Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid
Abstract
1. Introduction
2. Results
2.1. Antiradical and Antioxidant Activities Evaluated by DPPH, ABTS, and FRAP Assays
2.2. Reactive Oxygen Species Scavenging Capacity
2.3. Cell Viability Assays
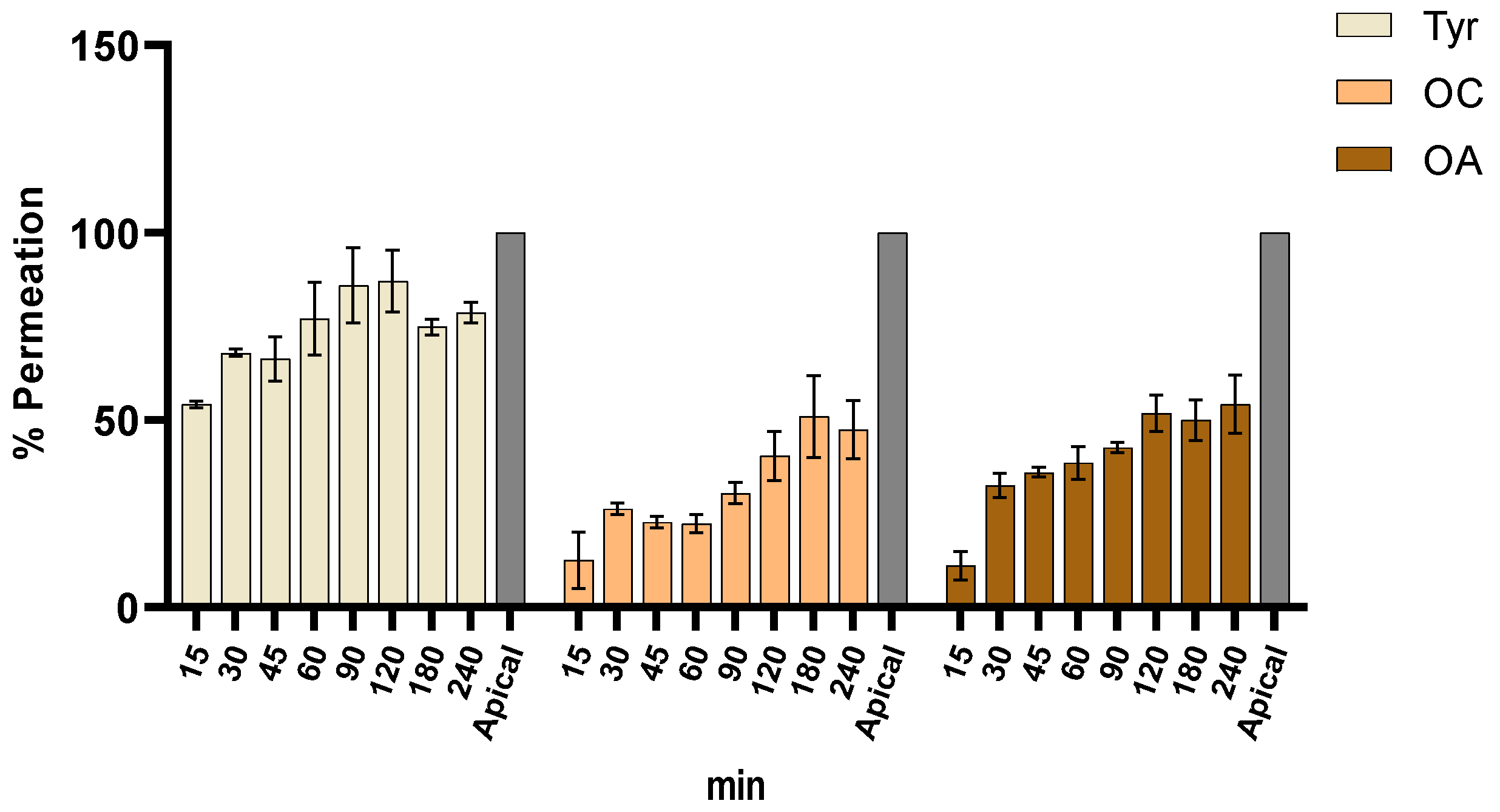
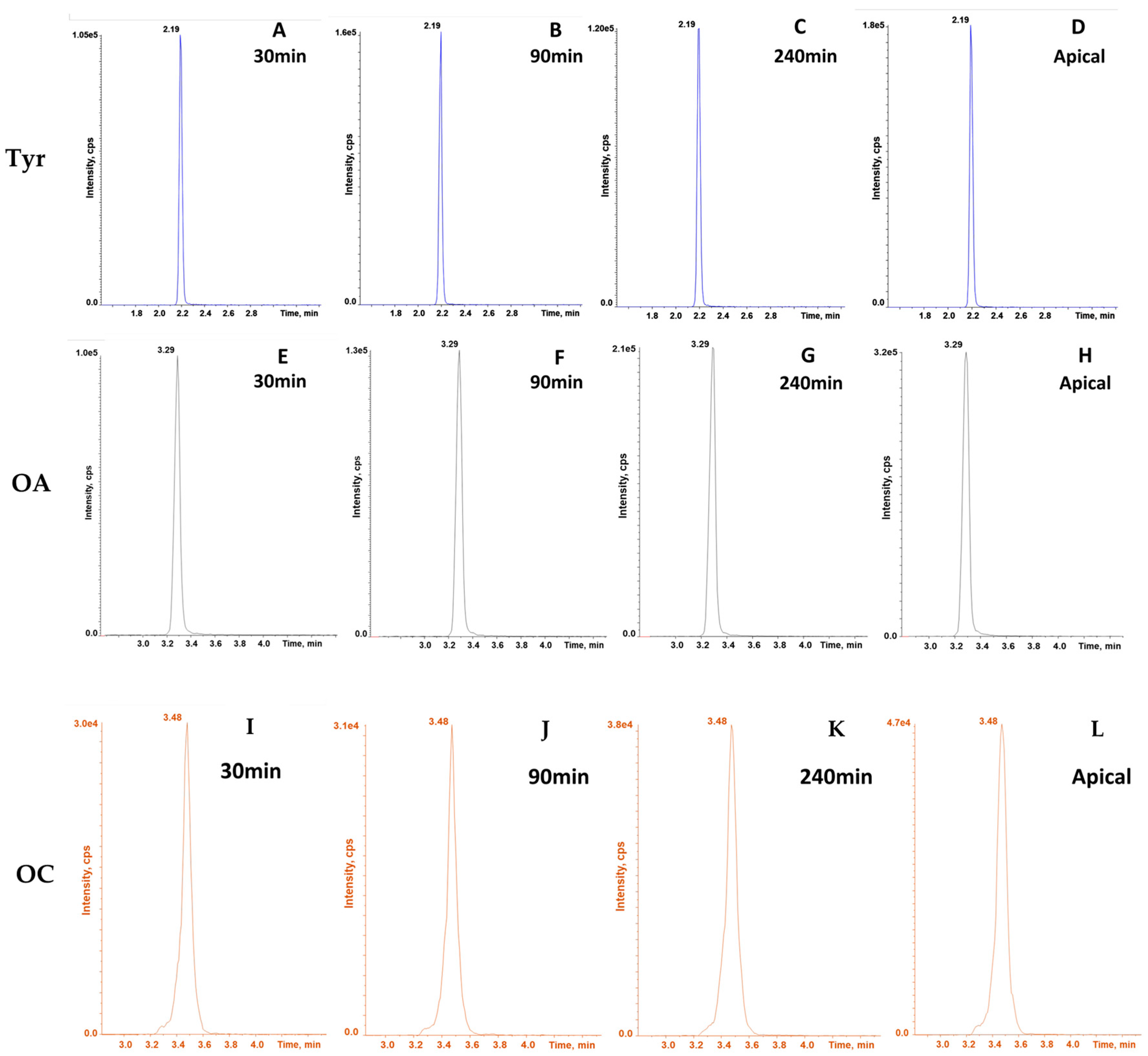
2.4. Intestinal Permeation Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Reactive Oxygen Species Scavenging Capacity Assays
4.2.1. Superoxide Anion Radical Scavenging Assay
4.2.2. Hypochlorous Acid Scavenging Assay
4.2.3. Peroxyl Radical Scavenging Assay
4.2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
4.2.5. ABTS Assay
4.2.6. Ferric Reducing/Antioxidant Power (FRAP) Assay
4.3. Cell Viability Assay
4.4. Intestinal Permeability Assay
4.5. LC-MS/MS Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean Diet and Health Status: Active Ingredients and Pharmacological Mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A Randomized Clinical Trial of Greek High Phenolic Early Harvest Extra Virgin Olive Oil in Mild Cognitive Impairment: The MICOIL Pilot Study. J. Alzheimers Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newman, J.W.; Holt, R.R. Oleocanthal-Rich Extra Virgin Olive Oil Demonstrates Acute Anti-Platelet Effects in Healthy Men in a Randomized Trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily Use of Extra Virgin Olive Oil with High Oleocanthal Concentration Reduced Body Weight, Waist Circumference, Alanine Transaminase, Inflammatory Cytokines and Hepatic Steatosis in Subjects with the Metabolic Syndrome: A 2-Month Intervention Study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea Europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Gianfrancesco, M.; Maurizio, S.; Maura, B.; Roberto, S.; Enrico, M.; Alceo, M. Simple and Hydrolyzable Compounds in Virgin Olive Oil. 3. Spectroscopic Characterizations of the Secoiridoid Derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like Activity in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- El Haouari, M.; Quintero, J.E.; Rosado, J.A. Anticancer Molecular Mechanisms of Oleocanthal. Phytother. Res. 2020, 34, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; Sayed, K.A.E.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of Tau Protein Fibrillization by Oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Giusti, L.; Hrelia, S. New Neuroprotective Perspectives in Fighting Oxidative Stress and Improving Cellular Energy Metabolism by Oleocanthal. Neural Regen. Res. 2019, 14, 1217. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Al Rihani, S.B.; Yang, E.; Kaddoumi, A. Chapter 56—Neuroprotective Effects of Oleocanthal in Neurological Disorders. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 671–679. ISBN 978-0-12-819528-4. [Google Scholar]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-ΚB Pathways. CPB 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef]
- Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Tsolakou, A.; Diamantakos, P.; Kalaboki, I.; Mena-Bravo, A.; Priego-Capote, F.; Abdallah, I.M.; Kaddoumi, A.; Melliou, E.; Magiatis, P. Oleocanthalic Acid, a Chemical Marker of Olive Oil Aging and Exposure to a High Storage Temperature with Potential Neuroprotective Activity. J. Agric. Food Chem. 2018, 66, 7337–7346. [Google Scholar] [CrossRef] [PubMed]
- Esposito Salsano, J.; Pinto, D.; Rodrigues, F.; Saba, A.; Manera, C.; Digiacomo, M.; Macchia, M. Oleocanthalic Acid from Extra-Virgin Olive Oil: Analysis, Preparative Isolation and Radical Scavenging Activity. J. Food Compos. Anal. 2022, 105, 104160. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-Assisted Extraction as a Green Technology Approach to Recover Polyphenols from Castanea Sativa Shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Silva, A.M.; Almeida, A.; Dall’Acqua, S.; Loschi, F.; Sarmento, B.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Insights into the 3D In Vitro Permeability and In Vivo Antioxidant Protective Effects of Kiwiberry Leaf Extract: A Step Forward to Human Nutraceutical Use. Int. J. Mol. Sci. 2022, 23, 14130. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Luís, A.S.; Moreira, M.M.; Ferraz, R.; Brezo-Borjan, T.; Švarc-Gajić, J.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Influence of Temperature on the Subcritical Water Extraction of Actinidia Arguta Leaves: A Screening of pro-Healthy Compounds. Sustain. Chem. Pharm. 2022, 25, 100593. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- González, F.; García-Martínez, E.; Del Mar Camacho, M.; Martínez-Navarrete, N.; Sarmento, B.; Fernandes, I.; Freitas, V.; Rodrigues, F.; Oliveira, B. Insights into the Development of Grapefruit Nutraceutical Powder by Spray Drying: Physical Characterization, Chemical Composition and 3D Intestinal Permeability. J. Sci. Food Agric. 2019, 99, 4686–4694. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Polyphenol Intake and Mortality Risk: A Re-Analysis of the PREDIMED Trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Pinto, D.; Braga, N.; Silva, A.M.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Chapter 6—Chestnut. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 127–144. ISBN 978-0-12-817106-6. [Google Scholar]
- Lee, O.-H.; Lee, B.-Y.; Kim, Y.-C.; Shetty, K.; Kim, Y.-C. Radical Scavenging-Linked Antioxidant Activity of Ethanolic Extracts of Diverse Types of Extra Virgin Olive Oils. J. Food Sci. 2008, 73, C519–C525. [Google Scholar] [CrossRef]
- Berto, A.; Ribeiro, A.B.; Sentandreu, E.; de Souza, N.E.; Mercadante, A.Z.; Chisté, R.C.; Fernandes, E. The Seed of the Amazonian Fruit Couepia Bracteosa Exhibits Higher Scavenging Capacity against ROS and RNS than Its Shell and Pulp Extracts. Food Funct. 2015, 6, 3081–3090. [Google Scholar] [CrossRef]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential Use of Cytisus Scoparius Extracts in Topical Applications for Skin Protection against Oxidative Damage. J. Photochem. Photobiol. B 2013, 125, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zheng, L.; Liu, R.; Chang, M.; Huang, J.; Zhao, C.; Jin, Q.; Wang, X. Physicochemical Property, Chemical Composition and Free Radical Scavenging Capacity of Cold Pressed Kernel Oils Obtained from Different Eucommia Ulmoides Oliver Cultivars. Ind. Crops Prod. 2018, 124, 912–918. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Fernandes, I.; Gonçalves Albuquerque, T.; Costa, H.S.; Freitas, V.; Rodrigues, F.; Oliveira, M.B.P.P. Infusions and Decoctions of Dehydrated Fruits of Actinidia Arguta and Actinidia Deliciosa: Bioactivity, Radical Scavenging Activity and Effects on Cells Viability. Food Chem. 2019, 289, 625–634. [Google Scholar] [CrossRef]
- Pinto, D.; Franco, S.D.; Silva, A.M.; Cupara, S.; Koskovac, M.; Kojicic, K.; Soares, S.; Rodrigues, F.; Sut, S.; Dall’Acqua, S.; et al. Chemical Characterization and Bioactive Properties of a Coffee-like Beverage Prepared from Quercus Cerris Kernels. Food Funct. 2019, 10, 2050–2060. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different Radical Scavenging Tests in Virgin Olive Oil and Their Relation to the Total Phenol Content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Valavanidis, A.; Nisiotou, C.; Papageorgiou, Y.; Kremli, I.; Satravelas, N.; Zinieris, N.; Zygalaki, H. Comparison of the Radical Scavenging Potential of Polar and Lipidic Fractions of Olive Oil and Other Vegetable Oils under Normal Conditions and after Thermal Treatment. J. Agric. Food Chem. 2004, 52, 2358–2365. [Google Scholar] [CrossRef]
- Choe, K.I.; Kwon, J.H.; Park, K.H.; Oh, M.H.; Kim, M.H.; Kim, H.H.; Cho, S.H.; Chung, E.K.; Ha, S.Y.; Lee, M.W. The Antioxidant and Anti-Inflammatory Effects of Phenolic Compounds Isolated from the Root of Rhodiola Sachalinensis A. BOR. Molecules 2012, 17, 11484–11494. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Liaki, V.; Stathopoulos, P.; Sklirou, A.D.; Tsakiri, E.N.; Jakschitz, T.; Bonn, G.; Trougakos, I.P.; Halabalaki, M.; Skaltsounis, L.A. Comparison Survey of EVOO Polyphenols and Exploration of Healthy Aging-Promoting Properties of Oleocanthal and Oleacein. Food Chem. Toxicol. 2019, 125, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-Induced Nitric Oxide Production in Intestinal Cells by Hydroxytyrosol and Tyrosol Metabolites: Insight into the Mechanism of Action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef]
- O’Shea, J.P.; Augustijns, P.; Brandl, M.; Brayden, D.J.; Brouwers, J.; Griffin, B.T.; Holm, R.; Jacobsen, A.-C.; Lennernäs, H.; Vinarov, Z.; et al. Best Practices in Current Models Mimicking Drug Permeability in the Gastrointestinal Tract—An UNGAP Review. Eur. J. Pharm. Sci. 2022, 170, 106098. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Camodeca, C.; D’Andrea, F.; Piragine, E.; Testai, L.; Calderone, V.; Orlandini, E.; Nuti, E.; Rossello, A. Matrix Metalloproteinase-12 Inhibitors: Synthesis, Structure-Activity Relationships and Intestinal Absorption of Novel Sugar-Based Biphenylsulfonamide Carboxylates. Bioorg. Med. Chem. 2018, 26, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- López-Yerena, A.; Vallverdú-Queralt, A.; Mols, R.; Augustijns, P.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Absorption and Intestinal Metabolic Profile of Oleocanthal in Rats. Pharmaceutics 2020, 12, 134, Reply in Pharmaceutics 2020, 12, 1221. [Google Scholar] [CrossRef]
- López-Yerena, A.; Pérez, M.; Vallverdú-Queralt, A.; Miliarakis, E.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Oleacein Intestinal Permeation and Metabolism in Rats Using an In Situ Perfusion Technique. Pharmaceutics 2021, 13, 719. [Google Scholar] [CrossRef]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of Health-Promoting Phenolic Compounds in Two Extra Virgin Olive Oils and Diversity of Associated Yeasts. J. Food Compos. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel Strong Scavengers of Reactive Oxygen and Nitrogen Species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertini, S.; Macchia, M.; Digiacomo, M. Enhanced Nutraceutical Properties of Extra Virgin Olive Oil Extract by Olive Leaf Enrichment. Nutrients 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; Abreu, B.V.d.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona Fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Borges, T.H.; Cabrera-Vique, C.; Seiquer, I. Antioxidant Properties of Chemical Extracts and Bioaccessible Fractions Obtained from Six Spanish Monovarietal Extra Virgin Olive Oils: Assays in Caco-2 Cells. Food Funct. 2015, 6, 2375–2383. [Google Scholar] [CrossRef]

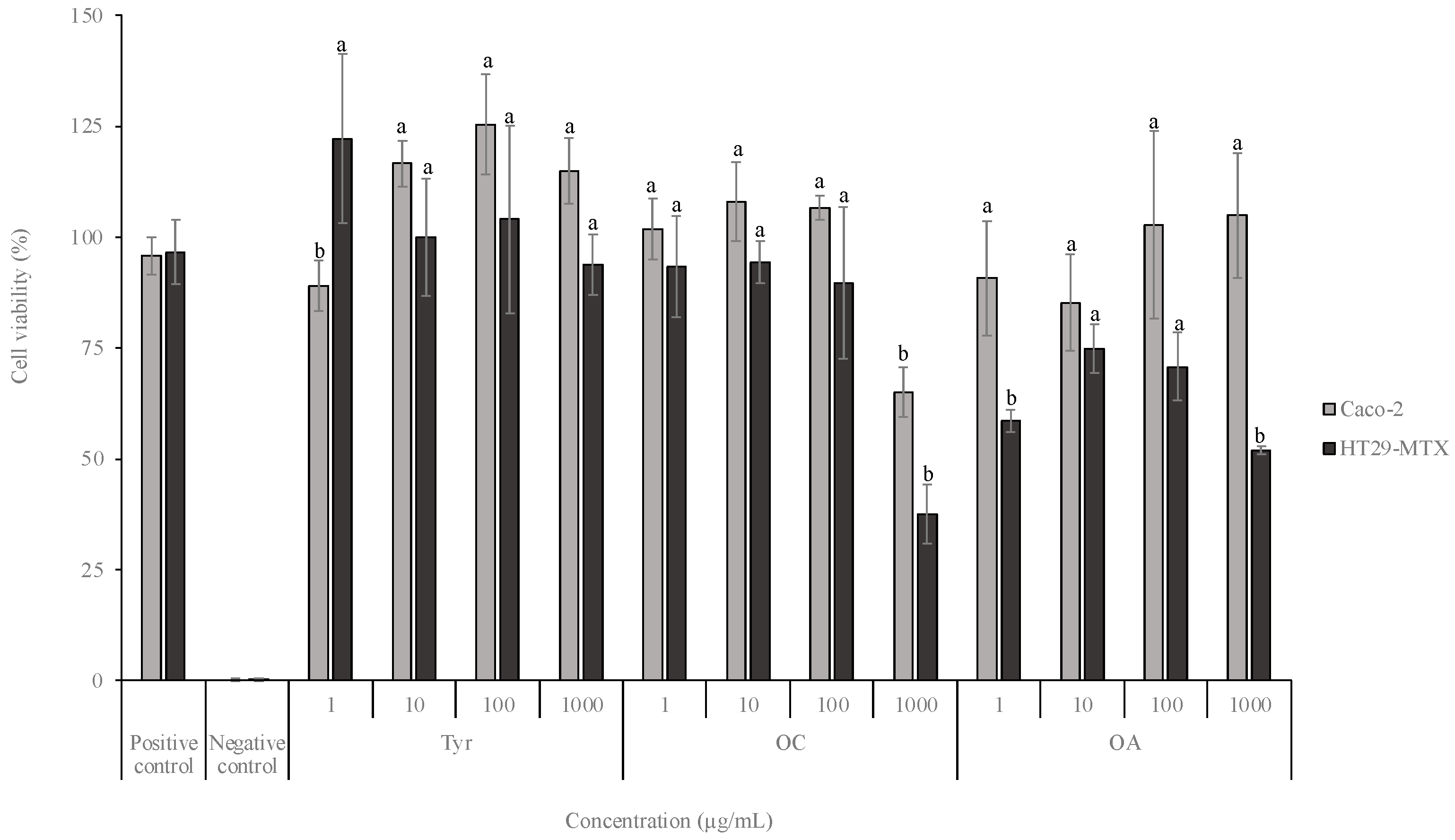
| FRAP | DPPH | ABTS | |
|---|---|---|---|
| µmol TE/mg dw | IC50 (µg/mL) | IC50 (µg/mL) | |
| OC | 0.021 ± 0.002 a | 660 ± 26 b | 70.37 ± 2.02 a |
| Tyr | 0.028 ± 0.0009 a | 1060 ± 47 a | 2.17 ± 0.07 c |
| OA | 0.009 ± 0.002 b | >2000 | 9.91 ± 1.05 b |
| Reactive Oxygen Species | ||||
|---|---|---|---|---|
| O2●− | HOCl | ROO● | ||
| IC50 (µg/mL) | % Inhibition | IC50 (µg/mL) | µmol TE/mg dw | |
| OC | 919.80 ± 34.30 a | - | 73.18 ± 1.43 b | 0.0152 ± 0.0029 c |
| Tyr | - | 17.05 ± 0.67 | 571.32 ± 8.50 a | 0.0046 ± 0.0007 c |
| OA | 19.09 ± 1.20 a,* | 360.87 ± 8.79 a,* | 0.0056 ± 0.0003 a,* | |
| Positive controls | ||||
| Catechin | 48.05 ± 0.78 b | - | 0.22 ± 0.01 c | 0.44 ± 0.07 b |
| Gallic acid | 12.04 ± 0.03 b | - | 4.80 ± 0.06 c | 1.39 ± 0.11 a |
| Analyte | SRM Transition (Da) | DP (V) | CE (V) | CxP (V) |
|---|---|---|---|---|
| Tyr | 137.0 → 106.0 (Q) | −20 | −5.0 | |
| 137.0 → 107.0 (q) | −35 | −21 | −5.4 | |
| 137.0 → 119.0 (q) | −20 | −5.9 | ||
| OC | 303.1 → 59.0 (Q) | −10 | −6.0 | |
| 303.1 → 165.0 (q) | −30 | −13 | −8.4 | |
| 303.1 → 182.6 (q) | −13 | −9.6 | ||
| OA | 319.2 → 110.8 (q) | −23 | −5.3 | |
| 319.2 → 155.0 (q) | −25 | −25 | −7.5 | |
| 319.2 → 199.0 (Q) | −19 | −9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. https://doi.org/10.3390/molecules28135150
Cuffaro D, Pinto D, Silva AM, Bertolini A, Bertini S, Saba A, Macchia M, Rodrigues F, Digiacomo M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules. 2023; 28(13):5150. https://doi.org/10.3390/molecules28135150
Chicago/Turabian StyleCuffaro, Doretta, Diana Pinto, Ana Margarida Silva, Andrea Bertolini, Simone Bertini, Alessandro Saba, Marco Macchia, Francisca Rodrigues, and Maria Digiacomo. 2023. "Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid" Molecules 28, no. 13: 5150. https://doi.org/10.3390/molecules28135150
APA StyleCuffaro, D., Pinto, D., Silva, A. M., Bertolini, A., Bertini, S., Saba, A., Macchia, M., Rodrigues, F., & Digiacomo, M. (2023). Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules, 28(13), 5150. https://doi.org/10.3390/molecules28135150










