The Strength of hERG Inhibition by Erythromycin at Different Temperatures Might Be Due to Its Interacting Features with the Channels
Abstract
1. Introduction
2. Results
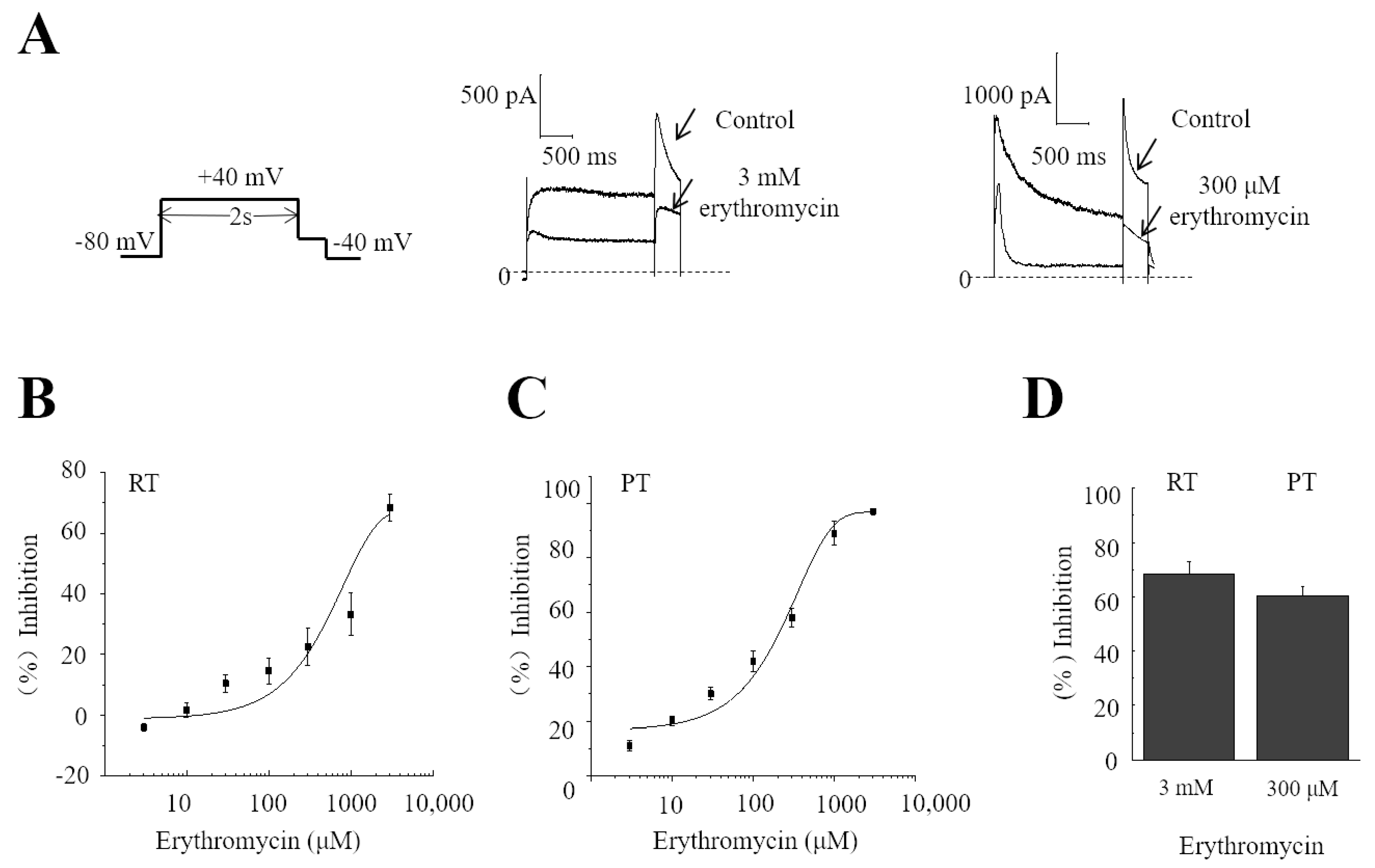
2.1. Temperature-Dependent Inhibition of hERG by Erythromycin
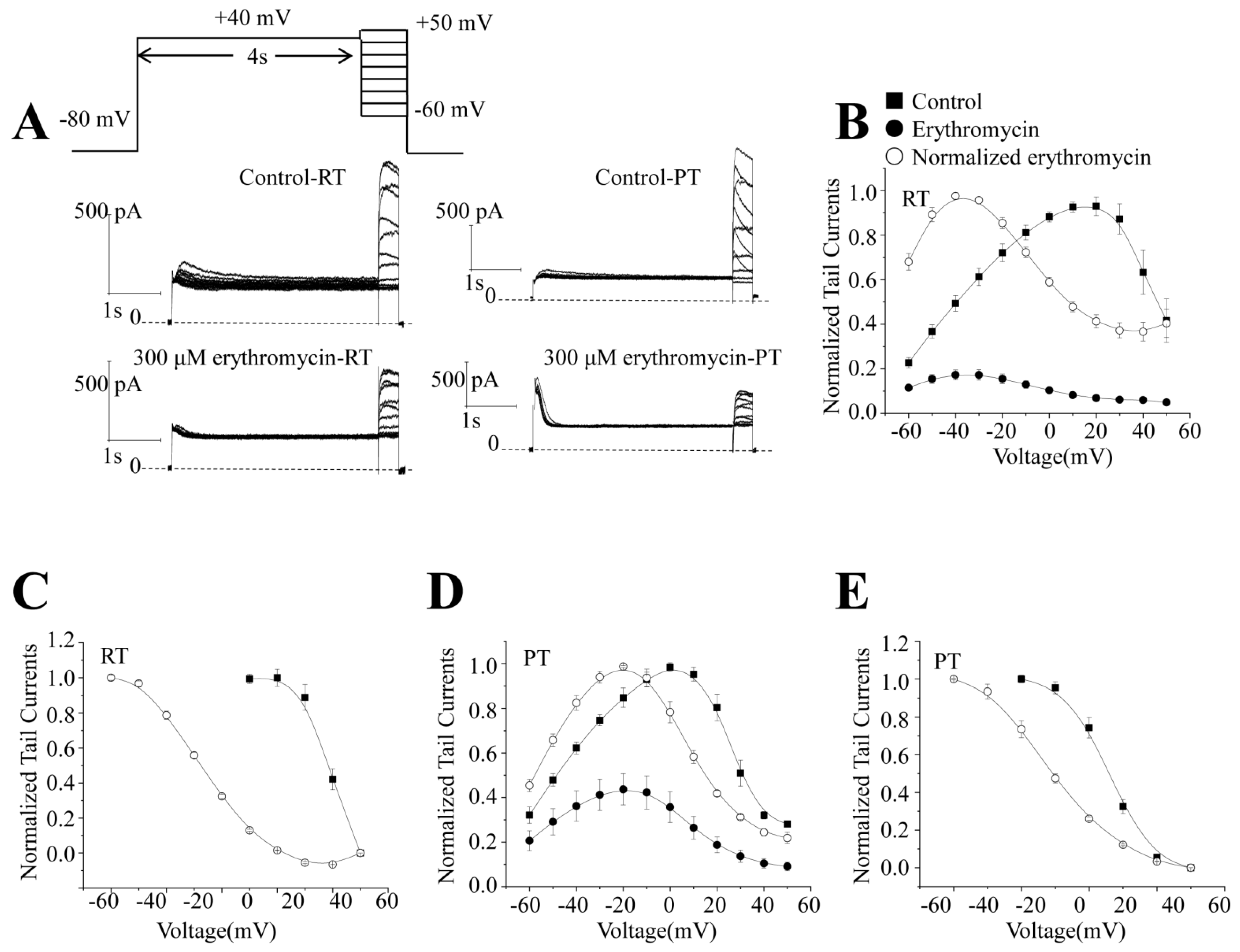
2.2. Effect of Erythromycin on the Steady-State Activation of RT and PT
2.3. Effect of Erythromycin on Steady-State Inactivation of hERG Channels at RT and PT
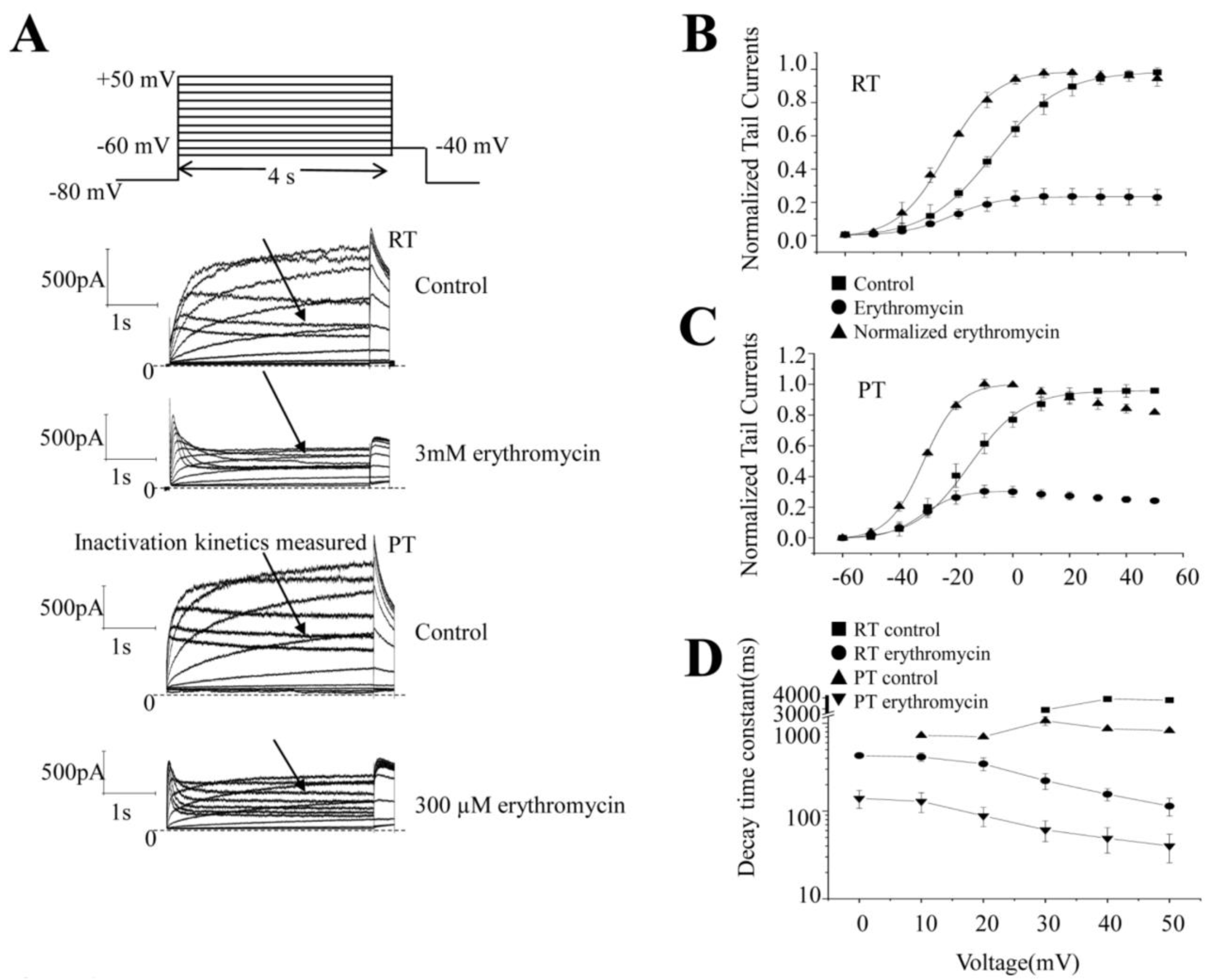
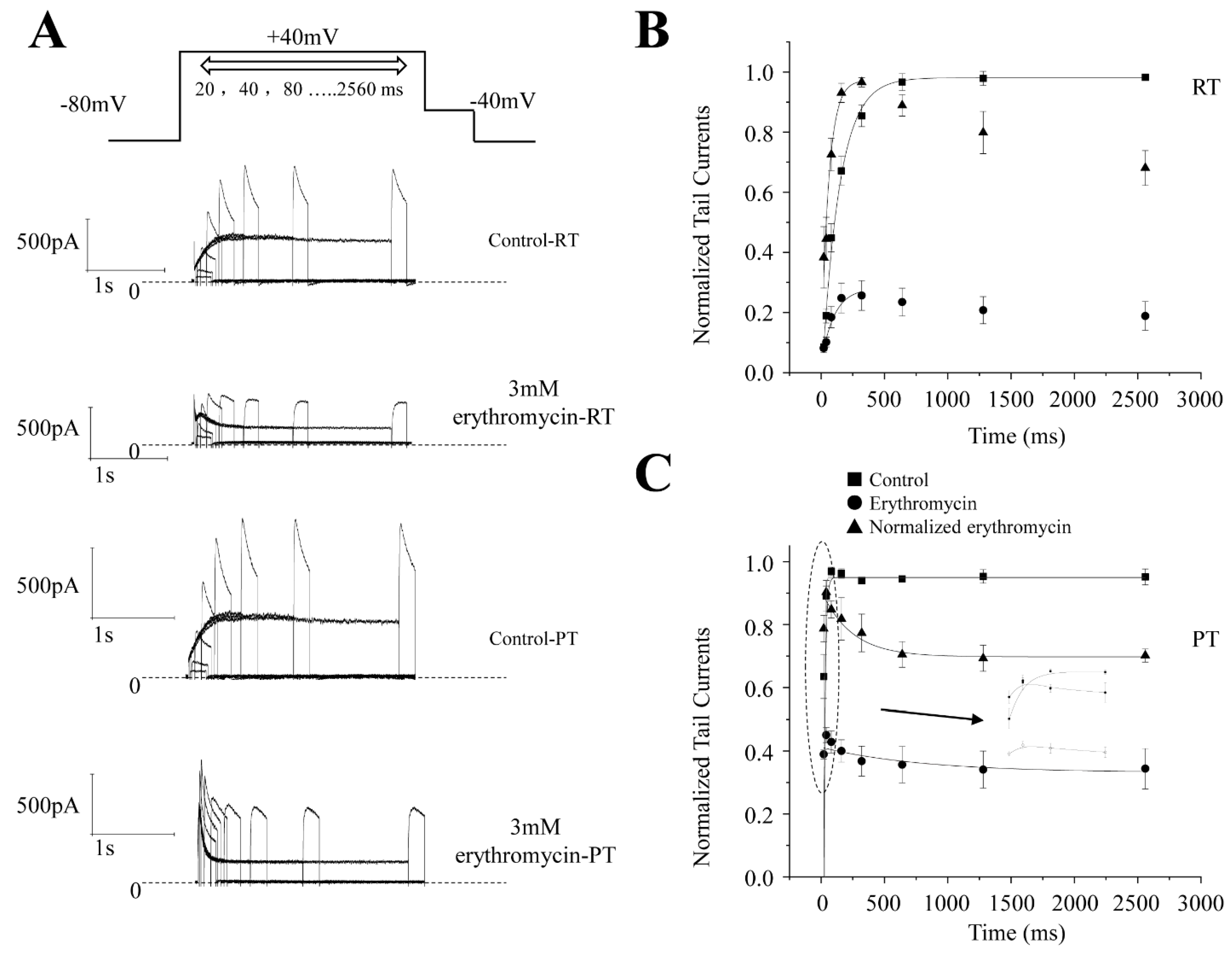
2.4. Effect of Erythromycin on the Onset of Inactivation of hERG Channel at RT and PT
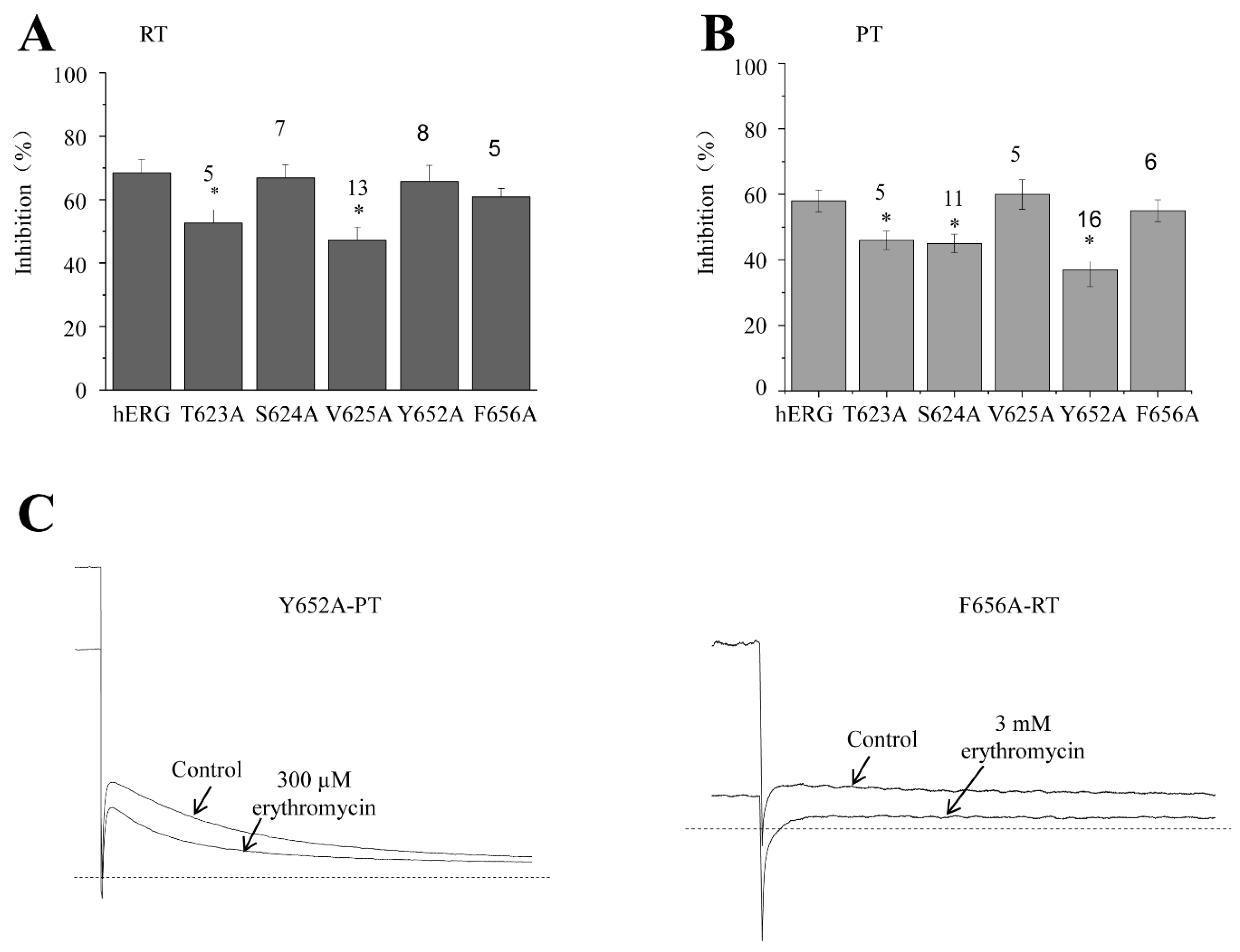
2.5. Inhibitory Effect of Erythromycin on Mutations of hERG Channel at RT and PT
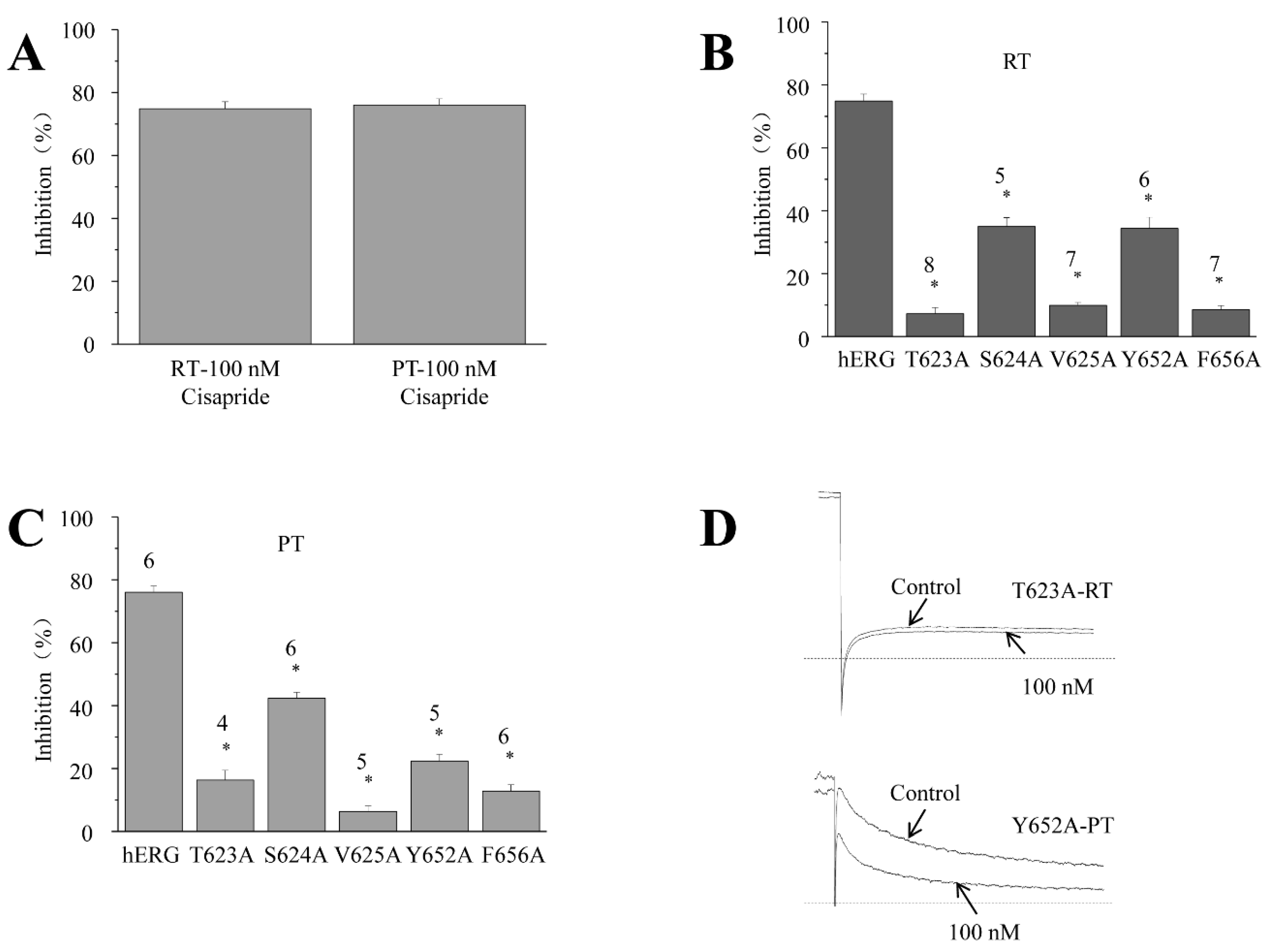
2.6. Inhibitory Effect of Cisapride on hERG Potassium Channels and Mutants at RT and PT
3. Discussion
4. Materials and Methods
4.1. Preparation of Erythromycin and Cisapride
4.2. Cell-Line Preparation
4.3. Electrophysiological Recordings
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanguinetti, M.C.; Jurkiewicz, N.K. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am. J. Physiol. Circ. Physiol. 1991, 260 Pt 2, H393–H399. [Google Scholar] [CrossRef]
- Virág, L.; Iost, N.; Opincariu, M.; Szolnoky, J.; Szécsi, J.; Bogáts, G.; Szenohradszky, P.; Varró, A.; Papp, J.G. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc. Res. 2001, 49, 790–797. [Google Scholar] [CrossRef]
- Helbing, W.A.; Roest, A.A.W.; Niezen, R.A.; Vliegen, H.W.; Hazekamp, M.G.; Ottenkamp, J.; De Roos, A.; Van Der Wall, E.E. ECG predictors of ventricular arrhythmias and biventricular size and wall mass in tetralogy of Fallot with pulmonary regurgitation. Heart 2002, 88, 515–519. [Google Scholar] [CrossRef]
- Jouven, X.; Hagege, A.; Charron, P.; Carrier, L.; Dubourg, O.; Langlard, J.M.; Aliaga, S.; Bouhour, J.B.; Schwartz, K.; Desnos, M.; et al. Relation between QT duration and maximal wall thickness in familial hypertrophic cardiomyopathy. Heart 2002, 88, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Schram, G.; Zhang, L.; Derakhchan, K.; Ehrlich, J.R.; Belardinelli, L.; Nattel, S. Ranolazine: Ion-channel-blocking actions and in vivo electrophysiological effects. Br. J. Pharmacol. 2004, 142, 1300–1308. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Delpón, E. Pharmacological approaches in the treatment of atrial fibrillation. Curr. Med. Chem. 2004, 11, 13–28. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Walker, B.D.; Campbell, T.J. HERG K+ channels: Friend and foe. Trends Pharmacol. Sci. 2001, 22, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hancox, J.C.; McPate, M.J.; El Harchi, A.; Zhang, Y.H. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol. Ther. 2008, 119, 118–132. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Jiang, C.; Curran, M.E.; Keating, M.T. A mechanistic link between an inherited and an acquird cardiac arrthytmia: HERG encodes the IKr potassium channel. Cell 1995, 81, 299–307. [Google Scholar] [CrossRef]
- Wang, W.; MacKinnon, R. Cryo-EM Structure of the Open Human Ether-à-go-go -Related K+ Channel hERG. Cell 2017, 169, 422–430.e10. [Google Scholar] [CrossRef] [PubMed]
- Mitcheson, J.S.; Chen, J.; Lin, M.; Culberson, C.; Sanguinetti, M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 12329–12333. [Google Scholar] [CrossRef]
- Chen, J.; Seebohm, G.; Sanguinetti, M.C. Position of aromatic residues in the S6 domain, not inactivation, dictates cisapride sensitivity of HERG and eag potassium channels. Proc. Natl. Acad. Sci. USA 2002, 99, 12461–12466. [Google Scholar] [CrossRef]
- Perry, M.; de Groot, M.J.; Helliwell, R.; Leishman, D.; Tristani-Firouzi, M.; Sanguinetti, M.C.; Mitcheson, J. Structural Determinants of HERG Channel Block by Clofilium and Ibutilide. Mol. Pharmacol. 2004, 66, 240–249. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef]
- Kirsch, G.E.; Trepakova, E.S.; Brimecombe, J.C.; Sidach, S.S.; Erickson, H.D.; Kochan, M.C.; Shyjka, L.M.; Lacerda, A.E.; Brown, A.M. Variability in the measurement of hERG potassium channel inhibition: Effects of temperature and stimulus pattern. J. Pharmacol. Toxicol. Methods 2004, 50, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhan, S.; Lees-Miller, J.P.; Teng, G.; Duff, H.J. Exaggerated block of hERG (KCNH2) and prolongation of action potential duration by erythromycin at temperatures between 37 °C and 42 °C. Heart Rhythm. 2005, 2, 860–866. [Google Scholar] [CrossRef]
- Fermini, B.; Hancox, J.C.; Abi-Gerges, N.; Bridgland-Taylor, M.; Chaudhary, K.W.; Colatsky, T.; Correll, K.; Crumb, W.; Damiano, B.; Erdemli, G.; et al. A New Perspective in the Field of Cardiac Safety Testing through the Comprehensive In Vitro Proarrhythmia Assay Paradigm. J. Biomol. Screen. 2016, 21, 1–11. [Google Scholar] [CrossRef]
- Duncan, R.S.; Ridley, J.M.; Dempsey, C.E.; Leishman, D.J.; Leaney, J.L.; Hancox, J.C.; Witchel, H.J. Erythromycin block of the HERG K+ channel: Accessibility to F656 and Y652. Biochem. Biophys. Res. Commun. 2006, 341, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Shi, C.; Li, L.; Du, Y.; Xu, Y. Compound ICA-105574 prevents arrhythmias induced by cardiac delayed repolarization. Eur. J. Pharmacol. 2013, 718, 87–97. [Google Scholar] [CrossRef]
- Crumb, W.J., Jr. Allosteric effects of erythromycin pretreatment on thioridazine block of hERG potassium channels. Br. J. Pharmacol. 2014, 171, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.P.; Rodriguez, E.; Galazzo, J.L.; Licari, P. Improved Bioconversion of 15-Fluoro-6-deoxyerythronolide B to 15-Fluoro-erythromycin A by Overexpression of the eryK Gene in Saccharopolyspora erythraea. Biotechnol. Prog. 2004, 20, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gong, Q.; Ye, B.; Fan, Z.; Makielski, J.C.; Robertson, G.A.; January, C.T. Properties of HERG Channels Stably Expressed in HEK 293 Cells Studied at Physiological Temperature. Biophys. J. 1998, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Mauerhöfer, M.; Bauer, C.K. Effects of Temperature on Heteromeric Kv11.1a/1b and Kv11.3 Channels. Biophys. J. 2016, 111, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Brooks, B.R.; Damjanovic, A. Determinants of conductance of a bacterial voltage-gated sodium channel. Biophys. J. 2021, 120, 3050–3069. [Google Scholar] [CrossRef] [PubMed]
- Windley, M.J.; Lee, W.; Vandenberg, J.; Hill, A.P. The Temperature Dependence of Kinetics Associated with Drug Block of hERG Channels Is Compound-Specific and an Important Factor for Proarrhythmic Risk Prediction. Mol. Pharmacol. 2018, 94, 760–769. [Google Scholar] [CrossRef]
- Lei, C.L.; Clerx, M.; Beattie, K.A.; Melgari, D.; Hancox, J.C.; Gavaghan, D.J.; Polonchuk, L.; Wang, K.; Mirams, G.R. Rapid Characterization of hERG Channel Kinetics II: Temperature Dependence. Biophys. J. 2019, 117, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, F.H.; Surmeier, D.J.; Scheuer, T.; Catterall, W.A. Neuromodulation of Na+ Channel Slow Inactivation via cAMP-Dependent Protein Kinase and Protein Kinase C. Neuron 2006, 49, 409–420. [Google Scholar] [CrossRef]
- Koltun, D.O.; Parkhill, E.Q.; Elzein, E.; Kobayashi, T.; Notte, G.T.; Kalla, R.; Jiang, R.H.; Li, X.; Perry, T.D.; Avila, B.; et al. Discovery of triazolopyridine GS-458967, a late sodium current inhibitor (Late INai) of the cardiac NaV 1.5 channel with improved efficacy and potency relative to ranolazine. Bioorganic Med. Chem. Lett. 2016, 26, 3202–3206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Wei, X.; Zhang, Y.; Zhang, Q.; Xu, J.; Yang, J.; Yu, J.; Stalin, A.; Liu, H.; Wang, J.; et al. The Strength of hERG Inhibition by Erythromycin at Different Temperatures Might Be Due to Its Interacting Features with the Channels. Molecules 2023, 28, 5176. https://doi.org/10.3390/molecules28135176
Cheng D, Wei X, Zhang Y, Zhang Q, Xu J, Yang J, Yu J, Stalin A, Liu H, Wang J, et al. The Strength of hERG Inhibition by Erythromycin at Different Temperatures Might Be Due to Its Interacting Features with the Channels. Molecules. 2023; 28(13):5176. https://doi.org/10.3390/molecules28135176
Chicago/Turabian StyleCheng, Dongrong, Xiaofeng Wei, Yanting Zhang, Qian Zhang, Jianwei Xu, Jiaxin Yang, Junjie Yu, Antony Stalin, Huan Liu, Jintao Wang, and et al. 2023. "The Strength of hERG Inhibition by Erythromycin at Different Temperatures Might Be Due to Its Interacting Features with the Channels" Molecules 28, no. 13: 5176. https://doi.org/10.3390/molecules28135176
APA StyleCheng, D., Wei, X., Zhang, Y., Zhang, Q., Xu, J., Yang, J., Yu, J., Stalin, A., Liu, H., Wang, J., Zhong, D., Pan, L., Zhao, W., & Chen, Y. (2023). The Strength of hERG Inhibition by Erythromycin at Different Temperatures Might Be Due to Its Interacting Features with the Channels. Molecules, 28(13), 5176. https://doi.org/10.3390/molecules28135176







