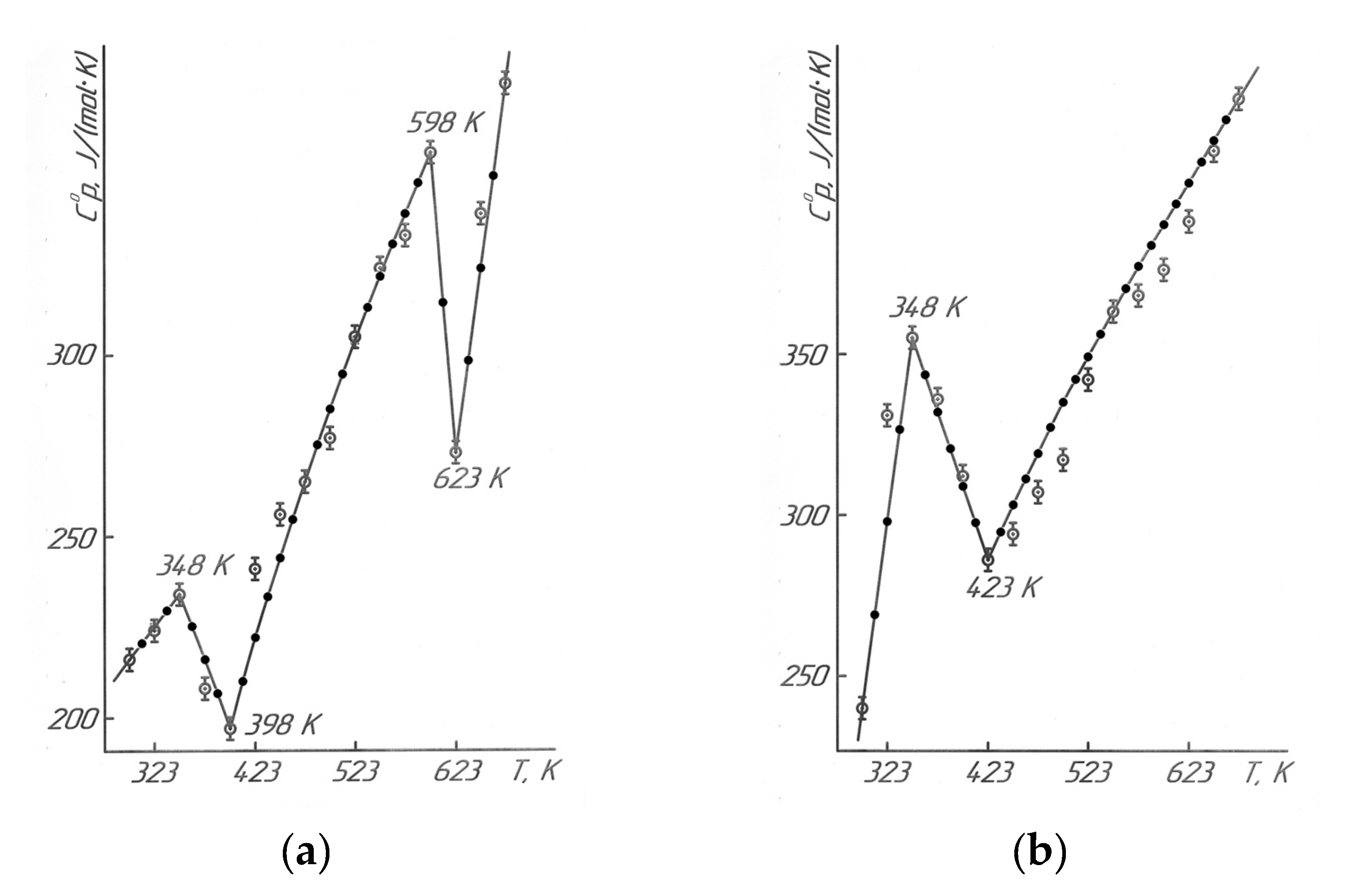
Results of the calorimetric studies in Figure 5 and Table 7 describe that LaLi2TiMnO6 (at 348 K, 598 K) and LaNa2TiMnO6 (at 348 K) had anomalous discontinuities of heat capacity on the C°p~f(T) curve; this is probably related to the second-order phase transition. These transitions can be caused by Schottky effects, changes in the magnetic resistance, the electrical conductivity, the dielectric permittivity, Curie points, and Néel points, etc. [19]. Based on temperatures of the second-order phase transition, the equations of the temperature dependence of the heat capacity of titanium-manganites were calculated. They are described by the following equations (Table 1).

Table 1.
Coefficients of equations for the temperature dependence of heat capacity.
The resulting calculated curves and lines sufficiently describe the experimental data (Figure 5).
The graphs in Figure 5 are based on the experimental data and equations in Table 1, using the KOMPAS-3D LT software. For the reliability and correctness of the obtained straight lines and curves of the dependences of C°p~f(T), the calculated values of the heat capacities are also shown in Figure 5 between the experimental ones. Then, after the thermodynamic studies, we demonstrated the results of the electrophysical investigations to determine the nature of the mentioned second-order phase transitions.
In order to compare the phase transitions on the dependence curve C°p~f(T) of titanium-manganites, the temperature dependence of the heat capacity on the IT-S-400 of the standard substance of barium titanate (BaTiO3) was investigated. BaTiO3 (“p.a.”) corresponding to TU 6-09-3963-84 (purity of BaTiO3-99.8448%) was chosen to explore. It was analyzed by X-ray phase analysis using DRON-2.0 to compare with the reference data.
All the diffraction maxima on the X-ray photograph of BaTiO3 were equal to 4.04, 2.87, 2.35, 2.05, 1.83, and 1.66; 1.44 Å corresponded to data in the ASTM database [20].
Figure 1 and Table 2 show the research results of BaTiO3 heat capacity in the range of 298.15–673 K.
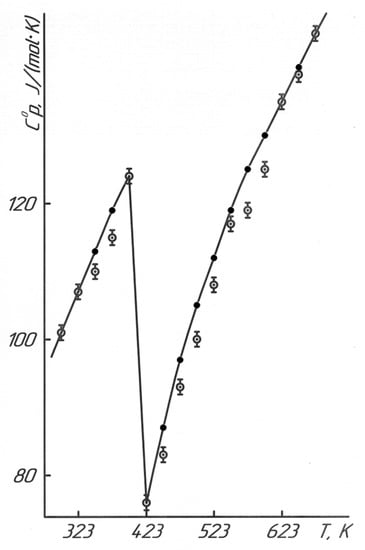
Figure 1.
Temperature dependence of the heat capacity of BaTiO3.  —experimental data, ●—calculated data.
—experimental data, ●—calculated data.
 —experimental data, ●—calculated data.
—experimental data, ●—calculated data.

Table 2.
The experimental values of BaTiO3 heat capacity [, J/(g·K); C°p ± , J/(mol·K)].
It should be stated that the experimental value of the standard heat capacity of BaTiO3 was equal to 101 ± 7 J/(mol·K), which is in good agreement with its reference data of 102.45 J/(mol·K), derived on the basis of the equation of the temperature dependence of the heat capacity (J/(mol·K)) [21]:
C°p = 121.46 + 8.535 × 10−3T − 19.163 × 105T−2 (298.15 − 1800 K).
The experimental value of C°p(298.15) for BaTiO3 also corresponds well with its calculated value equal to 100.3 J/(mol·K). It was calculated by the method of the ionic entropic increments [2] under the formula:
where SiBa2+ and SiTiO32−-ionic entropic increments equal 28.4 and 71.9 J/(mol·K), respectively [22].
S°(298.15) BaTiO3 = SiBa2+ + SiTiO32−,
Figure 1 illustrates the dependence diagram of C°p~f(T) for BaTiO3 in the interval of 298.15–673 K.
The data in Figure 1 and Table 2 demonstrate that the phase transition was observed in BaTiO3 at 398 K (125 °C). Referring to the literature data [23], this transition is observed at 393 K (120 °C) with a transition of its tetragonal modification to a cubic one with the appearance of the Curie point. The temperature dependence of the heat capacity of BaTiO3 was studied, and it depended on the heating rate of 1, 3, and 5 K/min, as described in [24]. This phase transition was observed at 395 K, 394.1 K, and 390.9 K, respectively. The technical capabilities of the IT-C-400 calorimeter can measure the heat capacities only per 25 K (in this interval of 373–398 K); thus, the temperature of this observed phase transition at 398 K is quite correct. Based on the temperature of the phase transition (398 K), we derived the equations describing this temperature dependence (J/(mol·K)):
C°p(1) = (45.2 ± 2.5) + (207.8 ± 11.5) × 10−3T − (5.64 ± 0.31) × 105T−2 (298.15–398 K),
C°p(2) = (885.6 ± 49.1) − (1912.8 ± 105.97) × 10−3T (398–423 K),
C°p(3) = (137.5 ± 7.6) + (62.1 ± 3.4) × 10−3T − (156.22 ± 8.65) × 105T−2 (423–673 K).
The technical capabilities of the calorimeter made it possible to calculate the standard entropies of compounds by using a system of ionic entropy increments [22]. These were equal to 203 ± 6 and 244 ± 7 J/(mol.K), respectively, for LaLi2TiMnO6 and LaNa2TiMnO6.
The temperature dependences of C°p(T) and the thermodynamic functions of S°(T), H°(T)–H°(298.15), and Φxx(T) (Table 3, Figure 2), in the interval of 298.15–675 K, were calculated by equations [25]:

Table 3.
The thermodynamic functions of the compounds.
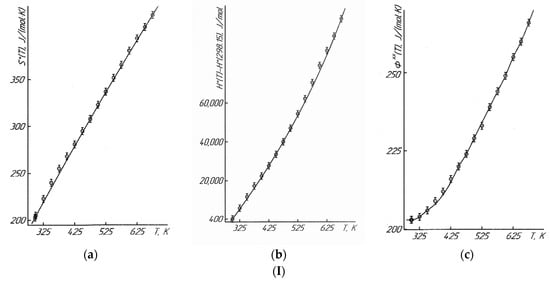
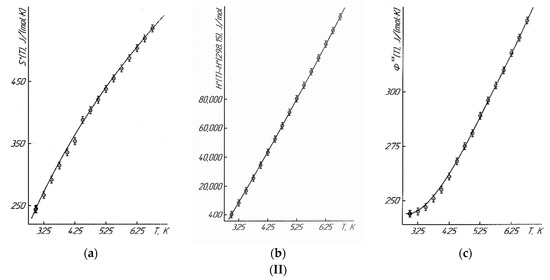
Figure 2.
Dependence of functions of S°(T) (a), H°(T)-H°(298.15) (b), Φxx(T) (c) of LaLi2TiMnO6 (I) and LaNa2TiMnO6 (II) on temperature.  —experimental data.
—experimental data.
 —experimental data.
—experimental data.
The temperature dependences of C°p(T) and the thermodynamic functions of S°(T), H°(T)–H°(298.15), and Φxx(T) were calculated using the known ratios in the range of 298.15–675 K (Table 1). This temperature range was chosen using the fact that the Φxx(T) function is only calculated from 298.15 K. It should be pointed out that the mentioned thermodynamic potential of Φxx(T) is an important thermodynamic function necessary to calculate the chemical equilibria under the third law of thermodynamics. The errors of functions S°(T) and Φxx(T) were calculated using the errors of S°(298.15) (±3.0%) [22] and the experimental data on C°p(T).
As a result, temperature dependences of the heat capacities of LaLi2TiMnO6 and LaNa2TiMnO6 in the range of 298.15–673 K were first studied.
To compare the values of the experimental data of the standard heat capacities of LaLi2TiMnO6 and LaNa2TiMnO6, they were calculated by independent calculation methods. According to [22], to calculate the C°p(298.15) of titanium-manganites, the following values of ion increments (Cip) of heat capacity [J/(mol·K] were used: Li+ = 20.7; Na+ = 26.8; La3+ = 29.3; Ti4+ = 25.5; Mn3+ = 25.0; O2− = 16.7.
The calculation of the C°p(298.15) of titanium-manganites was carried out according to the scheme:
where Cip(298.15) is the increment of the ion heat capacity at 298.15. The values of Cop(298.15) were calculated according to Equation (9), and were equal, respectively, to 219.4 J/(mol·K) for LaLi2TiMnO6 and 231.6 J/(mol·K) for LaNa2TiMnO6, were in satisfactory agreement with their experimental values of 216.0 and 240.0 J/(mol·K).
C°p(298.15)LaMI2TiMnO6 = Cip(298.15)La3+ + 2Cip(298.15)M+ + Cip(298.15)Ti4+ + Cip(298.15)Mn3+ + 6Cip(298.15)O2−,
The calculation of the standard heat capacity of LaNa2TiMnO6 by the Debye method [26] also gave good convergence with its experimental value. To calculate the Cop(298.15) LaNa2TiMnO6, the Debaev characteristic temperatures of the elements (QD, K) that make up this titanium-manganite and their melting temperatures (Tmelt., K) were used. For Tmelt. LaNa2TiMnO6, was 1473 K. The characteristic temperatures of the elements for LaNa2TiMnO6 (QD) were determined by the Korefan equation [26]:
where and Tmelt. are the melting temperatures of the compound and element, respectively. Then we calculate the isochoric heat capacities of the elements using Debye functions, and by summing them, we found the isochoric heat capacity of LaNa2TiMnO6. The transition from isochoric heat capacity to isobaric was carried out according to the Nernst-Lindeman equation:
Cp = Cv + 0.0051·T·Cp2/Tmelt.
Taking the above into account, the following data were used to calculate the standard heat capacity of LaNa2TiMnO6: Tmelt., K: La = 1193; Na = 370.7; Ti = 1941; Mn = 1517; O2 = 54.7; characteristic temperatures QD, K: La = 135; Na = 370.7; Ti = 380; Mn = 303; O2 = 89 [26]. Using Equation (11), we calculated QD for La = 150.01; Na = 308.97; Ti = 331.03; Mn = 298.57; O2 = 461.85. Then the arguments of the Debye function (/T) were calculated using tabular data in [26], equal to La = 0.503; Na = 1.036; Ti = 1.11; Mn = 1.001; O2 = 1.549. The corresponding isochoric heat capacities relative to Q’D/T based on tabular data [26] were equal for La = 24.64; Na = 23.75; Ti = 23.48; Mn = 23.76; O2 = 22.08 J/(mol·K).
Then by the equation:
we calculated the isochoric heat capacity of LaNa2TiMnO6, equal to 185.62 J/(mol·K).
Cv LaNa2TiMnO6 = Cv La + 2Cv Na + Cv Ti + Cv Mn + 3Cv O2,
Further, according to the Nernst–Lindeman Equation (11), we calculated the standard isobaric heat capacity of Cop(298.15) LaNa2TiMnO6, equal to 250.3 J/(mol·K). This calculated value was in satisfactory agreement with the experimental value of the Cop(298.15) LaNa2TiMnO6 (240 ± 11 250.3 J/(mol·K)), with an accuracy of 4.1%. Thus, it shows the correctness of our experimental data. Therefore, values of the calculated standard heat capacity of titanium manganites calculated by the independent methods confirmed the correctness and reliability of their experimental values.
In order to determine the nature of the second-order phase transition on the curves of dependences (C°p~f(T)) of LaLi2TiMnO6 and LaNa2TiMnO6 in the interval of 293–483 K per 10 K step, their electrical properties were studied, as described in [27] on an LCR-781 serial device (Taiwan) operating at a frequency of 1 kHz. The accuracy of measurements of the electric capacity, relative dielectric permittivity (ε), and electrical resistivity (R), according to the datasheet, is ±0.05% [27]. The research technique was described in detail in [28] and in our similar study [29]. The dielectric permittivity of a standard substance of barium titanate (BaTiO3) was measured at 1 kHz to confirm the validity of the obtained data. We have described before the purity of the used BaTiO3 in our thermodynamic studies.
The obtained value of the dielectric permittivity of BaTiO3 at 293 K is 1296, which conforms satisfactorily to its recommended value of 1400 ± 250 [30,31,32]. Table 4 and Figure 3 below demonstrate the results of the electrophysical measurements.

Table 4.
Results of the electrophysical measurements.
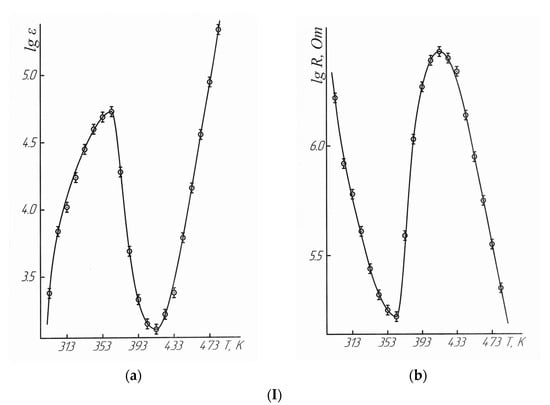
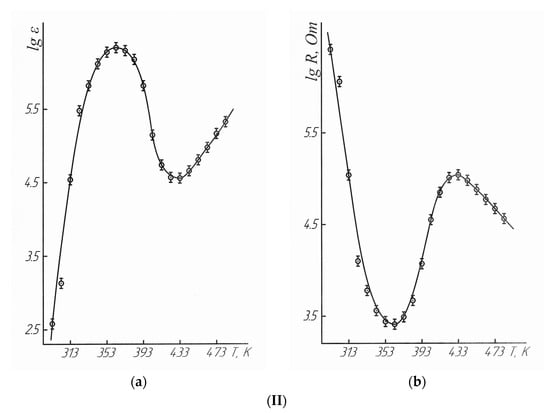
Figure 3.
Dependence of the dielectric permittivity (a), electrical resistivity (b) of LaLi2TiMnO6 (I) and LaNa2TiMnO6 (II) on temperature.  —experimental data.
—experimental data.
 —experimental data.
—experimental data.
The data in Table 4 and Figure 3 demonstrate that LaLi2TiMnO6 in the range of 293–363 K had semiconductor conductivity. It had metallic conductivity at 363–413 K, and it had semiconductor conductivity again at 413–483 K. LaNa2TiMnO6 in the range of 293–363 K shows the semiconductor conductivity. Then, it had the metallic conductivity at 363–433 K, and the semiconductor conductivity was again observed at 433–483 K. The above-mentioned changes from the semiconductor to metallic conductivity indicate the nature of the second-order phase transition on the dependence curves of LaLi2TiMnO6 and LaNa2TiMnO6 at 348 K. It should also be stated that LaLi2TiMnO6 and LaNa2TiMnO6 at 363 K have the maximum values of the dielectric permittivity equal to 53,224 and 2,182,878 respectively and, thus, this also explains the nature of phase transitions.
These experiments demonstrated that the phase transitions of LaLi2TiMnO6 and LaNa2TiMnO6 on the dependence curves of thermal capacity versus temperature at 348 K correspond in the tested temperature range of 293–363 K (maximum at 363 K) to a transition from the semiconductor to metallic conductivity. They are characterized by the maximum values of the dielectric permittivity. The high values of the dielectric permittivity of ferroelectrics near a temperature of the phase transition are described in [11]. The activation energies of conductivity were calculated for LaLi2TiMnO6 (21.44 kJ/mol) and LaNa2TiMnO6 (75.49 kJ/mol).
The widths of band gaps for LaLi2TiMnO6 in the range of 293–363 K and 413–483 K were equal to 0.69 and 2.31 eV, respectively. The widths of band gaps for LaNa2TiMnO6 between 293–363 K and 433–483 K were equal to 1.02 and 1.83 eV. Thus, they can be classified as narrow-band semiconductors.
In order to calculate the values of the standard enthalpies of formation of the test titanium-manganites, our developed method was used to calculate the standard enthalpy of formation of the double and triple manganites of the rare earth, alkali, and alkaline earth metals of the composition of LnMeI3MeII3Mn4O12 (MeI—alkali, MeII—alkaline earth, Ln—rare earth metals) [33,34].
The calculation method is as follows: the similarity coefficient K1 was calculated from the ratio of
where ΔfH°(298.15)Ln(MnO4)3 is a standard enthalpy of formation of permanganate of the rare-earth metals from the simple substances, ΔokH°(298.15)Ln(MnO4)3 is a sum of the enthalpy of formation from simple oxides or the conditionally taken standard enthalpy of formation of permanganate of the rare earth metals from oxides, and is equal to
K1 = ΔƒH°(298.15)Ln(MnO4)3/ΔokH°(298.15) Ln(MnO4)3,
ΔokH°(298.15) Ln(MnO4)3 = 0.5ΔfH°(298.15) Ln2O3 + 1.5ΔƒH°(298.15) Mn2O7.
Then the similarity coefficient K2 was calculated under the equation of
where ΔokH°(298.15)MeIMnO4—a standard enthalpy of formation of permanganate of alkali metal from oxides is equal to
K2 = ΔfH°(298.15) MeIMnO4/ΔokH°(298.15) MeIMnO4,
ΔokH°(298.15) MeIMnO4 = ΔfH°(298.15) Me2O + 0.5ΔfH°(298.15) Mn2O7.
The similarity coefficient K3 was calculated from the ratio of
where ΔokH°(298.15)MeII(MnO4)2 is a standard enthalpy of formation of permanganate of the alkaline earth metal from oxides is equal to
K3 = ΔfH°(298.15) MeII(MnO4)2/ΔokH°(298.15) MeII(MnO4)2,
ΔokH°(298.15) MeII(MnO4)2 = ΔfH°(298.15) MeO + ΔfH°(298.15) Mn2O7.
The average similarity coefficient was calculated from
ΔokH°(298.15) LnMeI3MeII3Mn4O12 was calculated from:
ΔokH°(298.15)LnMeI3MeII3Mn4O12 = 0.5ΔfH°(298.15)Ln2O3 +
1.5ΔfH°(298.15)Me2O + 3ΔfH°(298.15)MeO + 2ΔfH°(298.15)Mn2O3.
1.5ΔfH°(298.15)Me2O + 3ΔfH°(298.15)MeO + 2ΔfH°(298.15)Mn2O3.
Similar to Equations (13), (15) and (19), the ratio can be described as:
where it can be calculated as
In connection with the absence of the reference date on ΔfH°(298.15) manganites, the first approximate values were calculated from data on ΔfH°(298.15) permanganates, which were used to calculate the ΔfH°(298.15) of the test compounds. Thus, it was taken into account that the values of manganites are not much different from permanganates .
Based on the above and taking the ratios of (24, 25) for the titanium-manganites of lanthanum, the alkali, and alkaline earth metals, the following ratios can be demonstrated as:
ΔfH°(298.15) LnMeI3MeII3Mn4O12/ΔokH°(298.15) LnMeI3MeII3Mn4O12
= ΔfH°(298.15) LaMeI2TiMnO6/ΔokH°(298.15) LaMeI2TiMnO6,
= ΔfH°(298.15) LaMeI2TiMnO6/ΔokH°(298.15) LaMeI2TiMnO6,
Table 5 below shows the initial data for calculating the standard enthalpies of titanium-manganites formation, which are borrowed from [17,29,30,31].

Table 5.
Initial data for the calculation of standard enthalpy of titanium-manganites formation.
It should be noted that the coefficient for calculating ΔƒH°(298.15) LaLi2TiMnO6 will be equal to 1.2375, and for LaNa2TiMnO6 − 1.3084.
The calculated values based on the above data ΔfH°(298.15) will be equal to −3607.0 kJ/mol for LaLi2TiMnO6 and −3579.3 kJ/mol for LaNa2TiMnO6, respectively.
3. Experimental Part
Titanium-manganites of LaMeI2TiMnO6 (MeI–Li, Na) were obtained by high-temperature synthesis using ceramic technology. Oxides of lanthanum (III) (“puriss. spec.”), titanium (IV), manganese (III), and carbonates of lithium and sodium (“p.a.”) were applied to synthesize the titanium-manganites. These substances were pre-annealed at 300 °C to remove adsorption moisture. The calculated mole ratios of the starting reagents to obtain the final compound were thoroughly mixed and ground in an agate mortar. Synthesis was performed in stages in a SNOL laboratory furnace. The first stage was at 600 °C for 5 h, and the second step was to raise the temperature of the synthesis to 800 °C for 5 h. The third stage had a temperature at 1000 °C for 10 h and was repeated twice. The fourth stage was at 1200 °C for 4 h. After each temperature rise, the mixture was cooled down and milled. The final process had a low-temperature annealing at 400 °C for 10 h to obtain the low-temperature equilibrium phases. The formation of the equilibrium composition of the studied phases was monitored by X-ray diffraction analysis on the DRON-2.0 apparatus.
The indexing of X-ray photographs demonstrated that compounds were crystallized in the cubic syngony with the lattice parameters as follows: LaLi2TiMnO6–a = 13.48 ± 0.02 Å, V° = 2449.46 ± 0.06 Å3, Z = 4, Voel.cell = 612.87 ± 0.02 Å3, ρroent. = 3.81; ρpick. = 3.78 ± 0.03 g/cm3; LaNa2TiMnO6–a = 14.06 ± 0.02 Å, V° = 2779.43 ± 0.06 Å3, Z = 4, V°el.cell = 694.96 ± 0.02 Å3, ρroent. = 3.67; ρpick. = 3.65 ± 0.01 g/cm3 [38]. A pycnometric density was determined by using toluene as an indifferent liquid according to a well-known method [39]. Figure 4 (below) illustrates the diffractograms of the studied titanium-manganites. It should be pointed out that we present in detail the results of the indexing of X-ray photographs of the above compounds, and their correctness and reliability were confirmed by good conformity between the experimental and calculated values of 104/d2, and the pycnometric and X-ray densities of the theoretical and experimental values of their unit cells, as described in [38].
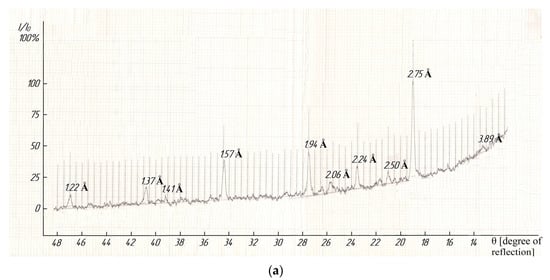
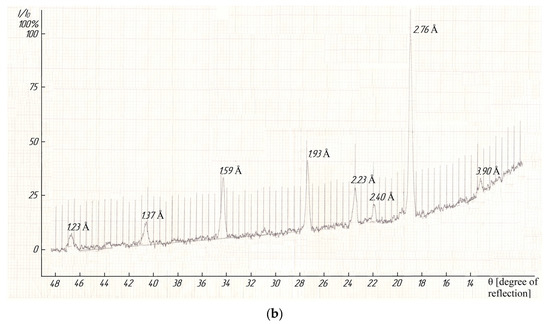
Figure 4.
The X-ray photographs of LaLi2TiMnO6 (a) and LaNa2TiMnO6 (b).
These compounds can be assigned to the perovskite structure according to the information below. The above-mentioned compounds can be represented as derivatives of lanthanum titanate (LaTiO3) and lanthanum manganate (LaMnO3), and they belong to a perovskite structure [40]. Secondly, in order to assign these compounds to the perovskite structure, we calculated the tolerance factor (t) by the formula [23]:
where –ion radii of A, B, and O2. In our case, A is a sum of ions of La3+, Li+, Ti4+ or La3+, Na+, Ti4+, and B-ion Mn3+. The radii of ions of La3+, Li+, Na+, Ti4+, Mn3+, and O2− at a coordination number equal to 6 according to the Goldschmidt system were used, according to [41]. The Goldschmidt system was preferred, i.e., the Pauling system has no ion radius of Mn3+. The tolerance factors (t) for LaLi2TiMnO6 (t = 0.97) and LaNa2TiMnO6 (t = 0.94) were calculated by Equation (24). Additionally, we calculated the “tolerance factor” t, based on the system of ion radii according to Shannon and Previtt, for LaLi2TiMnO6 0.94 and for LaNa2TiMnO6-0.90. The tolerance factor (t) is approximately in the range of 0.80 ÷ 1.00 for all perovskite-type compounds and t > 0.89 for an ideal cubic structure, as described in [41].
The parameter increment of “a” and the volume increase of the unit cells were observed during the elevating ionic radii from Li to Na.
Temperature dependence of the heat capacity of synthesized compounds of LaLi2TiMnO6 and LaNa2TiMnO6 was explored in the temperature range of 298.15–673 K by dynamic calorimetry using an IT-C-400 calorimeter. The device is based on the comparative method of a dynamic calorimeter with a heat meter and an adiabatic shell. During the experiment of heating (per 25 °C), the time lag of the ampoule temperature in relation to the base temperature was measured on an F136 device and stopwatch. Based on the specifications of the calorimeter, the heat capacity was measured per 25 K, and the limit temperature measurement was 673 K. The initial temperature for the measurement of heat capacity was 298.15 K and permitted to obtain the fundamental constant-standard heat capacity of the compound.
The measuring range of the volumetric heat capacity was not less than 1 × 106 J/K·m3. The time for the full temperature range with the experimental data processing was no more than 2.5 h. The measurement errors on the IT-C-400 device did not exceed ±10%. The device was calibrated using a calculation of the thermal conductivity of the heat meter (KT) [42,43].
A value of the molar heat capacity was calculated from the specific heat capacity using the molar mass. At each temperature after 25 K, five parallel experiments were conducted, the results of which were averaged and processed by methods of mathematical statistics.
For the averaged values of the specific heat capacity at each temperature, the standard deviation () was estimated according to [43]:
where n—number of experiments, Ci—a measured value of specific heat capacity, and —an arithmetic average of measured values of the specific heat capacity. A random error was calculated for averaged values of the molar heat capacity as described in [43]:
where —a random error in % and tp—Student coefficient (for n = 5, tp = 2.75 at p = 0.95 of the confidence range).
Operation of the device was verified with a calculation of the heat capacity of α-Al2O3 (“p.a.”, TU 6.09-426-75). The repeated (parallel) measurements in the range of 173–673 K (at 25 K, 5 times) were performed for calibration and verification. Therefore, five parallel measurements were made at each temperature at 25 K. The results were averaged and processed using mathematical statistics. Liquid nitrogen served as the refrigerant.
Our results, with the new literature data in [44], were compared for the accuracy of the heat capacity measurements of α-Al2O3 (Table 6).

Table 6.
Comparison of the heat capacity of α-Al2O3 with the literature data in [44] to verify the calorimeter operation.
The data in Table 6 demonstrates that our results for the temperature dependence of the heat capacity of -Al2O3, in the range of 173–673 K, satisfactorily conformed to the results in [44] within the operating accuracy of the IT-C-400 calorimeter.
It should be stated that in order to compare our values of the heat capacities of Al2O3 with the data in [44], our experimental values were used at 10 and 50 K based on equations of C°p~f(T) calculated from the experimental data because the data in [44] for C°p~f(T) were used at 10 and 50 K, while our experimental data were measured at ΔT = 25 K. It should be noted that the real errors of the experimental data on heat capacities calculated with Equations (25) and (26) were lower than a limiting accuracy of the device, i.e., it was less than 10%.

Table 7.
The experimental values of the heat capacity for the compounds.
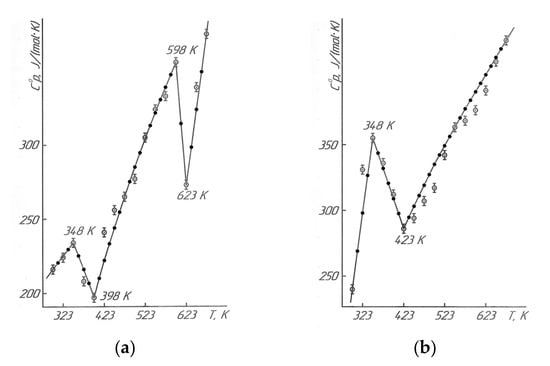
Figure 5.
Temperature dependence of the heat capacity LaLi2TiMnO6 (a) and LaNa2TiMnO6 (b).  —experimental data, ●—calculated data.
—experimental data, ●—calculated data.
 —experimental data, ●—calculated data.
—experimental data, ●—calculated data.
4. Conclusions
The temperature dependences of the heat capacity of LaMeI2TiMnO6 (MeI–Li, Na) were studied for the first time by experimental dynamic calorimetry in the range of 298.15–673 K.
The anomalous discontinuities in heat capacity of LaLi2TiMnO6 (348 K, 598 K) and LaNa2TiMnO6 (348 K) were observed on C°p~f(T) curve. There were probably related to the second-order phase transitions.
Based on the temperatures of the phase transitions, the equations for the temperature dependence of heat capacity were derived, and they sufficiently describe the experimental data.
The temperature dependences of C°p(T) and the thermodynamic functions of S°(T), H°(T)-H°(298.15), and Φxx(T) of the studied compounds were calculated using the experimental data on C°p(T) and the calculated values of S°(298.15) in the range of 298.15–673 K.
Using the methods of ion increments and Debye, the standard heat capacities of lithium and sodium lanthanum titanium-manganites were calculated, which were in satisfactory agreement with the experimental data.
According to the developed methodology, the standard enthalpy of the formation of LaLi2TiMnO6 and LaNa2TiMnO6 was calculated.
Based on the conducted electrophysical research, the nature of the second-order phase transitions on curves of C°p~f(T) dependences of the studied titanium-manganites was proved. Their semiconductor and metallic features of conductivity were revealed. The widths of their band gaps were calculated.
As a result, all the above-mentioned data on the experimental and calculated studies of the temperature dependence of heat capacity, thermodynamic functions, the results of calculation of the standard enthalpy of formation, and their revealed electrophysical characteristics of LaLi2TiMnO6 and LaNa2TiMnO6 are absolutely new, and they have no analogues.
The research results are also of practical importance for the directed synthesis of similar compounds with valuable physical and chemical properties, for prediction of the perspective physical and chemical characteristics of the studied phases, and for analysis of heterogeneous equilibria according to II and III laws of thermodynamics involving titanium-manganites. The new thermochemical constants are initial data to be loaded into fundamental guides for the thermodynamic values and information databanks. The conducted electrophysical studies determined the semiconducting character of conductivity of LaLi2TiMnO6 and LaNa2TiMnO6 and the maximum values of the dielectric permittivity near the temperature of the phase transitions.
Author Contributions
Conceptualization, B.K.K., S.B. and A.N.; methodology, S.B.K., Z.I.S. and E.E.K.; formal analysis, B.K.K., S.B., A.N. and N.Y.L.; writing—review and editing, B.K.K., S.B.K., Z.I.S. and E.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of program-targeted financing under the program 057 “Applied scientific research of a technological nature in the field of industry” of the Committee for Industrial Development of the Ministry of Industry and Infrastructure Development of the Republic of Kazakhstan within the framework of the scientific and technical project “Creation of new composite materials with high performance properties based on rare and rare earth elements” for 2021–2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Tumarkin, A.V.; Al’myashev, V.I.; Razumov, S.V.; Gaidukov, M.M.; Gagarin, A.G.; Altynnikov, A.G.; Kozyrev, A.B. Structural properties of barium strontium titanate films grown under different technological conditions. Phys. Solid State 2015, 57, 553–557. [Google Scholar] [CrossRef]
- Volkov, N.B. Spintronics: Manganite-based magnetic tunnel structures. Physics-Uspekhi 2012, 55, 250–269. [Google Scholar] [CrossRef]
- Seliverstova, Y.V.; Ibraev, N.K.; Zhumabekov, A.Z. The Effect of Silver Nanoparticles on the Photodetecting Properties of the TiO2/Graphene Oxide Nanocomposite. Opt. Spectrosc. 2020, 128, 1449–1457. (In Russian) [Google Scholar] [CrossRef]
- Tretyakov, Y.D.; Brylev, O.A. New generations of inorganic functional materials. J. Russ. Chem. Soc. 2000, 45, 10–16. (In Russian) [Google Scholar]
- Protasov, N.M. Structural Modeling of Complex Oxides with a Perovskite Structure in a Partially Covalent Approximation; Publishing House of Moscow State University: Moscow, Russia, 2011; 51p. (In Russian) [Google Scholar]
- Aksenova, T.V.; Gavrilova, L.Y.; Cherepanov, V.A. Crystal structure and physicochemical properties of doped lanthanum manganites. Russ. J. Phys. Chem. A 2012, 86, 1862–1868. [Google Scholar] [CrossRef]
- Yanchevskii, O.Z.; V’yunov, O.I.; Belous, A.G.; Tovstolytkin, A.I.; Kravchik, V.P. Synthesis and Characterization of La0,7Sr0,3Mn1-xTixO3. Phys. Solid State 2006, 48, 709–716. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Agarkov, D.A.; Burmistrov, I.N.; Kudrenko, E.A.; Bredikhin, S.I.; Kharton, V.V. Synthesis and Properties of Fuel Cell Anodes Based on (La0,5+xSr0,5–x)1–yMn0,5Ti0,5O3–δ (x = 0–0.25, y = 0–0.03). Russ. J. Electrochem. 2014, 50, 730–736. [Google Scholar] [CrossRef]
- Qasim, I.; Blanchard, E.R.; Kennedy, J.B.; Ling, D.C.; Ling-Yun, J.; Kamiyama, T.; Miao, P.; Torii, S. Soft ferromagnetism in mixed valence Sr1-xLaxTi0.5Mn0.5O3 perovskites. Dalton Trans. 2014, 18, 6909–6918. [Google Scholar] [CrossRef] [PubMed]
- Mesilov, V.V.; Galakhov, V.R.; Yermakov, A.Y.; Uimin, M.A.; Gubkin, A.F.; Sherstobitova, E.A.; Udintseva, M.S.; Zakharova, G.S.; Smirnov, D.A. Soft X-ray absorption spectroscopy of titanium dioxide nanopowders with cobalt impurities. J. Exp. Theor. Phys. 2017, 124, 908–913. [Google Scholar] [CrossRef]
- Barfoot, J. Introduction to the Physics of Ferroelectric Phenomena; Mir: Moscow, Russia, 1970; 352p. (In Russian) [Google Scholar]
- Yao, Z.; Zhu, K.; Zhang, J.; Li, J.; Li, X.; Wang, J.; Yan, K.; Liu, J. LiF-Assisted Synthesis of Perovskite-Type Li0.35La0.55TiO3 Solid Electrolyte for Rechargeable Lithium-Metal Batteries. J. Electron. Mater. 2022, 51, 736–744. [Google Scholar] [CrossRef]
- Denissova, L.T.; Molokeyev, M.S.; Galiakhmetova, N.A.; Denissov, V.M.; Vassilev, G.V. Synthesis, crystal structure and thermophysical properties of substituted titanates Bi2Pr2Ti3O12 and Bi2Nd2Ti3O12. Solid State Phys. 2021, 63, 1056–1061. (In Russian) [Google Scholar]
- Sankovich, A.M.; Markin, A.V.; Smirnova, N.N.; Zvereva, I.A. Heat capacity and thermodynamic properties of layered perovskite-like oxides of K2La2Ti3O10 and K2Nd2Ti3O10. J. Phys. Chem. 2019, 93, 407–416. (In Russian) [Google Scholar] [CrossRef]
- Denissova, L.T.; Molokeyev, M.S.; Ryabov, V.V.; Kargin, Y.F.; Chumalina, L.G.; Denissov, V.M. Crystal structure and thermodynamic properties of titanium, structure and thermodynamic properties of titanium ErGaTi2O7. J. Inorg. Chem. 2021, 66, 492–497. (In Russian) [Google Scholar]
- Denissova, L.T.; Molokeyev, M.S.; Kargin, Y.F.; Ryabov, V.V.; Chumalina, L.G.; Beloussova, N.V.; Denissov, V.M. Structure and thermodynamic properties of titanates of DyGaTi2O7 and EuGaTi2O7. Inorg. Mater. 2021, 57, 768–775. (In Russian) [Google Scholar] [CrossRef]
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Yermagambet, B.T.; Bekturganov, N.S.; Oskembekov, I.M. Double and Triple Manganites, Ferrites and Chromites of Alkaline, Alkaline Earth and Rare Earth Metals; Nauchnyi Mir: Moscow, Russia, 2017; 416p. (In Russian) [Google Scholar]
- Fedorov, V.A.; Kuznetsov, N.T. Analysis and Study of Semiconductor Materials; Samara State University: Samara, Russia, 2008; 84p. (In Russian) [Google Scholar]
- Reznitsky, L.A. Calorimetry of a Solid; Publishing House of Moscow State University: Moscow, Russia, 1981; 184p. (In Russian) [Google Scholar]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Pyrros, N.P.; Hubbard, C.R. Standard X-ray Diffraction Powder Patterns; National Bureau of Standards: Washington, DC, USA, 1984; 158p, Available online: https://archive.org/details/standardxraydiff2520morr/page/8/mode/2up (accessed on 13 March 2023).
- Kubascewski, O.; Alcock, C.B. Metallurgical Thermochemistry; Pergamon Press: Oxford, UK, 1982; 390p. [Google Scholar]
- Kumok, V.N. Problem of Harmonization of Methods to Estimate Thermodynamic Characteristics. In Direct and Inverse Problems of Chemical Thermodynamics; Nauka: Moscow, Russia, 1987; p. 108. (In Russian) [Google Scholar]
- Wells, A.F. Structural Inorganic Chemistry; Oxford University Press: Oxford, UK, 1987; Volume 2, 69p. [Google Scholar]
- Bui, M.T. Investigation of Temperature Dependences of Electrophysical Properties of Ferroelectric Materials; Dissertation for Degree of Candidate of Physical and Mathematical Sciences; St. Petersburg National Research University: St. Petersburg, Russia, 2019; 143p. (In Russian) [Google Scholar]
- Gerasimov, Y.I.; Krestovnikov, A.N.; Shakhov, A.S. Chemical Thermodynamics in Non–Ferrous Metallurgy; Metallurgy: Moscow, Russia, 1960; Volume 1, 230p. (In Russian) [Google Scholar]
- Morachevsky, A.S.; Sladkov, I.B. Thermodynamic Calculations in Metallurgy: Handbook; Metallurgy: Moscow, Russia, 1985; 137p. (In Russian) [Google Scholar]
- Okazaki, K. Technology of Ceramic Dielectrics; Energy: Moscow, Russia, 1976; 327p. (In Russian) [Google Scholar]
- User Manual. RLC Meter (LCR-781). M.: CJSC “PriST”. 2012, p. 3. Available online: https://saprd.ru/grsi/53914-13/2013-53914-13.pdf (accessed on 13 March 2023).
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Kuanyshbekov, E.E.; Mukhtar, A.A. Thermodynamic and Electrophysics of New LaCaCuZnMnO6 Copper—Zinc Manganite of Lanthanum and Calcium. High Temp. 2022, 60, 474–478. (In Russian) [Google Scholar] [CrossRef]
- Fessenko, Y.G. Perovskite Family and Ferroelectricity; Atomizdat: Moscow, Russia, 1972; 248p. (In Russian) [Google Scholar]
- Venevtsev, Y.N.; Politova, Y.D.; Ivanov, S.A. Ferro- and Anti-Ferroelectrics of the Barium Titanate Family; Chemistry: Moscow, Russia, 1985; 256p. (In Russian) [Google Scholar]
- Lines, M.; Glass, A. Ferroelectrics and Their Related Materials; Mir: Moscow, Russia, 1981; 736p. (In Russian) [Google Scholar]
- Kasenov, B.K.; Edilbaeva, S.T.; Mustafin, E.S.; Zhumadilov, E.K. Evaluation of thermodynamic functions of ternary oxides LnMeMn2O5 (Ln—rare earth metal, Me—alkali metal). J. Phys. Chem. 1999, 73, 1116–1118. (In Russian) [Google Scholar]
- Kasenov, B.K.; Kasenova, S.B.; Sagintayeva, Z.I.; Kuanyshbekov, E.E.; Mukhtar, A.A.; Kakenov, K.S.; Esenbayeva, G.A. Evaluation of thermodynamic characteristics of nickel (cobalt)-cuprate-manganites LaMeI2Ni(Co)CuMnO6 and LaMeIINi(Co)CuMnO6 (MeI—Li, Na, K.; MeII—Mg, Ca, Sr, Ba). Chem. J. Kazakhstan 2019, 1, 149–156. (In Russian) [Google Scholar]
- Glushko, V.P. (Ed.) Thermal Constants of Substances/Handbook; Issue X, part 1; Nauka: Moscow, Russia, 1981; 300p. (In Russian) [Google Scholar]
- Glushko, V.P. (Ed.) Thermal Constants of Substances/Handbook; Issue XIII, part 1; Nauka: Moscow, Russia, 1978; 536p. (In Russian) [Google Scholar]
- Glushko, V.P. (Ed.) Thermal Constants of Substances/Handbook; Issue VII, part 1; Nauka: Moscow, Russia, 1974; 344p. (In Russian) [Google Scholar]
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Baisanov, S.O.; Lu, N.Y.; Kuanyshbekov, E.E.; Turtubaeva, M.O. Novel Titanium-Manganites of Lanthanum and Alkali Metals. Bull. Karaganda Univ.–Chem. 2022, 108, 136–141. [Google Scholar] [CrossRef]
- Kivilis, S.S. Technique of Measuring the Density of Liquids and Solids; Standardgiz: Moscow, Russia, 1959; 191p. (In Russian) [Google Scholar]
- Portnoi, K.I.; Timofeyeva, N.I. Oxygen Compounds of Rare Earth Elements. Guide; Metallurgy: Moscow, Russia, 1986; 480p. (In Russian) [Google Scholar]
- Kostrominka, N.A.; Kumok, V.N.; Skorik, N.A. Chemistry of Coordination Compounds; Higher school: Moscow, Russia, 1990; 432p. (In Russian) [Google Scholar]
- Platunov, Y.S.; Buravoi, S.Y.; Kurepin, V.V.; Petrov, G.S. Thermophysical Measurements and Devices; Mashinostroenie: Moscow, Russia, 1986; 256p. (In Russian) [Google Scholar]
- Specification and Operating Instructions of IT-C-400. Aktobe. Aktobe Plant “Etalon”. 1986. 48p. (In Russian). Available online: https://nd-gsi.ru/grsi/060xx/06000-77.pdf (accessed on 13 March 2023).
- Bodryakov, V.Y.; Bykov, A.A. Correlation Characteristics of the Volumetric Thermal Expansion Coefficient and Specific Heat of Corundum. Glass Ceram. 2015, 72, 67–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).