Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair
Abstract
1. Introduction
2. Experimental Results
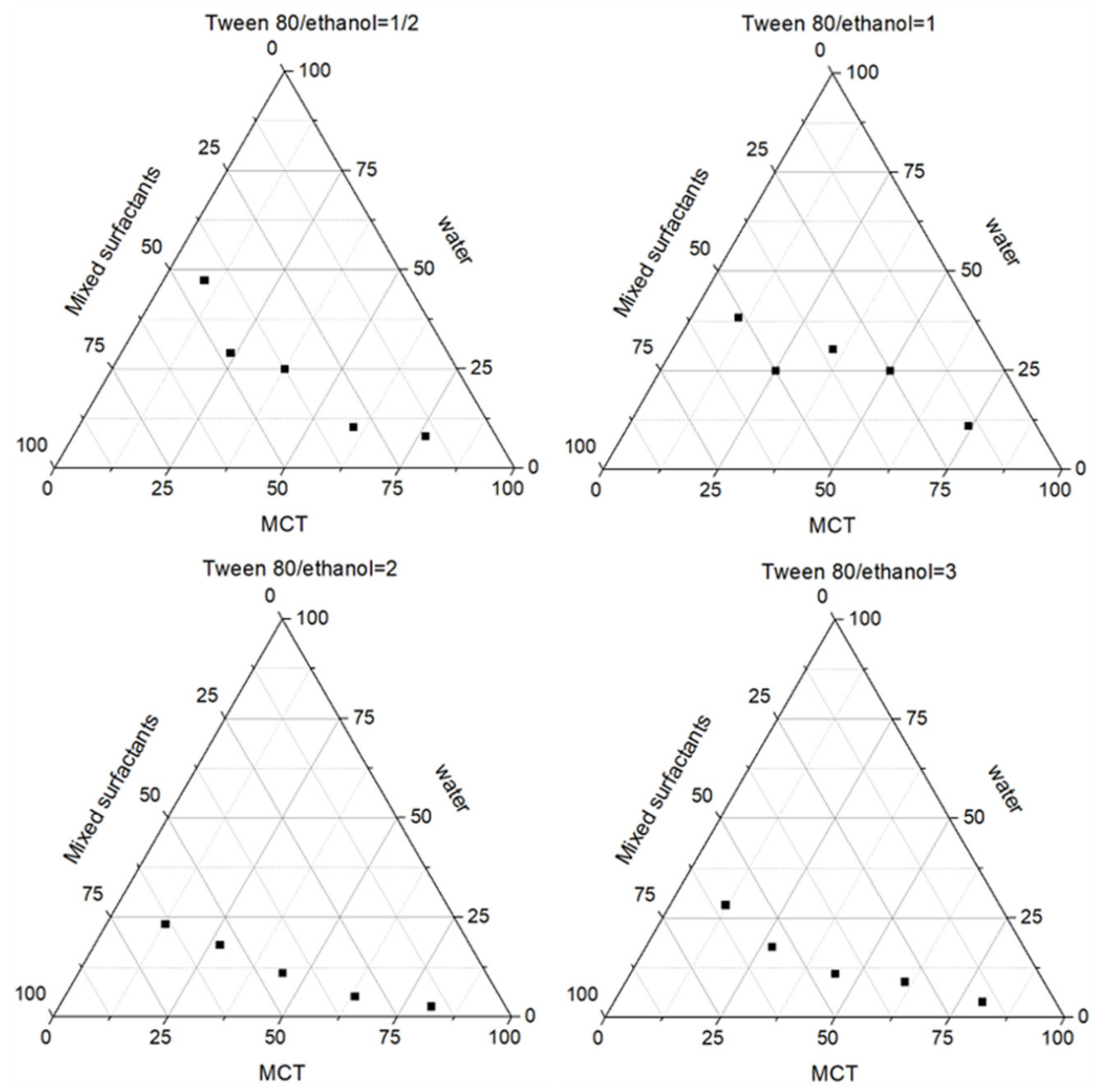
2.1. Preparation of Salidroside Inflammation Targeting Emulsion Gel
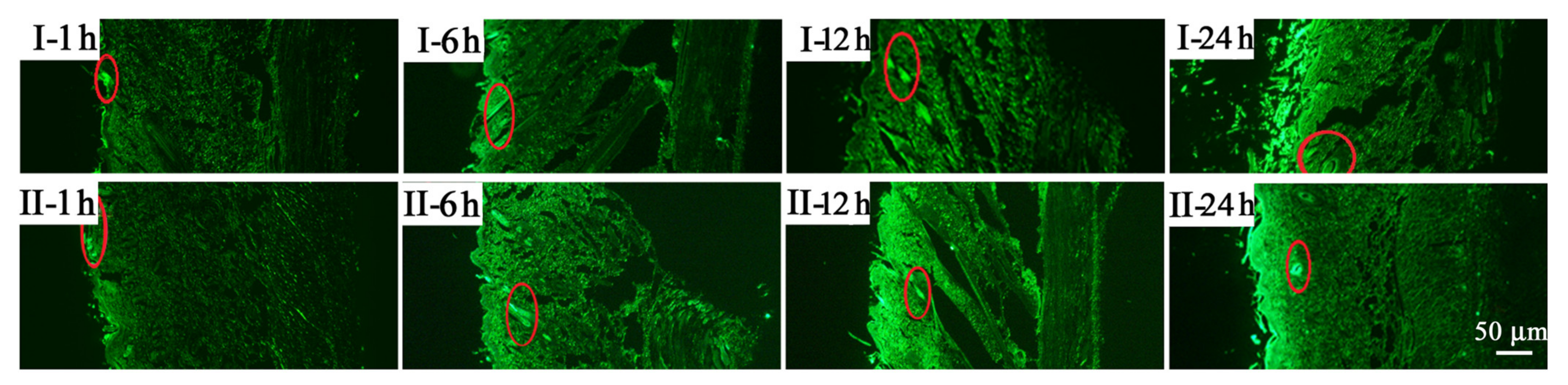
2.2. Percutaneous Penetration
2.3. Morphological Changes in Rats
2.4. Wound Healing Rate
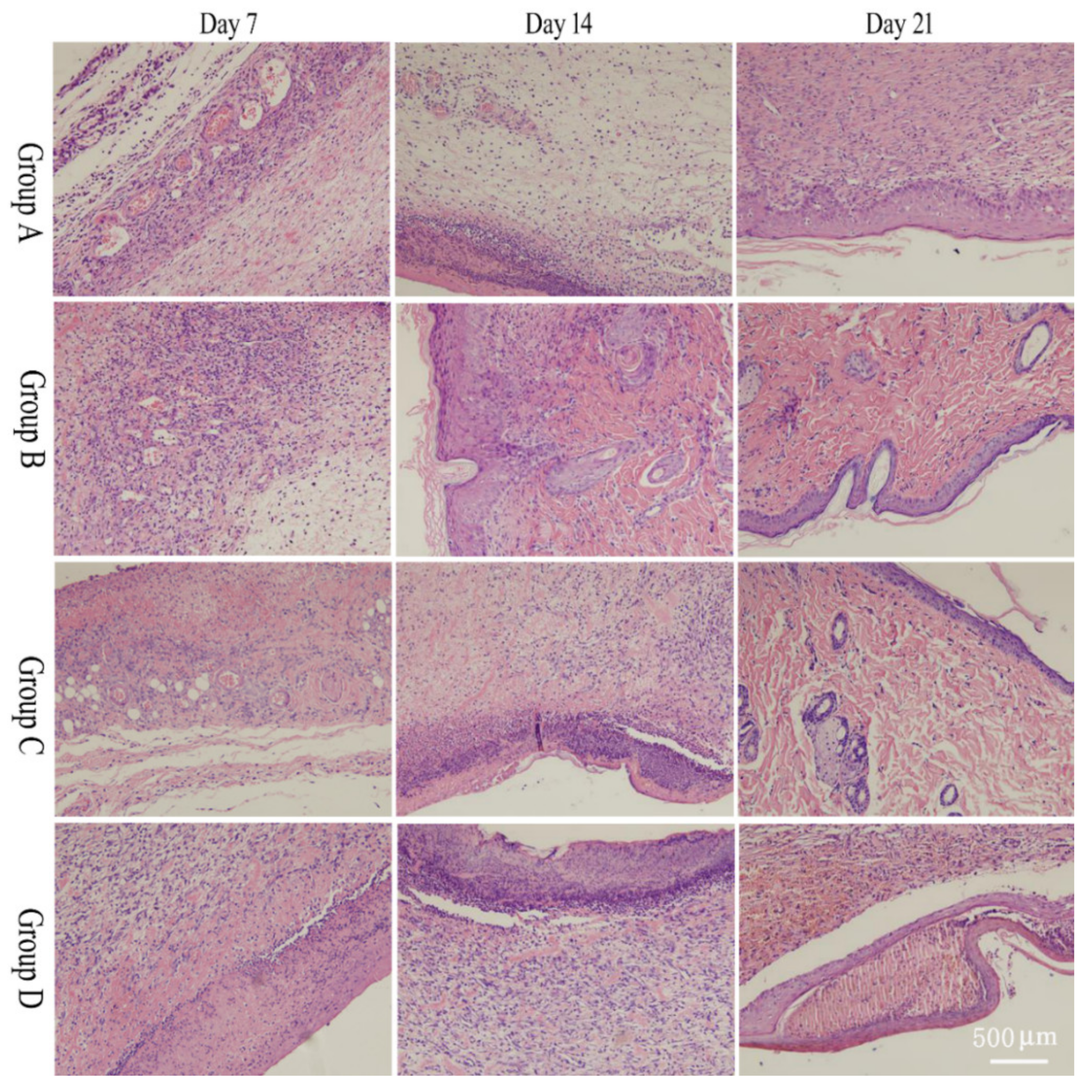
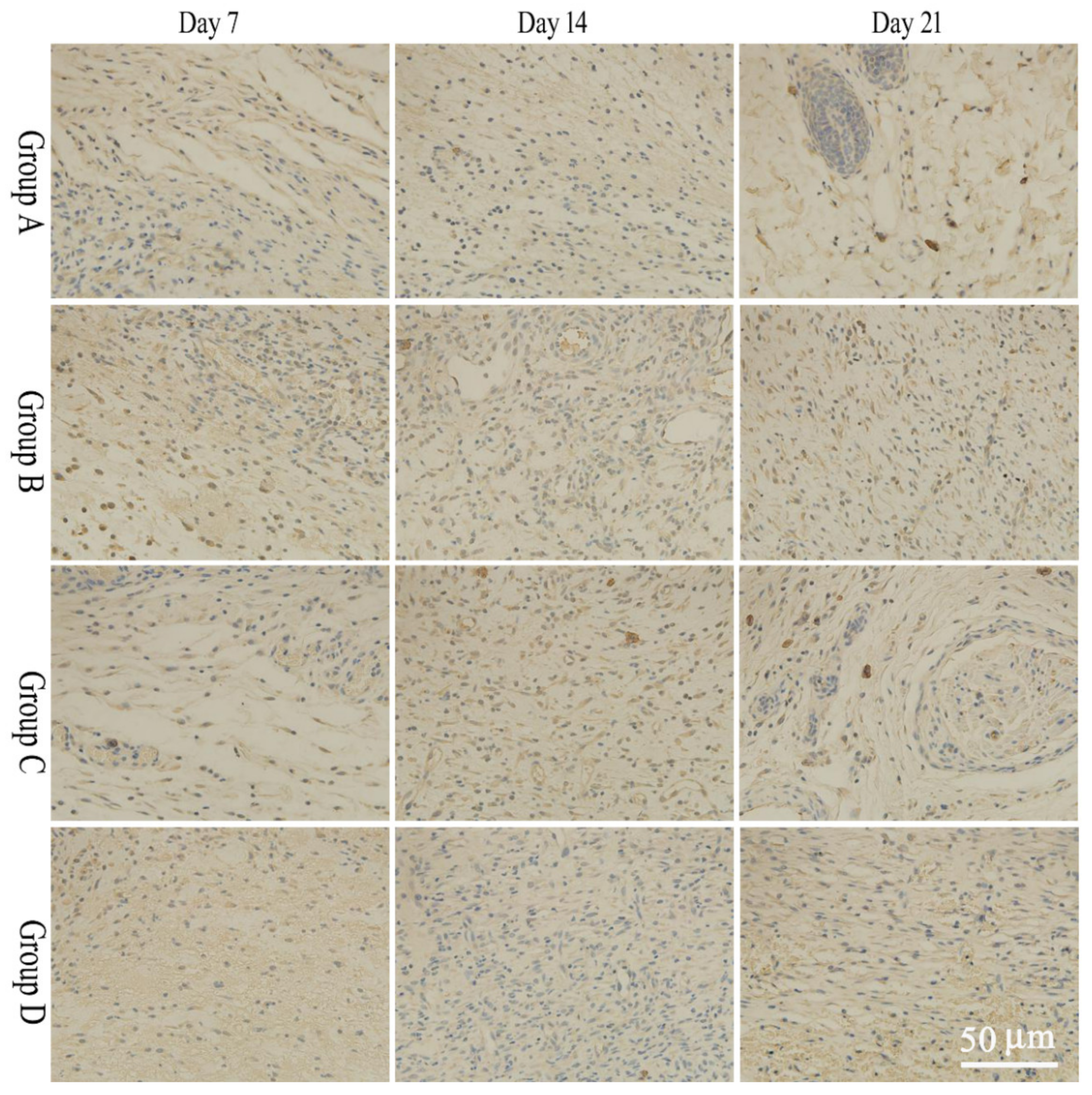
2.5. Pathological Section
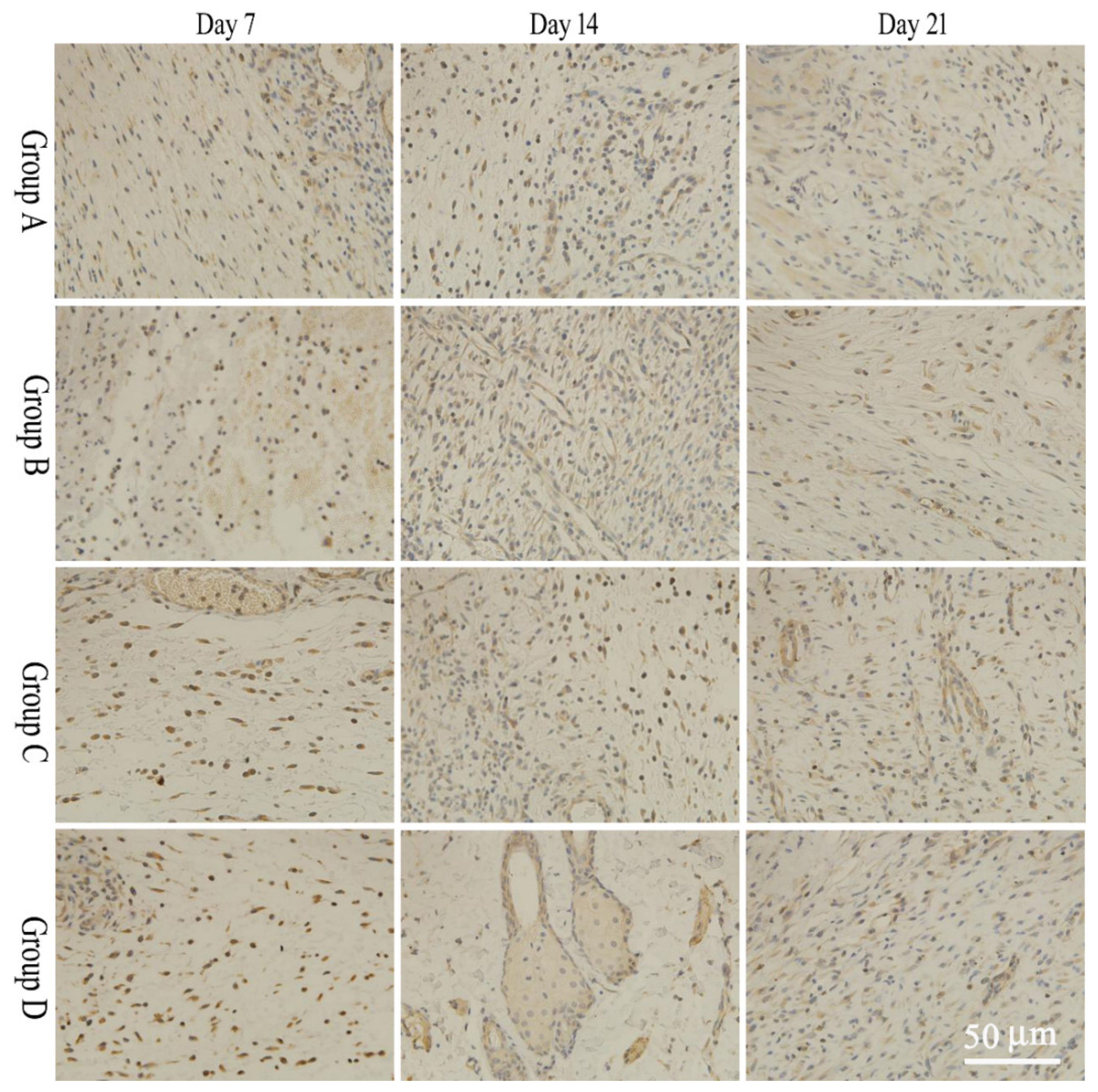
2.6. bFGF and EGF Expression
3. Materials and Methods
3.1. Animals
3.2. Materials
3.3. Instruments
3.4. Preparation of Salidroside Inflammation Targeting Emulsion Gel
3.5. Preparation of Non-Targeted Salidroside Emulsion Gel
3.6. Zeta Potential Measurement
3.7. In Vivo Fluorescence Transdermal Experiment
3.7.1. Preparation of Fluorescently Labeled Emulsion Gel
3.7.2. Preparation and Observation of Microscopic Samples
3.8. Preparation of Rat Wound Model and Experimental Grouping
3.9. Wound Efficacy Indicators
3.9.1. Gross Observation of Wounds
3.9.2. Wound Healing Rates
3.9.3. Histopathological Observation
3.9.4. Immunohistochemistry
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, X.B.; Hou, Y.; Wang, W.X.; Ai, X.P.; Hu, Y.; Chen, X.R.; Zhang, J.; Meng, X.L.; Zhang, Y. Research progress on the pharmacological effects and mechanism of tibetan medicine Rhodiola rosea. China Pharm. 2019, 30, 851–856. [Google Scholar]
- Wang, Q.; Ruan, X.; Li, H.D.; Wang, G.J. Research status, questions and strategies of rare medicinal plant rhodiola L. J. Nat. Resour. 2007, 22, 880–889. [Google Scholar]
- Zhang, Z.; Yang, W.; Ma, F.; Ma, Q.; Zhang, B.; Zhang, Y.; Liu, Y.; Liu, H.; Hua, Y. Enhancing the chemotherapy effect of apatinib on gastric cancer by co-treating with salidroside to reprogram the tumor hypoxia micro-environment and induce cell apoptosis. Drug Deliv. 2020, 27, 691–702. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, J.; Chen, Y.; Jin, X.; Wang, Y. Salidroside improves angiogenesis-osteogenesis coupling by regulating the HIF-1α/VEGF signalling pathway in the bone environment. Eur. J. Pharmacol. 2020, 884, 173394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, F.; Yan, Z.; Chen, Z.; Zhao, G. Salidroside downregulates microRNA-133a and inhibits endothelial cell apoptosis induced by oxidized low-density lipoprotein. Int. J. Mol. Med. 2020, 46, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.J.; Zhang, Z.Y.; Jin, J.; Han, J.X.; Wang, Y.; Yang, K.; Yang, Y.Y.; Wang, H.Q.; Dai, X.T.; Yao, C.; et al. Salidroside can target both P4HB-mediated inflammation and melanogenesis of the skin. Theranostics 2020, 10, 11110–11126. [Google Scholar] [CrossRef]
- Sun, A.Q.; Yan, T.H.; Ju, X.L. Research progress on the pharmacological effects and molecular mechanism of salidroside. Lishizhen Med. Mater. Med. Res. 2018, 29, 1440–1443. [Google Scholar]
- Guan, S.; Feng, H.; Song, B.; Guo, W.; Xiong, Y.; Huang, G.; Zhong, W.; Huo, M.; Chen, N.; Lu, J.; et al. Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int. Immunopharmacol. 2011, 11, 2194–2199. [Google Scholar] [CrossRef]
- Tirosh, B.; Khatib, N.; Barenholz, Y.; Nissan, A.; Rubinstein, A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol. Pharm. 2009, 6, 1083–1091. [Google Scholar] [CrossRef]
- Gomes, R.C.; Guirro, E.C.O.; Gonçalves, A.C.; Junior, J.A.F.; Junior, L.O.M.; Guirro, R.R.J. High-voltage electric stimulation of the donor site of skin grafts accelerates the healing process. A randomized blinded clinical trial. Burns 2018, 44, 636–645. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.Y.; Du, J. Effect of bioelectric field on proliferation and migration of epidermal stem cells. Chin. J. Trauma 2015, 31, 249–253. [Google Scholar]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering emulsion gels as functional colloids emphasizing food applications: A review. Front. Nutr. 2022, 9, 890188. [Google Scholar]
- Liu, F.; Liang, X.; Yan, J.; Zhao, S.; Li, S.; Liu, X.; Ngai, T.; McClements, D.J. Tailoring the properties of double-crosslinked emulsion gels using structural design principles: Physical characteristics, stability, and delivery of lycopene. Biomaterials 2022, 280, 121265. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Luan, X.; Hao, Z.; Wang, Y. Robust superhydrophobic/superoleophilic filter paper with TiO2 nanoparticles for separating oil-water mixtures and surfactant-stabilized water-oil emulsions. ACS Appl. Nano Mater. 2022, 5, 16687–16693. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, X.; Liu, L.; Gong, W.; Kong, X.; Yao, J. Robust and versatile cellulose aerogel with a self-wettable surface for efficient dual separations of oil-in-water and water-in-oil emulsions. ACS Appl. Polym. Mater. 2022, 4, 1657–1665. [Google Scholar] [CrossRef]
- Pandey, S.; Senthilguru, K.; Uvanesh, K.; Sagiri, S.S.; Behera, B.; Babu, N.; Bhattacharyya, M.K.; Pal, K.; Banerjee, I. Natural gum modified emulsion gel as single carrier for the oraldelivery of probiotic-drug combination. Int. J. Biol. Macromol. 2016, 92, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Mir, S.R.; Kohli, K.; Amin, S. Effect of oil and co-surfactant on the formation of Solutol HS 15 based colloidal drug carrier by Box–Behnken statistical design. Colloid. Surface A 2014, 453, 68–77. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, G.; Liu, C.; Zhang, L.; Gao, H.; Zhang, Y.; Dai, C.; Huang, L.; Meng, X.; Zhang, W.; et al. Draconitine perchlorate induced human breast cancer MCF-7 apoptosis through mitochondrial pathway. Int. J. Med. Sci. 2013, 10, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Di, T.T.; Zhang, C.L.; Chen, L. Danhuang powder promotes wound capillary angiogenesis in rats with diabetic foot ulcer. Diabetes New World 2017, 20, 165–166. [Google Scholar]
- Litwiniuk, M.; Krejner, A.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.J.; Coutinho-Netto, J. Cellular aspects of wound healing. Bras. Dermatol. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Jubeh, T.T.; Nadler-Milbauer, M.; Barenholz, Y.; Rubinstein, A. Local treatment of experimental colitis in the rat by negatively charged liposomes of catalase, TMN and SOD. J. Drug Target. 2006, 14, 155–163. [Google Scholar] [CrossRef]
- Jubeh, T.T.; Barenholz, Y.; Rubinstein, A. Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharm. Res. 2004, 21, 447–453. [Google Scholar] [CrossRef]
- Mohajeri, S.; Moayedi, S.; Mohajeri, S.; Yadegar, A.; Haririan, I. Targeting pathophysiological changes using biomaterials-based drug delivery systems: A key to managing inflammatory bowel disease. Front. Pharmacol. 2022, 13, 1045575. [Google Scholar] [CrossRef]
- Harel, E.; Rubinstein, A.; Nissan, A.; Khazanov, E.; Milbauer, M.N.; Barenholz, Y.; Tirosh, B. Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS ONE 2011, 6, e24202. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.K.S.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Bhat, G.J.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control. Release 2018, 290, 165–179. [Google Scholar] [CrossRef]
- Song, B.; Gu, Y.; Pu, J.; Reid, B.; Zhao, Z.; Zhao, M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007, 2, 1479–1489. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J.S. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008, 16, 432–441. [Google Scholar] [CrossRef]
- Wang, C.; Sani, E.S.; Gao, W. Wearable bioelectronics for chronic wound management. Adv. Funct. Mater. 2022, 32, 2111022. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.W.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Wang, W.; Li, Q.; Liu, J.; Xu, T.; Zhao, X. Effect of ultrasound debridement on serum inflammatory factors and bFGF, EGF expression in wound tissues. J. Coll. Physicians Surg. Pak. 2019, 29, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.M.; Han, S.H.; Ko, E.A.; Yoon, D.S.; Park, I.K.; Shin, H.C.; Park, K.H.; Lee, J.W. Development of stabilized dual growth factor-loaded hyaluronate collagen dressing matrix. J. Tissue Eng. 2021, 12, 2041731421999750. [Google Scholar] [CrossRef] [PubMed]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef]
- Shah, V.P.; Elkins, J.S.; Williams, R.L. Evaluation of the test system used for in vitro release of drugs for topical dermatological drug products. Pharm. Dev. Technol. 1999, 4, 377–385. [Google Scholar] [CrossRef]
- Hou, D.X.; Wang, D.K. Advances in in vitro drug release from semisolid preparations. Chin. J. Pharm. 2019, 17, 135–140. [Google Scholar]
| 7 d | 14 d | 21 d | |
|---|---|---|---|
| Group A | Exudate, dark red wound | Exudate, red wound | No exudate, light red, hairless growth |
| Group B | No exudate, dark red wound | Exudate, red wound | No exudate, light red, hairless growth |
| Group C | No exudate, dark red wound | No exudate, light red wound; smooth and light white healing area with little pigmentation | No exudate, light red, hairy growth |
| Group D | No exudate, dark red wound | No exudate, light red wound; smooth and light white healing area | No exudate, light red, hairless growth |
| Group | Wound Healing Rate | ||
|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |
| Group A | 0.50 ± 0.09 | 0.71 ± 0.17 | 0.83 ± 0.23 |
| Group B | 0.57 ± 0.10 | 0.76 ± 0.19 | 0.84 ± 0.19 |
| Group C | 0.68 ± 0.12 * | 0.83 ± 0.21 * | 0.87 ± 0.20 |
| Group D | 0.64 ± 0.13 | 0.82 ± 0.20 | 0.86 ± 0.21 |
| Group | Expression of bFGF in Granulation Tissue (pg/mL) | ||
|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |
| Group A | 7.14 ± 1.23 | 10.21 ± 2.36 | 7.41 ± 1.91 |
| Group B | 11.53 ± 2.67 | 11.79 ± 2.55 | 10.00 ± 2.03 |
| Group C | 16.87 ± 2.42 *,∇ | 16.63 ± 2.57 *,∇ | 15.68 ± 4.52 * |
| Group D | 19.53 ± 8.37 *,∇ | 11.36 ± 2.37 | 12.96 ± 3.31 * |
| Group | Expression of EGF in Granulation Tissue (pg/mL) | ||
|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |
| Group A | 6.32 ± 1.06 | 4.49 ± 0.86 | 7.21 ± 0.88 |
| Group B | 6.42 ± 1.34 | 6.08 ± 0.58 | 7.91 ± 1.88 |
| Group C | 6.43 ± 1.76 | 11.62 ± 3.04 *,∇ | 18.86 ± 0.94 *,∇ |
| Group D | 9.47 ± 2.46 | 8.67 ± 2.15 * | 11.03 ± 3.06 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yang, J.; Luo, S.; Zhang, H.; Liu, B.; Pan, Z. Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair. Molecules 2023, 28, 5151. https://doi.org/10.3390/molecules28135151
Wang X, Yang J, Luo S, Zhang H, Liu B, Pan Z. Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair. Molecules. 2023; 28(13):5151. https://doi.org/10.3390/molecules28135151
Chicago/Turabian StyleWang, Xiaojie, Jun Yang, Shuai Luo, Hucheng Zhang, Bo Liu, and Zhiquan Pan. 2023. "Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair" Molecules 28, no. 13: 5151. https://doi.org/10.3390/molecules28135151
APA StyleWang, X., Yang, J., Luo, S., Zhang, H., Liu, B., & Pan, Z. (2023). Study of Salidroside and Its Inflammation Targeting Emulsion Gel for Wound Repair. Molecules, 28(13), 5151. https://doi.org/10.3390/molecules28135151






