An AIE-Active NIR Fluorescent Probe with Good Water Solubility for the Detection of Aβ1–42 Aggregates in Alzheimer’s Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
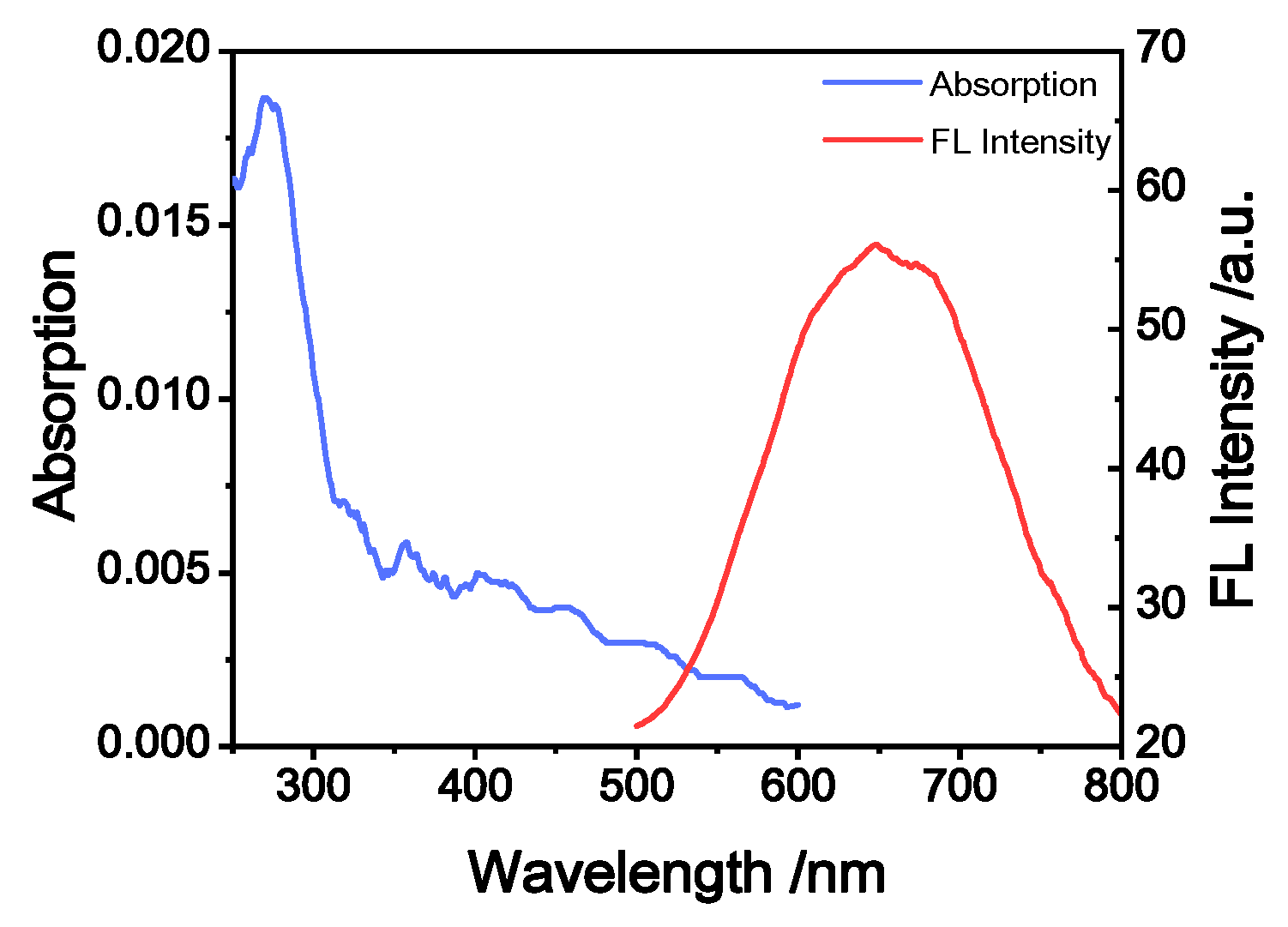
2.2. Photophysical Characterization of TMNL
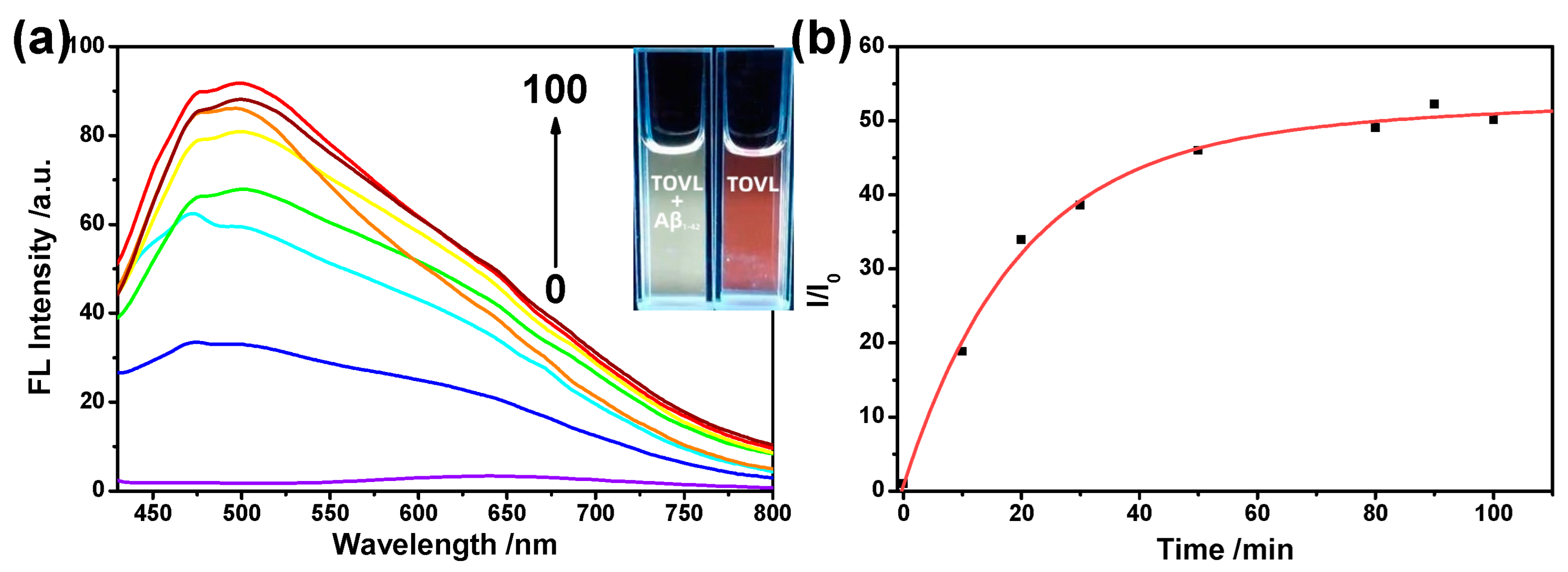
2.3. The Performance of Aβ1–42 Fibrils Detection
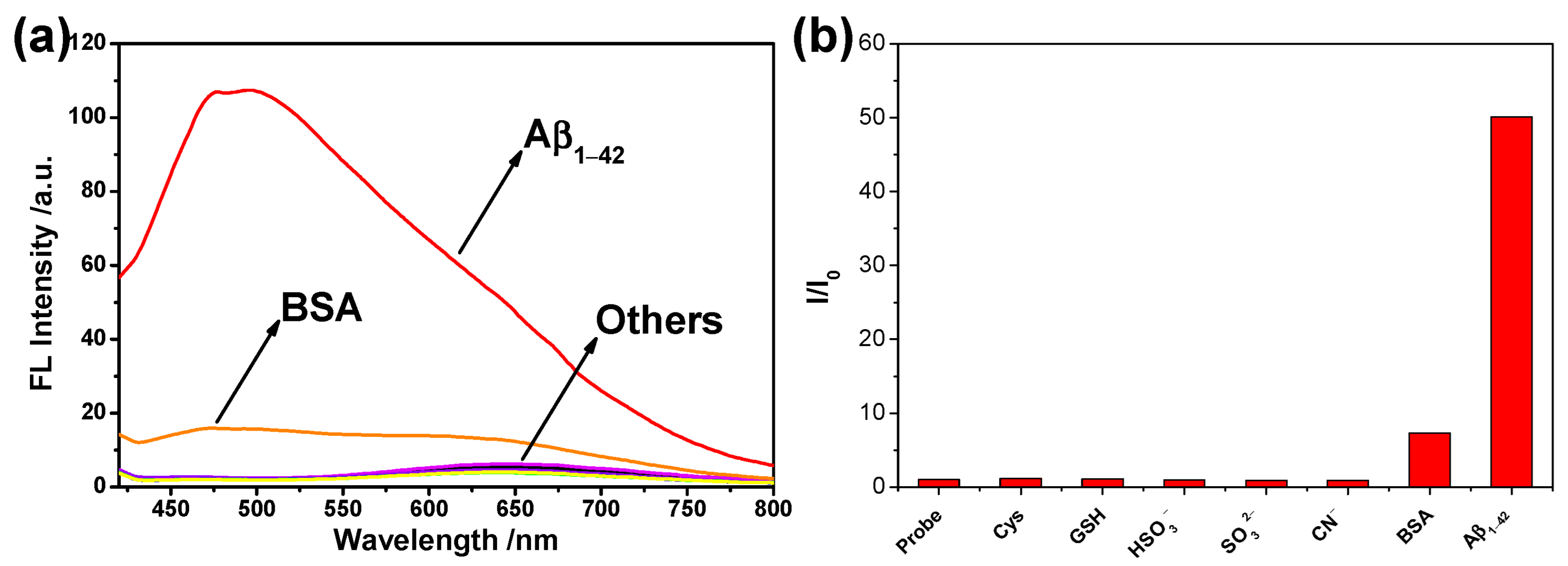
2.4. Selectivity Study
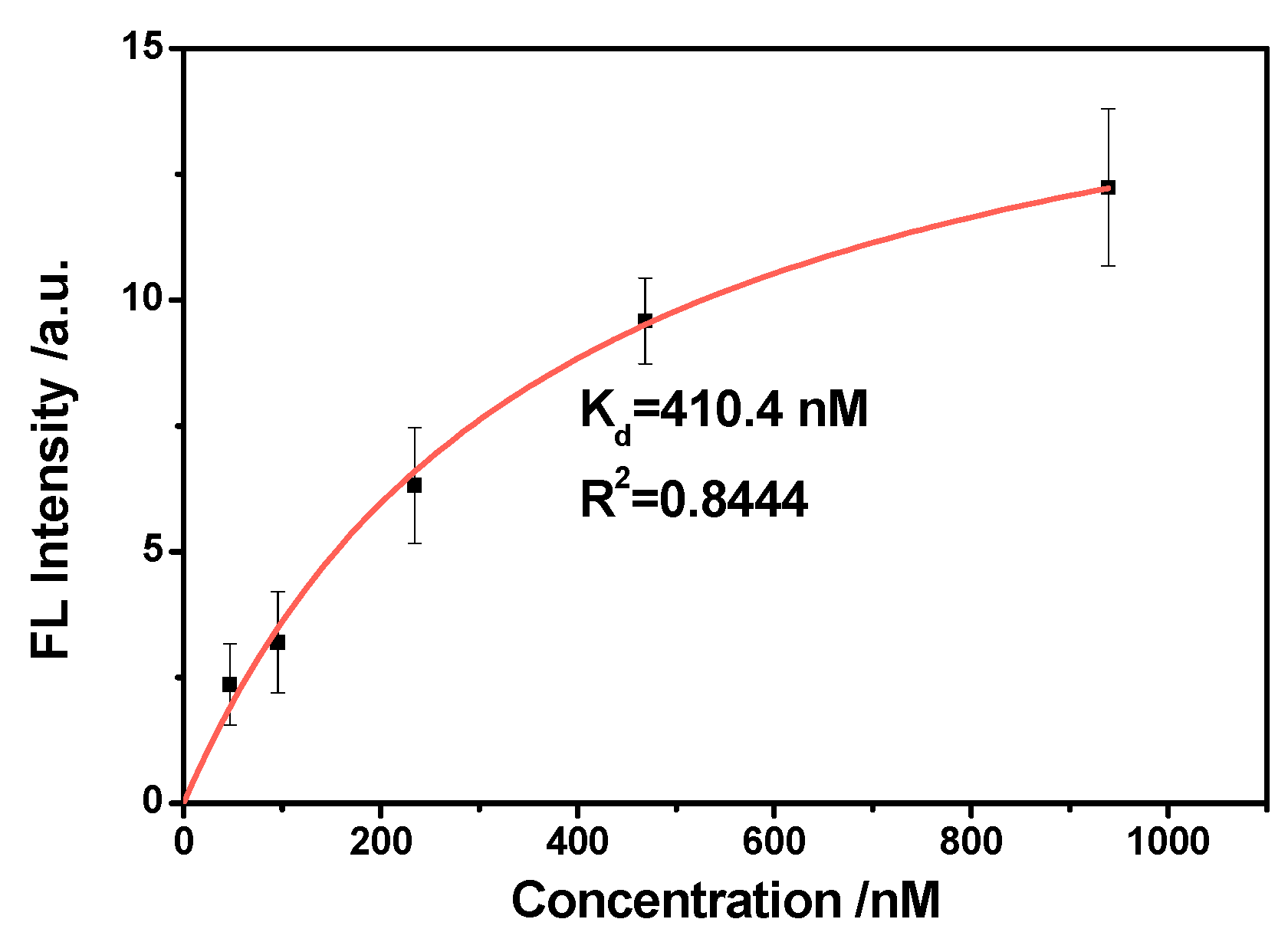
2.5. The Dissociation Constant Study
2.6. Appearance Observations
2.7. In Vitro Mapping with High-Fidelity Aβ1–42 Plaque Information
3. Materials and Methods
3.1. Materials and Instrumentations
3.2. Preparation of AD Transgenic Mouse Brain Tissue Paraffin Sections
3.3. Preparation of Aβ1–42 Fibrils
3.4. Coincubation Preparation Process of TMNL with Aβ1–42 Fibrils
3.5. Synthesis of Compound 4
3.6. Synthesis of Compound 13
3.7. Synthesis of Compound TMNL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gravitz, L. Drugs: A tangled web of targets. Nature 2011, 475, S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Resolving controversies on the path to Alzheimer’s therapeutics. Nat. Med. 2011, 17, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Bhasikuttan, A.C.; Mohanty, J. Detection, inhibition and disintegration of amyloid fibrils: The role of optical probes and macrocyclic receptors. Chem. Commun. 2017, 53, 2789–2809. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Goedert, M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Morris, K.; Serpell, L. From natural to designer self-assembling biopolymers, the structural characterisation of fibrous proteins & peptides using fibre diffraction. Chem. Soc. Rev. 2010, 39, 3445–3453. [Google Scholar]
- Hamley, I.W. The Amyloid Beta Peptide: A Chemist’s Perspective. Role in Alzheimer’s and Fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef]
- Kepp, K.P. Bioinorganic Chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef]
- Schroeter, E.H.; Ilagan, M.X.G.; Brunkan, A.L.; Hecimovic, S.; Li, Y.M.; Xu, M.; Lewis, H.D.; Saxena, M.T.; Strooper, B.D.; Coonrod, A.; et al. A presenilin dimer at the core of the γ-secretase enzyme: Insights from parallel analysis of Notch 1 and APP proteolysis. Proc. Natl. Acad. Sci. USA 2003, 100, 13075–13080. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T. The carboxy terminus of the.beta. amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ishikawa, M.; Kimura, H.; Hayashi, S.; Matsumura, K.; Watanabe, H.; Shimizu, Y.; Cheng, Y.; Cui, M.; Kawashima, H.; et al. Development of dual functional SPECT/fluorescent probes for imaging cerebral β-amyloid plaques. Bioorg. Med. Chem. Lett. 2010, 20, 3885–3888. [Google Scholar] [CrossRef]
- Hintersteiner, M.; Enz, A.; Frey, P.; Jaton, A.-L.; Kinzy, W.; Kneuer, R.; Neumann, U.; Rudin, M.; Staufenbiel, M.; Stoeckli, M.; et al. In vivo detection of amyloid-β deposits by near-infrared imaging using an oxazine-derivative probe. Nat. Biotechnol. 2005, 23, 577–583. [Google Scholar] [CrossRef]
- Tong, H.; Lou, K.; Wang, W. Near-infrared fluorescent probes for imaging of amyloid plaques in Alzheimer’s disease. Acta Pharm. Sin. B 2015, 5, 25–33. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, L.; Chen, S.; Leung, C.W.T.; Da, L.-T.; Faisal, M.; Silva, D.-A.; Liu, J.; Lam, J.W.Y.; Huang, X.; et al. Monitoring and Inhibition of Insulin Fibrillation by a Small Organic Fluorogen with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 1680–1689. [Google Scholar] [CrossRef]
- Marzano, N.R.; Wray, K.M.; Johnston, C.L.; Paudel, B.P.; Hong, Y.; van Oijen, A.; Ecroyd, H. An α-Cyanostilbene Derivative for the Enhanced Detection and Imaging of Amyloid Fibril Aggregates. ACS Chem. Neurosci. 2020, 11, 4191–4202. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Kim, H.-K.; Baek, A.R.; Kim, S.; Kim, M.J.; Kim, M.; Cho, A.E.; Lee, G.-H.; Jung, H.; Yang, J.-u.; et al. Multifunctional imaging of amyloid-beta peptides with a new gadolinium-based contrast agent in Alzheimer’s disease. J. Ind. Eng. Chem. 2020, 83, 214–223. [Google Scholar] [CrossRef]
- Das, A.; Dutta, T.; Gadhe, L.; Koner, A.L.; Saraogi, I. Biocompatible Fluorescent Probe for Selective Detection of Amyloid Fibrils. Anal. Chem. 2020, 92, 10336–10341. [Google Scholar] [CrossRef]
- Zhou, K.; Bai, H.; Feng, L.; Dai, J.; Cui, M. Smart D-π-A Type Near-Infrared Aβ Probes: Effects of a Marked π Bridge on Optical and Biological Properties. Anal. Chem. 2017, 89, 9432–9437. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Nesterov, E.E.; Skoch, J.; Hyman, B.T.; Klunk, W.E.; Bacskai, B.J.; Swager, T.M. In Vivo Optical Imaging of Amyloid Aggregates in Brain: Design of Fluorescent Markers. Angew. Chem. Int. Ed. 2005, 44, 5452–5456. [Google Scholar] [CrossRef]
- Raymond, S.B.; Skoch, J.; Hills, I.D.; Nesterov, E.E.; Swager, T.M.; Bacskai, B.J. Smart optical probes for near-infrared fluorescence imaging of Alzheimer’s disease pathology. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 93–98. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Kimura, H.; Saji, H. BODIPY-Based Molecular Probe for Imaging of Cerebral β-Amyloid Plaques. ACS Chem. Neurosci. 2012, 3, 319–324. [Google Scholar] [CrossRef]
- Cui, M.; Ono, M.; Watanabe, H.; Kimura, H.; Liu, B.; Saji, H. Smart Near-Infrared Fluorescence Probes with Donor-Acceptor Structure for in Vivo Detection of β-Amyloid Deposits. J. Am. Chem. Soc. 2014, 136, 3388–3394. [Google Scholar] [CrossRef]
- Fu, H.; Tu, P.; Zhao, L.; Dai, J.; Liu, B.; Cui, M. Amyloid-β Deposits Target Efficient Near-Infrared Fluorescent Probes: Synthesis, in vitro Evaluation, and in vivo Imaging. Anal. Chem. 2016, 88, 1944–1950. [Google Scholar] [CrossRef]
- Yan, J.W.; Zhu, J.Y.; Zhou, K.X.; Wang, J.S.; Tan, H.Y.; Xu, Z.Y.; Chen, S.B.; Lu, Y.T.; Cui, M.C.; Zhang, L. Neutral merocyanine dyes: For in vivo NIR fluorescence imaging of amyloid-β plaques. Chem. Commun. 2017, 53, 9910–9913. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, K.; Yan, J.; Sun, H.; Pistolozzi, M.; Cui, M.; Zhang, L. Dual-functional red-emitting fluorescent probes for imaging beta-amyloid plaques and viscosity. Sens. Actuators B 2019, 298, 126903. [Google Scholar] [CrossRef]
- Zhou, K.; Yuan, C.; Dai, B.; Wang, K.; Chen, Y.; Ma, D.; Dai, J.; Liang, Y.; Tan, H.; Cui, M. Environment-Sensitive Near-Infrared Probe for Fluorescent Discrimination of Aβ and Tau Fibrils in AD Brain. J. Med. Chem. 2019, 62, 6694–6704. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Fang, S.Q.; Tang, Y.W.; Wang, C.; Luo, H.; Qu, L.L.; Zhao, J.H.; Shi, C.J.; Yin, F.C.; Wang, X.B.; et al. A hemicyanine derivative for near-infrared imaging of β-amyloid plaques in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 179, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and Synthesis of Curcumin Analogues for in Vivo Fluorescence Imaging and Inhibiting Copper-Induced Cross-Linking of Amyloid Beta Species in Alzheimer’s Disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Zhang, Q.; Kuang, Y.; Qin, A.; Gao, M.; Li, F.; Tang, B.Z. An AIE-active theranostic probe for light-up detection of Aβ aggregates and protection of neuronal cells. J. Mater. Chem. B 2019, 7, 2434–2441. [Google Scholar] [CrossRef]
- Fu, W.; Yan, C.; Guo, Z.; Zhang, J.; Zhang, H.; Tian, H.; Zhu, W.H. Rational Design of Near-Infrared Aggregation-Induced-Emission-Active Probes: In Situ Mapping of Amyloid-β Plaques with Ultrasensitivity and High-Fidelity. J. Am. Chem. Soc. 2019, 141, 3171–3177. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Sun, A.; Xiong, Y.; Tan, H.; Shi, Y.; Yu, P.; Roy, G.; Zhang, L.; Yan, J. Dual-functional AIE fluorescent probes for imaging β-amyloid plaques and lipid droplets. Anal. Chim. Acta 2020, 1133, 109–118. [Google Scholar] [CrossRef]
- Xu, M.; Li, R.; Li, X.; Lv, G.; Li, S.; Sun, A.; Zhou, Y.; Yi, T. NIR fluorescent probes with good water-solubility for detection of amyloid beta aggregates in Alzheimer’s disease. J. Mater. Chem. B 2019, 7, 5535–5540. [Google Scholar] [CrossRef]
- Patel, K.; Shah, S.K.H.; Prabhakaran, P. Chapter Five—Aggregation-induced emission materials for protein fibrils imaging. Prog. Mol. Biol. Transl. Sci. 2021, 185, 113–136. [Google Scholar]
- Zems, Y.; Moiseev, A.G.; Perepichka, D.F. Convenient Synthesis of a Highly Soluble and Stable Phosphorescent Platinum Porphyrin Dye. Org. Lett. 2013, 15, 5330–5333. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Dong, J.; Zhang, Y.; Zhu, J.; Gao, J. Rational design of stable near-infrared cyanine-based probe with remarkable large Stokes Shift for monitoring Carbon monoxide in living cells and in vivo. Dyes Pigm. 2019, 171, 107753. [Google Scholar] [CrossRef]
- Levine, H. Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Groenning, M.; Norrman, M.; Flink, J.M.; van de Weert, M.; Bukrinsky, J.T.; Schluckebier, G.; Frokjaer, S. Binding mode of Thioflavin T in insulin amyloid fibrils. J. Struct. Biol. 2007, 159, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, C.; Rimola, A.; Rodríguez-Santiago, L.; Ugliengo, P.; Álvarez-Larena, Á.; Gutiérrez-de-Terán, H.; Sodupe, M.; González-Duarte, P. Crystal structure of thioflavin-T and its binding to amyloid fibrils: Insights at the molecular level. Chem. Commun. 2010, 46, 1156–1158. [Google Scholar] [CrossRef]
- Ning, Z.W.; Wu, S.Z.; Liu, G.J.; Ji, Y.M.; Jia, L.Y.; Niu, X.X.; Ma, R.F.; Zhang, Y.; Xing, G.W. Water-soluble AIE-Active Fluorescent Organic Nanoparticles: Design, Preparation and Application for Specific Detection of Cysteine over Homocysteine and Glutathione in Living Cells. Chem. Asian J. 2019, 14, 2220–2224. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.-M.; Hou, M.; Zhou, W.; Ning, Z.-W.; Zhang, Y.; Xing, G.-W. An AIE-Active NIR Fluorescent Probe with Good Water Solubility for the Detection of Aβ1–42 Aggregates in Alzheimer’s Disease. Molecules 2023, 28, 5110. https://doi.org/10.3390/molecules28135110
Ji Y-M, Hou M, Zhou W, Ning Z-W, Zhang Y, Xing G-W. An AIE-Active NIR Fluorescent Probe with Good Water Solubility for the Detection of Aβ1–42 Aggregates in Alzheimer’s Disease. Molecules. 2023; 28(13):5110. https://doi.org/10.3390/molecules28135110
Chicago/Turabian StyleJi, Yan-Ming, Min Hou, Wei Zhou, Zhang-Wei Ning, Yuan Zhang, and Guo-Wen Xing. 2023. "An AIE-Active NIR Fluorescent Probe with Good Water Solubility for the Detection of Aβ1–42 Aggregates in Alzheimer’s Disease" Molecules 28, no. 13: 5110. https://doi.org/10.3390/molecules28135110
APA StyleJi, Y.-M., Hou, M., Zhou, W., Ning, Z.-W., Zhang, Y., & Xing, G.-W. (2023). An AIE-Active NIR Fluorescent Probe with Good Water Solubility for the Detection of Aβ1–42 Aggregates in Alzheimer’s Disease. Molecules, 28(13), 5110. https://doi.org/10.3390/molecules28135110





