Investigation of the Origin of High Photoluminescence Quantum Yield in Thienyl-S,S-dioxide AIEgens Oligomers by Temperature Dependent Optical Spectroscopy
Abstract
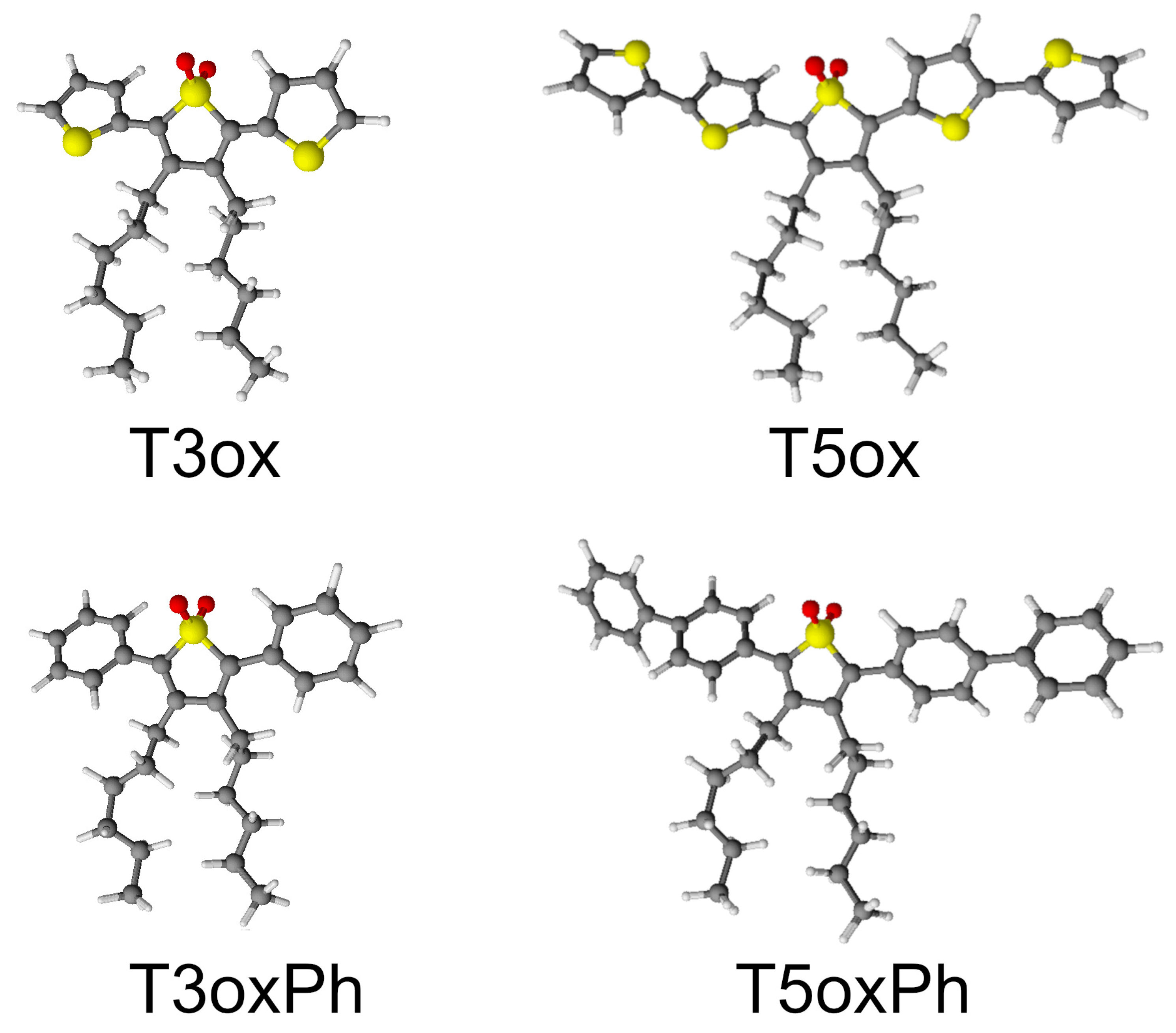
1. Introduction
2. Results
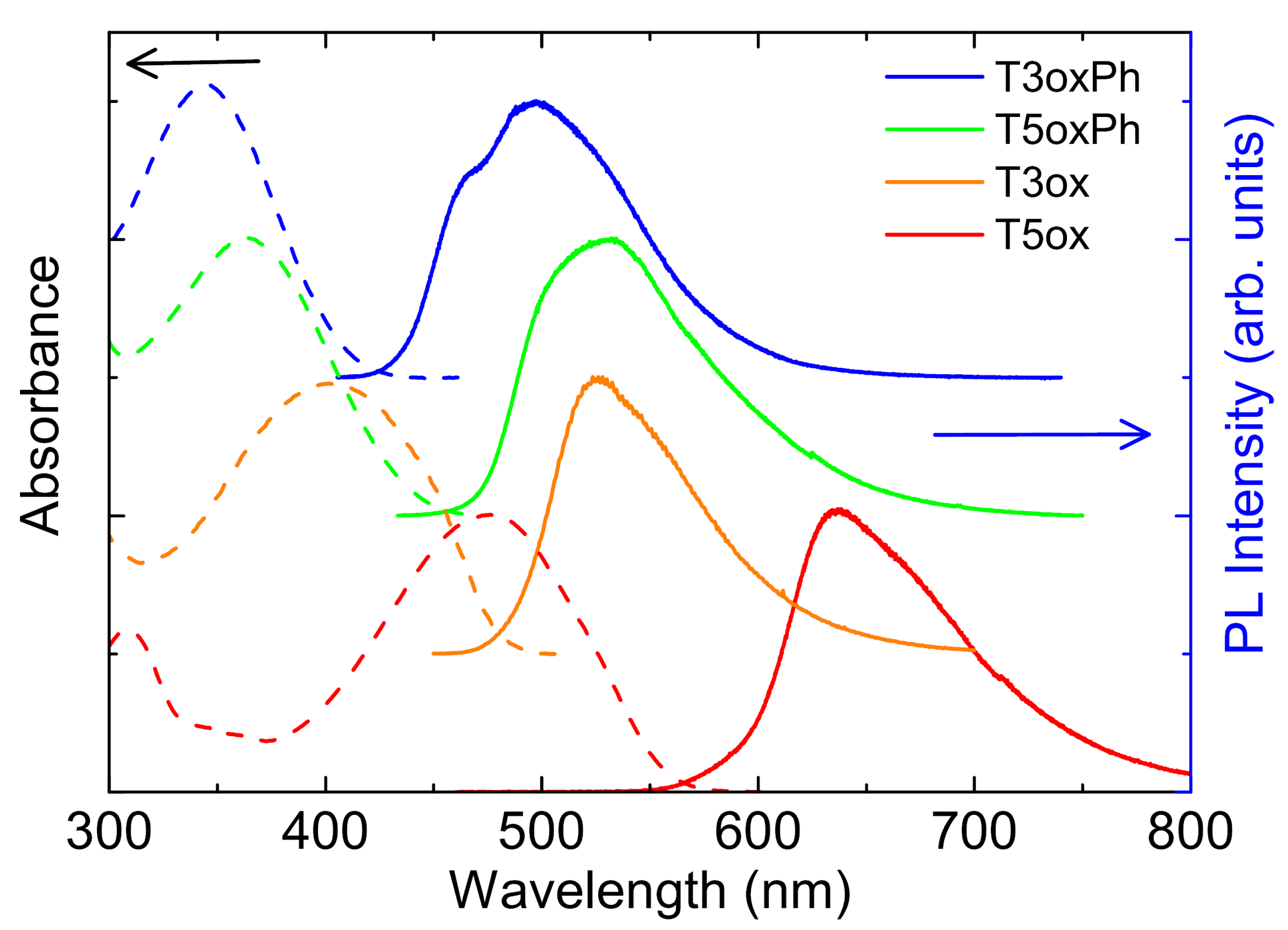
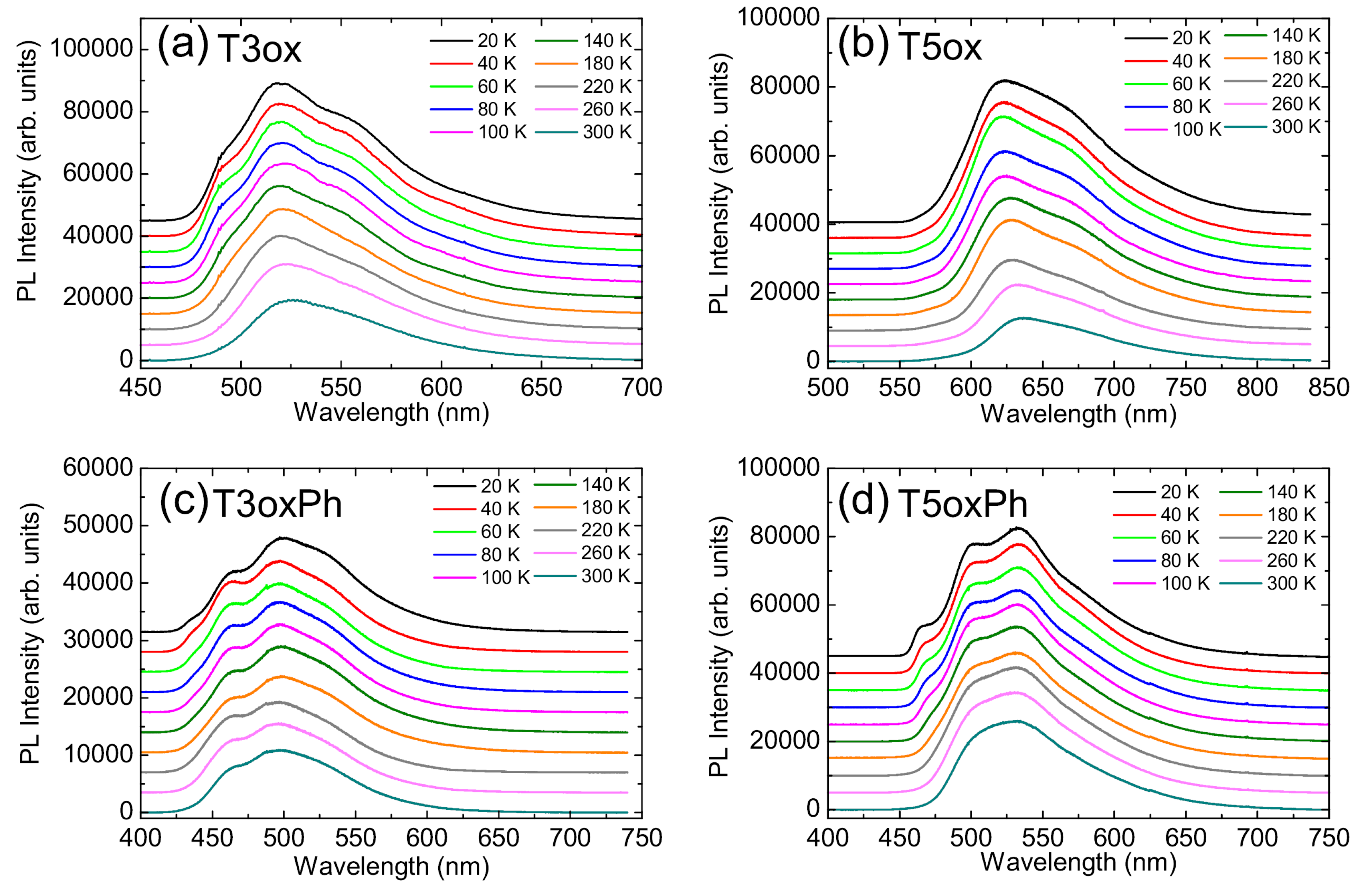
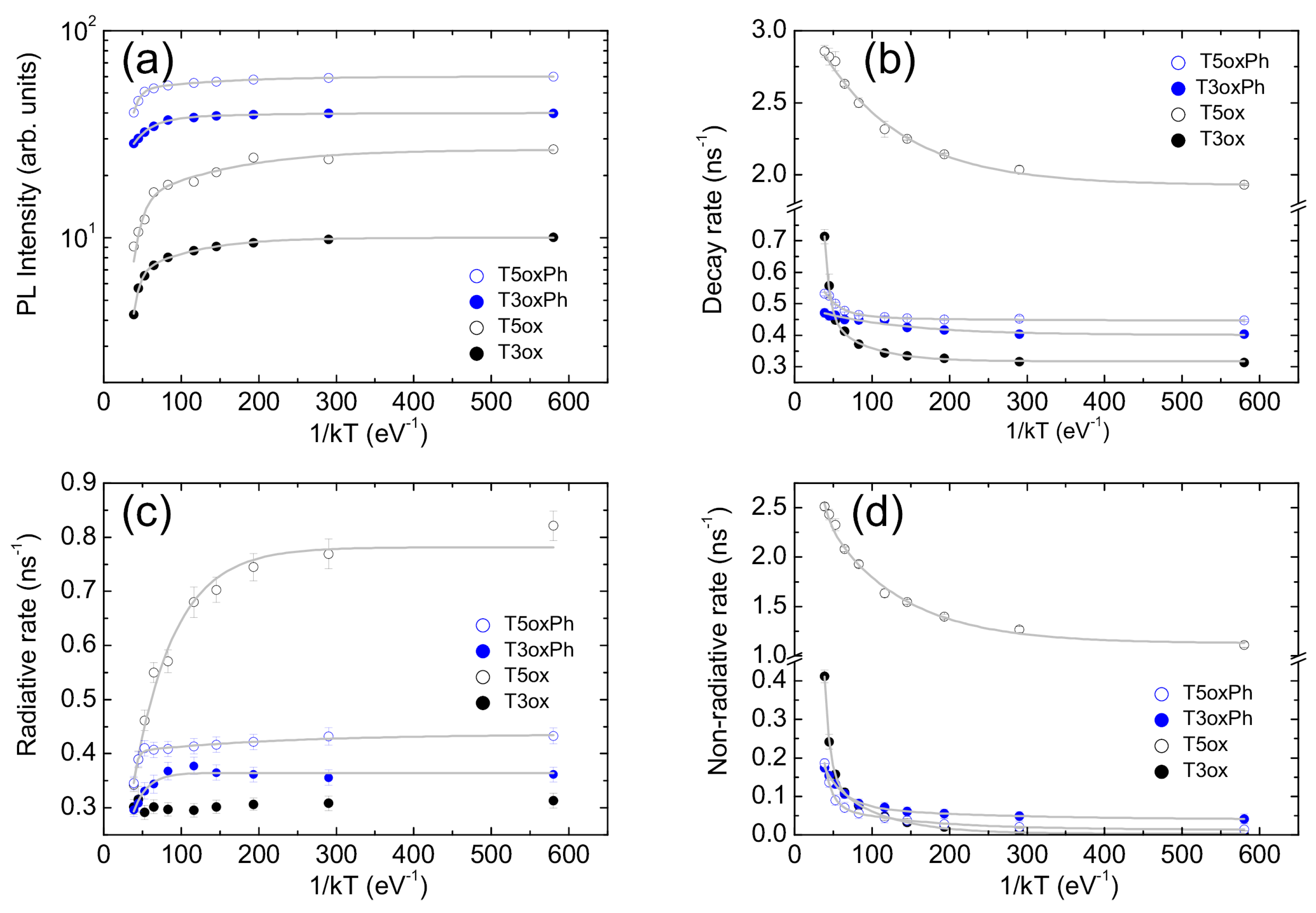
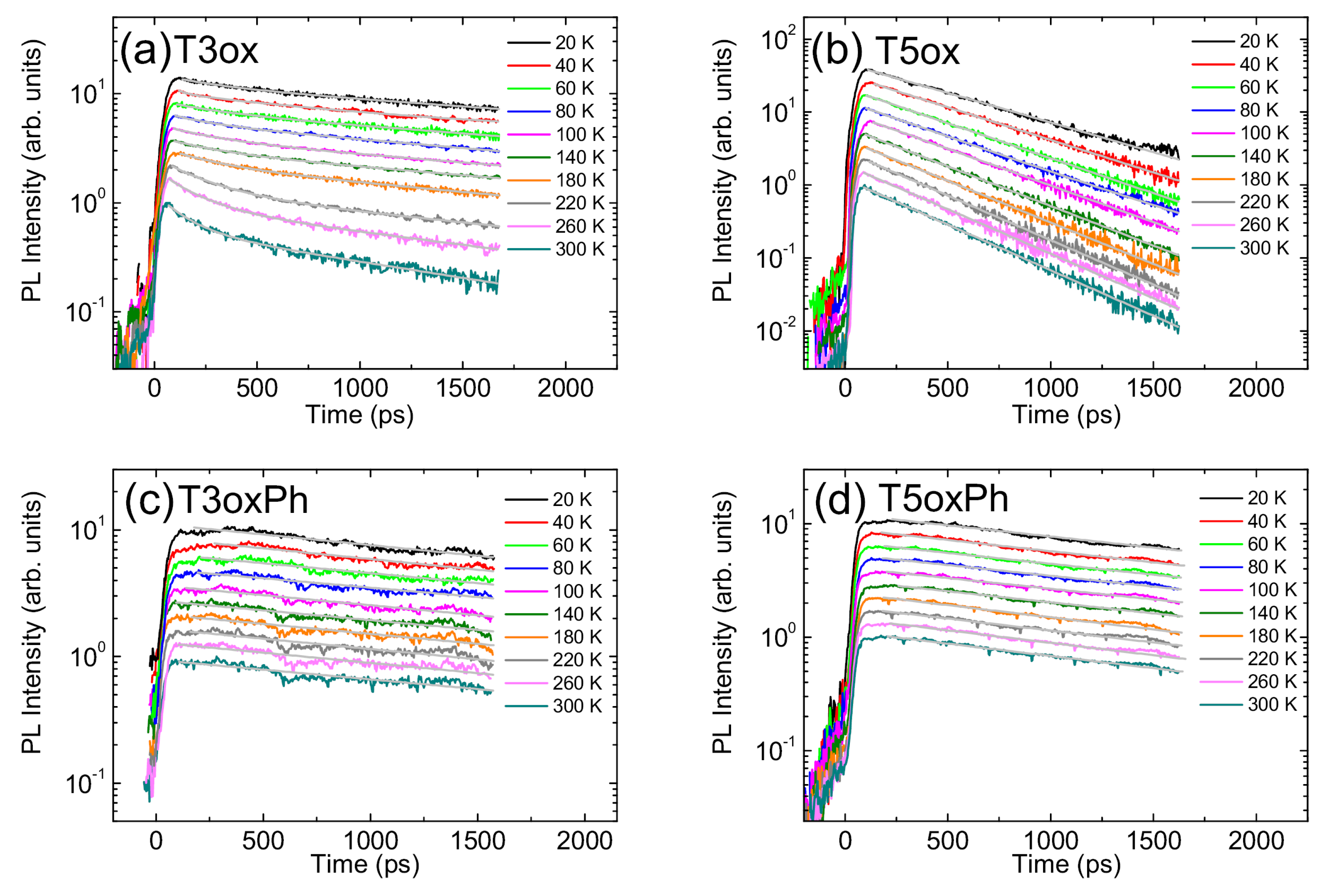
3. Conclusions
4. Materials and Methods
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, L.X. Organic Solar Cells: Recent Progress and Challenges. ACS Energy Lett. 2019, 4, 2537–2539. [Google Scholar] [CrossRef]
- Ren, H.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent Progress in Organic Photodetectors and their Applications. Adv. Sci. 2021, 8, 2002418. [Google Scholar] [CrossRef]
- Chang, J.; Lin, Z.; Zhang, C.; Hao, Y. Organic Field-Effect Transistor: Device Physics, Materials, and Process. In Different Types of Field-Effect Transistors; Pejovic, M.M., Pejovic, M.M., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 7. [Google Scholar] [CrossRef]
- Zou, S.J.; Shen, Y.; Xie, F.M.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Anni, M.; Lattante, S. Organic Lasers: Fundamentals, Developments, and Applications; Pan Stanford Publishing: Singapore, 2018; pp. 1–324. [Google Scholar]
- Zhang, Q.; Tao, W.; Huang, J.; Xia, R.; Cabanillas-Gonzalez, J. Toward Electrically Pumped Organic Lasers: A Review and Outlook on Material Developments and Resonator Architectures. Adv. Photonics Res. 2021, 2, 2000155. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, Z. Recent progress in organic field-effect transistor-basedbchem/bio-sensors. View 2022, 3, 20200115. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Glackin, J.M.; Filipi, J.; Kezic, N.; Samuel, I.D.; Turnbull, G.A. Preconcentration techniques for trace explosive sensing. Sci. Total Environ. 2019, 658, 650–658. [Google Scholar] [CrossRef]
- Capobianco, M.L.; Barbarella, G.; Manetto, A. Oligothiophenes as Fluorescent Markers for Biological Applications. Molecules 2012, 17, 910–933. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Doan, V.; Schwartz, B.J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 110, 4068–4078. [Google Scholar] [CrossRef]
- Belletete, M.; Bouchard, J.; Leclerc, M.; Durocher, G. Photophysics and Solvent-Induced Aggregation of 2,7-Carbazole-Based Conjugated Polymers. Macromolecules 2005, 38, 880–887. [Google Scholar] [CrossRef]
- Palsson, L.O.; Wang, C.; Russell, D.L.; Monkman, A.P.; Bryce, M.R.; Rumbles, G.; Samuel, I.D. Photophysics of a fluorene co-polymer in solution and films. Chem. Phys. 2002, 279, 229–237. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. AIEgens based on main group heterocycles. J. Mater. Chem. C 2018, 6, 11835–11852. [Google Scholar] [CrossRef]
- Granström, M.; Harrison, M.G.; Friend, R.H. Handbook of Oligo- and Polythiophenes; Wiley VHC: Weinheim, Germany, 1999. [Google Scholar]
- Rasmussen, S.C.; Ogawa, K.; Rothstein, S.D. Handbook of Organic Electronics and Photonics; American Scientific Publisher: Valencia, CA, USA, 2008; Chapter 1. [Google Scholar]
- Barbarella, G.; Melucci, M.; Sotgiu, G. The Versatile Thiophene: An Overview of Recent Research on Thiophene-Based Materials. Adv. Mater. 2005, 17, 1581–1593. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Thiophene-Based Organic Semiconductors. Top. Curr. Chem. 2017, 375, 84. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent thiophene-based materials and their outlook for emissive applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M.; et al. Small-molecule solar cells with efficiency over 9%. Nat. Photonics 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A Series of Simple Oligomer-like Small Molecules Based on Oligothiophenes for Solution-Processed Solar Cells with High Efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.; Fang, J.; Guo, X.; Zhu, L.; Guo, B.; Wang, Y.; Zhang, G.; Arunagiri, L.; Liu, F.; et al. Random terpolymer based on thiophene-thiazolothiazole unit enabling efficient non-fullerene organic solar cells. Nat. Commun. 2020, 11, 4612. [Google Scholar] [CrossRef]
- Horowitz, G.; Hajlaoui, M.E. Mobility in Polycrystalline Oligothiophene Field-Effect Transistors Dependent on Grain Size. Adv. Mater. 2000, 12, 1046–1050. [Google Scholar] [CrossRef]
- Amna, B.; Isci, R.; Siddiqi, H.M.; Majewski, L.A.; Faraji, S.; Ozturk, T. High-performance, low-voltage organic field-effect transistors using thieno[3,2-b]thiophene and benzothiadiazole co-polymers. J. Mater. Chem. C 2022, 10, 8254–8265. [Google Scholar] [CrossRef]
- Larik, F.A.; Faisal, M.; Saeed, A.; Abbas, Q.; Kazi, M.A.; Abbas, N.; Thebo, A.A.; Khan, D.M.; Channar, P.A. Thiophene-based molecular and polymeric semiconductors for organic field effect transistors and organic thin film transistors. J. Mater. Sci. Mater. Electron. 2018, 29, 17975–18010. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Suzuki, K.; Masumoto, Y.; Tomiuchi, Y.; Shiraishi, Y.; Kuroda, M. Optical properties of quasi-one-dimensional thiophene-based oligomers. Phys. Rev. B 1994, 50, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Yassar, A.; Horowitz, G.; Valat, P.; Wintgens, V.; Hmyene, M.; Deloffre, F.; Srivastava, P.; Lang, P.; Garnier, F. Exciton Coupling Effects in the Absorption and Photoluminescence of Sexithiophene Derivatives. J. Phys. Chem. 1995, 99, 9155–9159. [Google Scholar] [CrossRef]
- Perepichka, I.; Perepichka, D.F.; Meng, H.; Wudl, F. Light-Emitting Polythiophenes. Adv. Mater. 2005, 17, 2281–2305. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Leok Chan, K.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of Light-Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Becker, R.S.; Seixas de Melo, J.; Macanita, A.L.; Elisei, F. Comprehensive Evaluation of the Absorption, Photophysical, Energy Transfer, Structural, and Theoretical Properties of α-Oligothiophenes with One to Seven Rings. J. Phys. Chem. 1996, 100, 18683–18695. [Google Scholar] [CrossRef]
- Beljonne, D.; Shuai, Z.; Pourtois, G.; Bredas, J.L. Spin-Orbit Coupling and Intersystem Crossing in Conjugated Polymers: A Configuration Interaction Description. J. Phys. Chem. A 2001, 105, 3899–3907. [Google Scholar] [CrossRef]
- Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Bongini, A.; Arbizzani, C.; Mastragostino, M.; Anni, M.; Gigli, G.; Cingolani, R. Tuning Solid-State Photoluminescence Frequencies and Efficiencies of Oligomers Containing One Central Thiophene-S,S-dioxide Unit. J. Am. Chem. Soc. 2000, 122, 11971–11978. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chirdon, D.N.; Maurer, A.B.; Bernhard, S.; Noonan, K.J.T. Synthesis of Thiophene 1,1-Dioxides and Tuning Their Optoelectronic Properties. Org. Lett. 2013, 15, 5230–5233. [Google Scholar] [CrossRef]
- Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Fattori, V.; Cocchi, M.; Cacialli, F.; Gigli, G.; Cingolani, R. Modified Oligothiophenes with High Photo- and Electroluminescence Efficiencies. Adv. Mater. 1999, 11, 1375–1379. [Google Scholar] [CrossRef]
- Anni, M.; Gigli, G.; Paladini, V.; Cingolani, R.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M. Color engineering by modified oligothiophene blends. Appl. Phys. Lett. 2000, 77, 2458–2460. [Google Scholar] [CrossRef]
- Barbarella, G.; Favaretto, L.; Zambianchi, M.; Pudova, O.; Arbizzani, C.; Bongini, A.; Mastragostino, M. From Easily Oxidized to Easily Reduced Thiophene-Based Materials. Adv. Mater. 1998, 10, 551–554. [Google Scholar] [CrossRef]
- Barbarella, G.; Favaretto, L.; Sotgiu, G.; Antolini, L.; Gigli, G.; Cingolani, R.; Bongini, A. Rigid-Core Oligothiophene-S,S-dioxides with High Photoluminescence Efficiencies Both in Solution and in the Solid State. Chem. Mater. 2001, 13, 4112–4122. [Google Scholar] [CrossRef]
- Anni, M.; Della Sala, F.; Raganato, M.F.; Fabiano, E.; Lattante, S.; Cingolani, R.; Gigli, G.; Barbarella, G.; Favaretto, L.; Görling, A. Nonradiative Relaxation in Thiophene-S,S-dioxide Derivatives: The Role of the Environment. J. Phys. Chem. B 2005, 109, 6004–6011. [Google Scholar] [CrossRef]
- Xie, L.H.; Hou, X.Y.; Hua, Y.R.; Huang, Y.Q.; Zhao, B.M.; Liu, F.; Peng, B.; Wei, W.; Huang, W. An Effective Strategy to Tune Supramolecular Interaction via a Spiro-Bridged Spacer in Oligothiophene-S,S-dioxides and Their Anomalous Photoluminescent Behavior. Org. Lett. 2007, 9, 1619–1622. [Google Scholar] [CrossRef]
- Osken, I.; Gundogan, A.S.; Tekin, E.; Eroglu, M.S.; Ozturk, T. Fluorene-Dithienothiophene-S,S-dioxide Copolymers. Fine-Tuning for OLED Applications. Macromolecules 2013, 46, 9202–9210. [Google Scholar] [CrossRef]
- Barbarella, G.; Zambianchi, M.; Antolini, L.; Ostoja, P.; Maccagnani, P.; Bongini, A.; Marseglia, E.A.; Tedesco, E.; Gigli, G.; Cingolani, R. Solid-State Conformation, Molecular Packing, and Electrical and Optical Properties of Processable β-Methylated Sexithiophenes. J. Am. Chem. Soc. 1999, 121, 8920–8926. [Google Scholar] [CrossRef]
- Antolini, L.; Tedesco, E.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Casarini, D.; Gigli, G.; Cingolani, R. Molecular Packing and Photoluminescence Efficiency in Odd-Membered Oligothiophene S,S-Dioxides. J. Am. Chem. Soc. 2000, 122, 9006–9013. [Google Scholar] [CrossRef]
- Lanzani, G.; Cerullo, G.; De Silvestri, S.; Barbarella, G.; Sotgiu, G. Influence of the environment on the excited state deactivation in functionalized quinque-thienyls. J. Chem. Phys. 2001, 115, 1623–1625. [Google Scholar] [CrossRef]
- Lattante, S.; De Giorgi, M.; Barbarella, G.; Favaretto, L.; Gigli, G.; Cingolani, R.; Anni, M. Interplay between stimulated emission and singlet-singlet annihilation in oligothiophene dioxide thin films. J. Appl. Phys. 2006, 100, 023530. [Google Scholar] [CrossRef]
- Anni, M.; Lattante, S.; Cingolani, R.; Gigli, G.; Barbarella, G.; Favaretto, L. Far-field emission and feedback origin of random lasing in oligothiophene dioxide neat films. Appl. Phys. Lett. 2003, 83, 2754–2756. [Google Scholar] [CrossRef]
- Anni, M.; Lattante, S.; Stomeo, T.; Cingolani, R.; Gigli, G.; Barbarella, G.; Favaretto, L. Modes interaction and light transport in bidimensional organic random lasers in the weak scattering limit. Phys. Rev. B 2004, 70, 195216. [Google Scholar] [CrossRef]
- Ghofraniha, N.; Viola, I.; Di Maria, F.; Barbarella, G.; Gigli, G.; Leuzzi, L.; Conti, C. Experimental evidence of replica symmetry breaking in random lasers. Nat. Commun. 2015, 6, 6058. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, S.; Wang, H.C.; Bai, S.J.; Wang, Z.; Ren, X.; Xu, Y.X. Distinct luminescent properties between thiophene-S-oxide and Thiophene-S, S-dioxides incorporated ladder-type molecules. Dyes Pigm. 2020, 175, 108147. [Google Scholar] [CrossRef]
- Nakahama, T.; Kitagawa, D.; Sotome, H.; Ito, S.; Miyasaka, H.; Kobatake, S. Optical properties and solvatofluorochromism of fluorene derivatives bearing S,S-dioxidized thiophene. Photochem. Photobiol. Sci. 2016, 15, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, H.; Luo, W.; Nie, H.; Hu, R.; Qin, A.; Zhao, Z.; Tang, B.Z. Oxidation-enhanced emission: Exploring novel AIEgens from thieno[3,2-b]thiophene S,S-dioxide. J. Mater. Chem. C 2017, 5, 960–968. [Google Scholar] [CrossRef]
- Sala, F.D.; Heinze, H.; Görling, A. Excitation energies of terthiophene and its dioxide derivative: A first-principles study. Chem. Phys. Lett. 2001, 339, 343–350. [Google Scholar] [CrossRef]
- Della Sala, F.; Gigli, G.; Raganato, M.; Anni, M.; Pisignano, D.; Cingolani, R.; Favaretto, L.; Sotgiu, G.; Barbarella, G.; Antolini, L. Effects of intermolecular interactions on photoluminescence efficiency of crystalline thienylene-S,S-dioxide molecular semiconductors. Org. Electron. 2004, 5, 129–134. [Google Scholar] [CrossRef]
- Gigli, G.; Barbarella, G.; Favaretto, L.; Cacialli, F.; Cingolani, R. High-efficiency oligothiopene-based light-emitting diodes. Appl. Phys. Lett. 1999, 75, 439–441. [Google Scholar] [CrossRef]
- Louarn, G.; Buisson, J.P.; Lefrant, S.; Fichou, D. Vibrational Studies of a Series of .alpha.-Oligothiophenes as Model Systems of Polythiophene. J. Phys. Chem. 1995, 99, 11399–11404. [Google Scholar] [CrossRef]
- Ariu, M.; Sims, M.; Rahn, M.D.; Hill, J.; Fox, A.M.; Lidzey, D.G.; Oda, M.; Cabanillas-Gonzalez, J.; Bradley, D.D.C. Exciton migration in β-phase poly(9,9-dioctylfluorene). Phys. Rev. B 2003, 67, 195333. [Google Scholar] [CrossRef]
- In this case the temperature dependence of the slow process, related to exciton recombination, has been considered for the following analysis.
- Hermet, P.; Bantignies, J.L.; Maurin, D.; Sauvajol, J.L. Terahertz spectroscopy of the crystalline α-quaterthiophene: A combined experimental and density functional theory study. Chem. Phys. Lett. 2007, 445, 47–50. [Google Scholar] [CrossRef]
- Cirmi, G.; Brida, D.; Gambetta, A.; Piacenza, M.; Sala, F.D.; Favaretto, L.; Cerullo, G.; Lanzani, G. Observation and control of coherent torsional dynamics in a quinquethiophene molecule. Phys. Chem. Chem. Phys. 2010, 12, 7917–7923. [Google Scholar] [CrossRef]
- Johnston, M.; Herz, L.; Khan, A.; Köhler, A.; Davies, A.; Linfield, E. Low-energy vibrational modes in phenylene oligomers studied by THz time-domain spectroscopy. Chem. Phys. Lett. 2003, 377, 256–262. [Google Scholar] [CrossRef]
- Macchi, G.; Medina, B.M.; Zambianchi, M.; Tubino, R.; Cornil, J.; Barbarella, G.; Gierschner, J.; Meinardi, F. Spectroscopic signatures for planar equilibrium geometries in methyl-substituted oligothiophenes. Phys. Chem. Chem. Phys. 2009, 11, 984–990. [Google Scholar] [CrossRef]
- Chenouf, J.; Boutahir, M.; Fakrach, B.; Rahmani, A.; Chadli, H.; Hermet, P.; Mejia-Lopez, J.; Rahmani, A. Encapsulation effect of π-conjugated quaterthiophene on the radial breathing and tangential modes of semiconducting and metallic single-walled carbon nanotubes. J. Comput. Chem. 2020, 41, 2420–2428. [Google Scholar] [CrossRef]
- Moreno Castro, C.; Ruiz Delgado, M.C.; Hernandez, V.; Hotta, S.; Casado, J.; Lopez Navarrete, J.T. Efficiency of the π conjugation in a novel family of α,α’-bisphenyl end-capped oligothiophenes by means of Raman spectroscopy. J. Chem. Phys. 2002, 116, 10419–10427. [Google Scholar] [CrossRef]
- Sharafy, S.; Muszkat, K.A. Viscosity dependence of fluorescence quantum yields. J. Am. Chem. Soc. 1971, 93, 4119–4125. [Google Scholar] [CrossRef]
- Bongini, A.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Casarini, D. Conformational profile, energy barriers and optical properties of quinquethiophene-S,S-dioxides. Tetrahedron 2002, 58, 10151–10158. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Greenham, N.; Samuel, I.; Hayes, G.; Phillips, R.; Kessener, Y.; Moratti, S.; Holmes, A.; Friend, R. Measurement of absolute photoluminescence quantum efficiencies in conjugated polymers. Chem. Phys. Lett. 1995, 241, 89–96. [Google Scholar] [CrossRef]
| Molecule | Decay Time (ns) | PLQY (%) | Radiative Time (ns) | Non-Radiative Time (ns) |
|---|---|---|---|---|
| T3ox | 1.65 ± 0.05 | 46.0 ± 1.4 | 3.60 ± 0.18 | 3.10 ± 0.18 |
| T3oxPh | 2.13 ± 0.05 | 63 ± 2 | 3.38 ± 0.14 | 5.8 ± 0.5 |
| T5ox | 0.350 ± 0.010 | 12.0 ± 0.4 | 2.92 ± 0.13 | 0.398 ± 0.013 |
| T5oxPh | 1.88 ± 0.02 | 70 ± 2 | 2.90 ± 0.11 | 5.4 ± 0.3 |
| Molecule | E (meV) | E (meV) | E (meV) | E (meV) | E (meV) | E (meV) | E (meV) | E (meV) |
|---|---|---|---|---|---|---|---|---|
| T3ox | 14.7 ± 1.6 | 148 ± 21 | 19 ± 4 | 150 ± 30 | - | - | 15.0 ± 1.3 | 145 ± 11 |
| T3oxPh | 9 ± 3 | 48 ± 6 | 8.9 ± 1.6 | - | 55 ± 13 | - | 10 ± 2 | 39 ± 6 |
| T5ox | 7 ± 2 | 45 ± 10 | 9.5 ± 0.3 | - | 6 ± 2 | 36 ± 5 | 8 ± 2 | 34 ± 6 |
| T5oxPh | 8.5 ± 1.2 | 50 ± 6 | 39 ± 5 | - | 1.5 ± 0.4 | 35 ± 4 | 8.2 ± 1.1 | 39 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anni, M. Investigation of the Origin of High Photoluminescence Quantum Yield in Thienyl-S,S-dioxide AIEgens Oligomers by Temperature Dependent Optical Spectroscopy. Molecules 2023, 28, 5161. https://doi.org/10.3390/molecules28135161
Anni M. Investigation of the Origin of High Photoluminescence Quantum Yield in Thienyl-S,S-dioxide AIEgens Oligomers by Temperature Dependent Optical Spectroscopy. Molecules. 2023; 28(13):5161. https://doi.org/10.3390/molecules28135161
Chicago/Turabian StyleAnni, Marco. 2023. "Investigation of the Origin of High Photoluminescence Quantum Yield in Thienyl-S,S-dioxide AIEgens Oligomers by Temperature Dependent Optical Spectroscopy" Molecules 28, no. 13: 5161. https://doi.org/10.3390/molecules28135161
APA StyleAnni, M. (2023). Investigation of the Origin of High Photoluminescence Quantum Yield in Thienyl-S,S-dioxide AIEgens Oligomers by Temperature Dependent Optical Spectroscopy. Molecules, 28(13), 5161. https://doi.org/10.3390/molecules28135161






