Fracturing Fluid Polymer Thickener with Superior Temperature, Salt and Shear Resistance Properties from the Synergistic Effect of Double-Tail Hydrophobic Monomer and Nonionic Polymerizable Surfactant
Abstract
1. Introduction
2. Results and Discussion
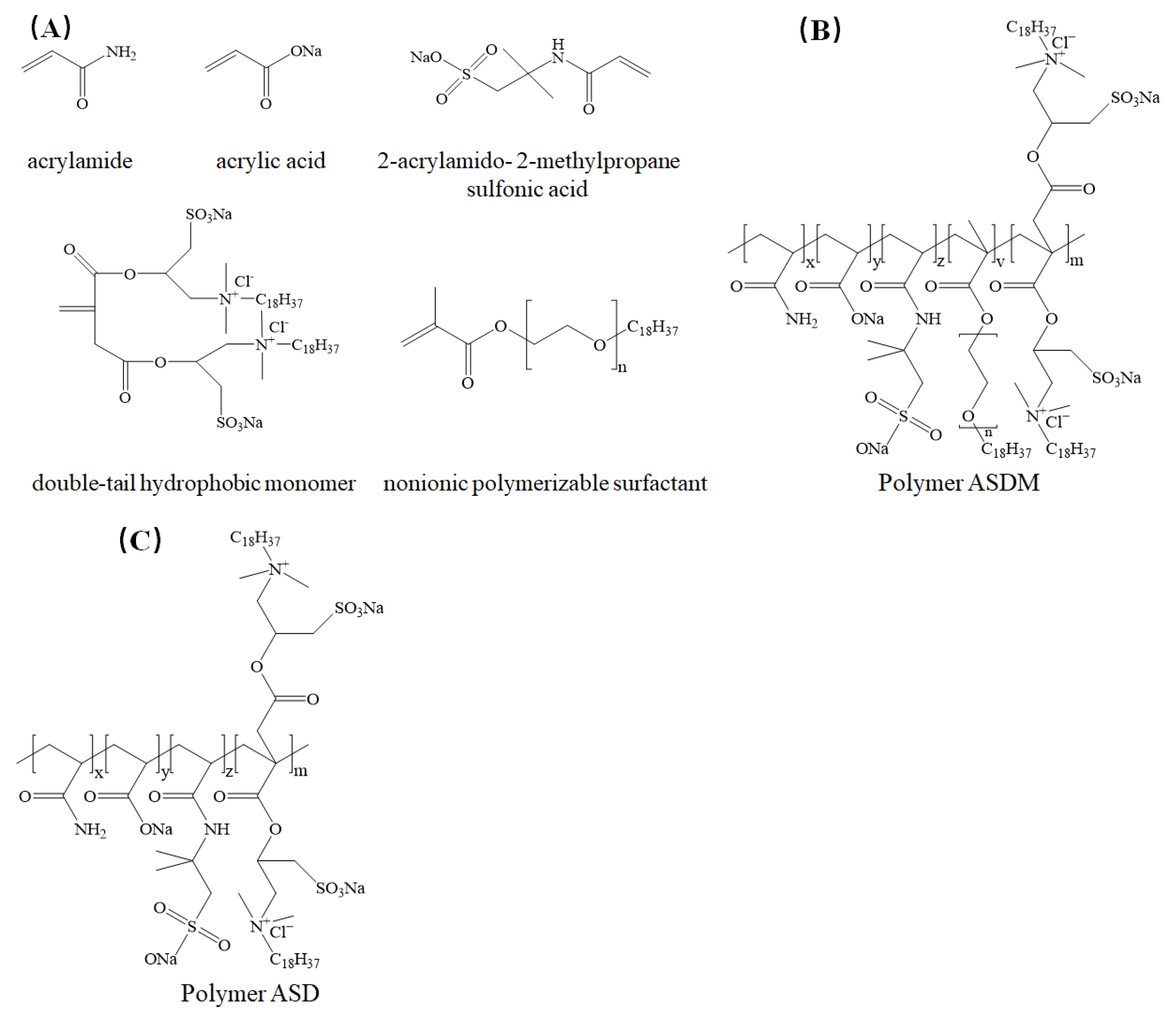
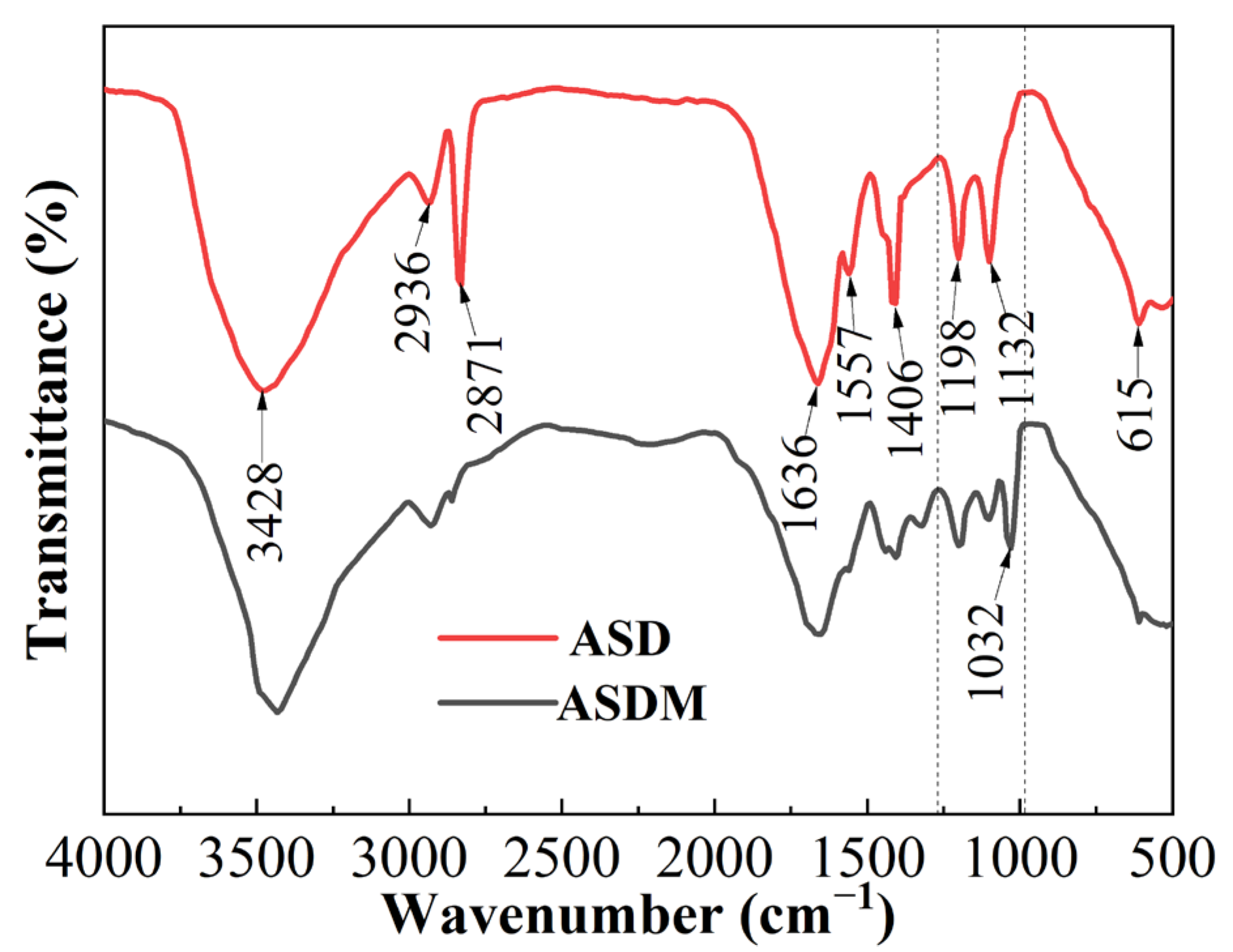
2.1. Characterization of Thickeners
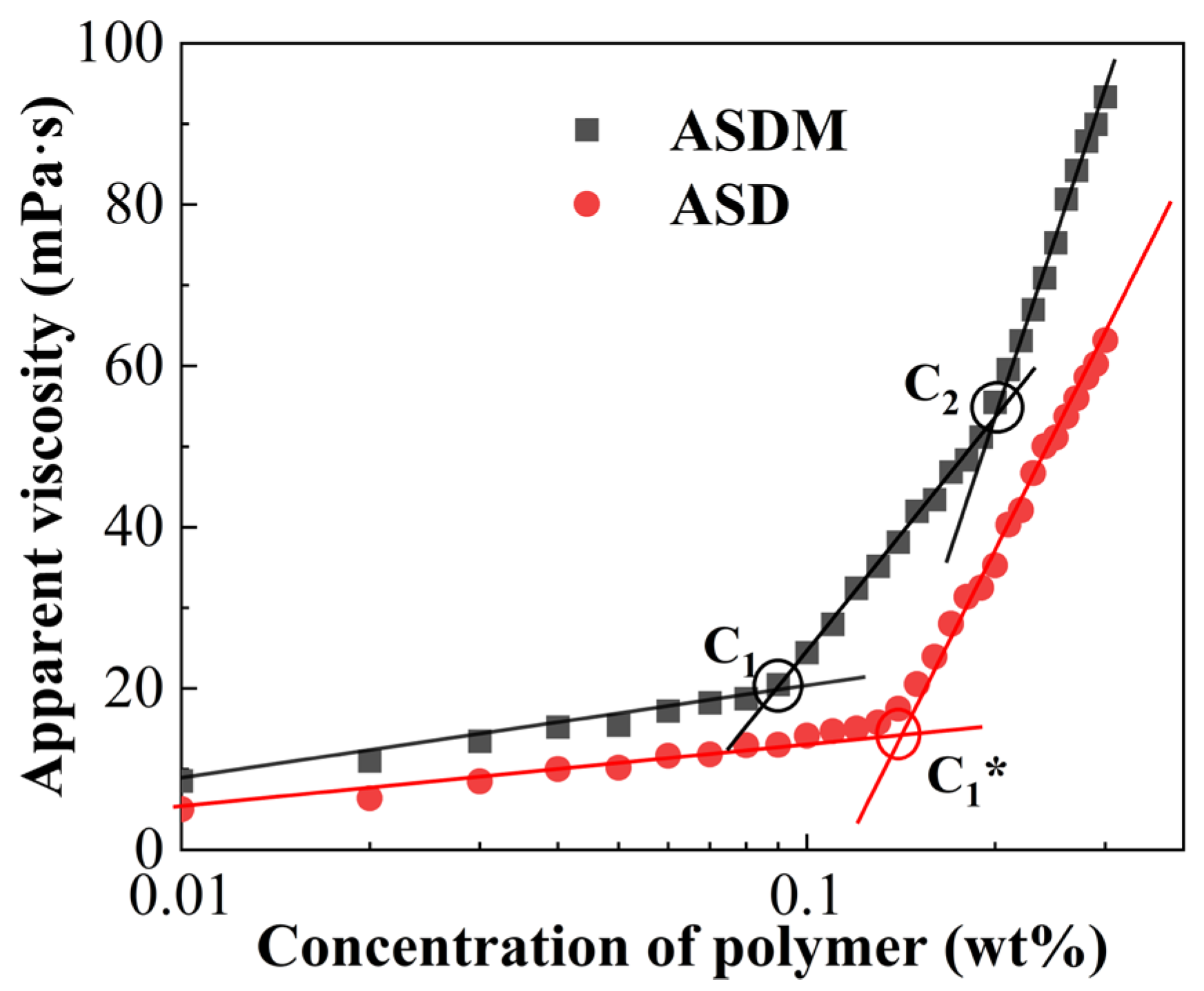
2.2. Critical Association Concentration
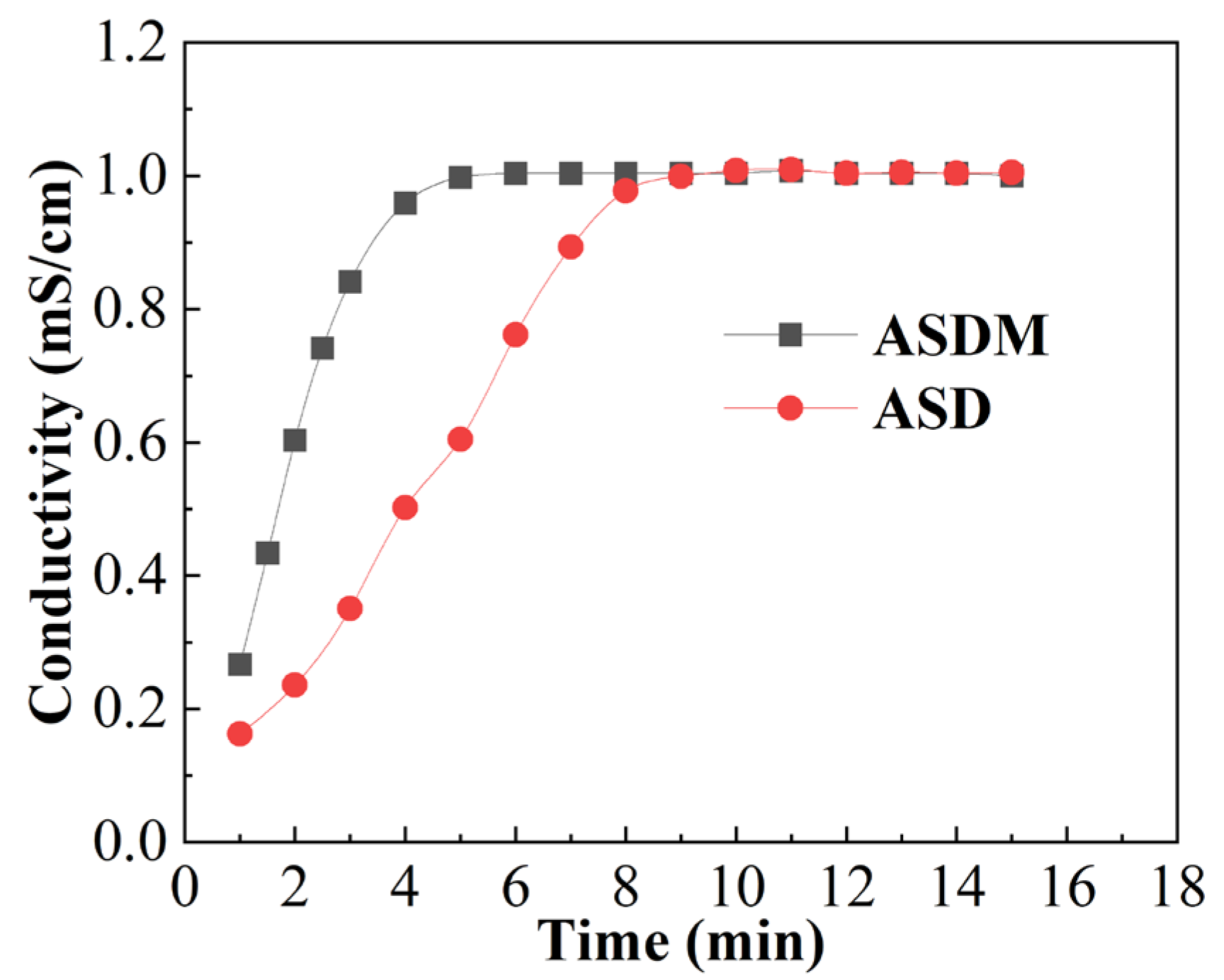
2.3. Dissolution Rate
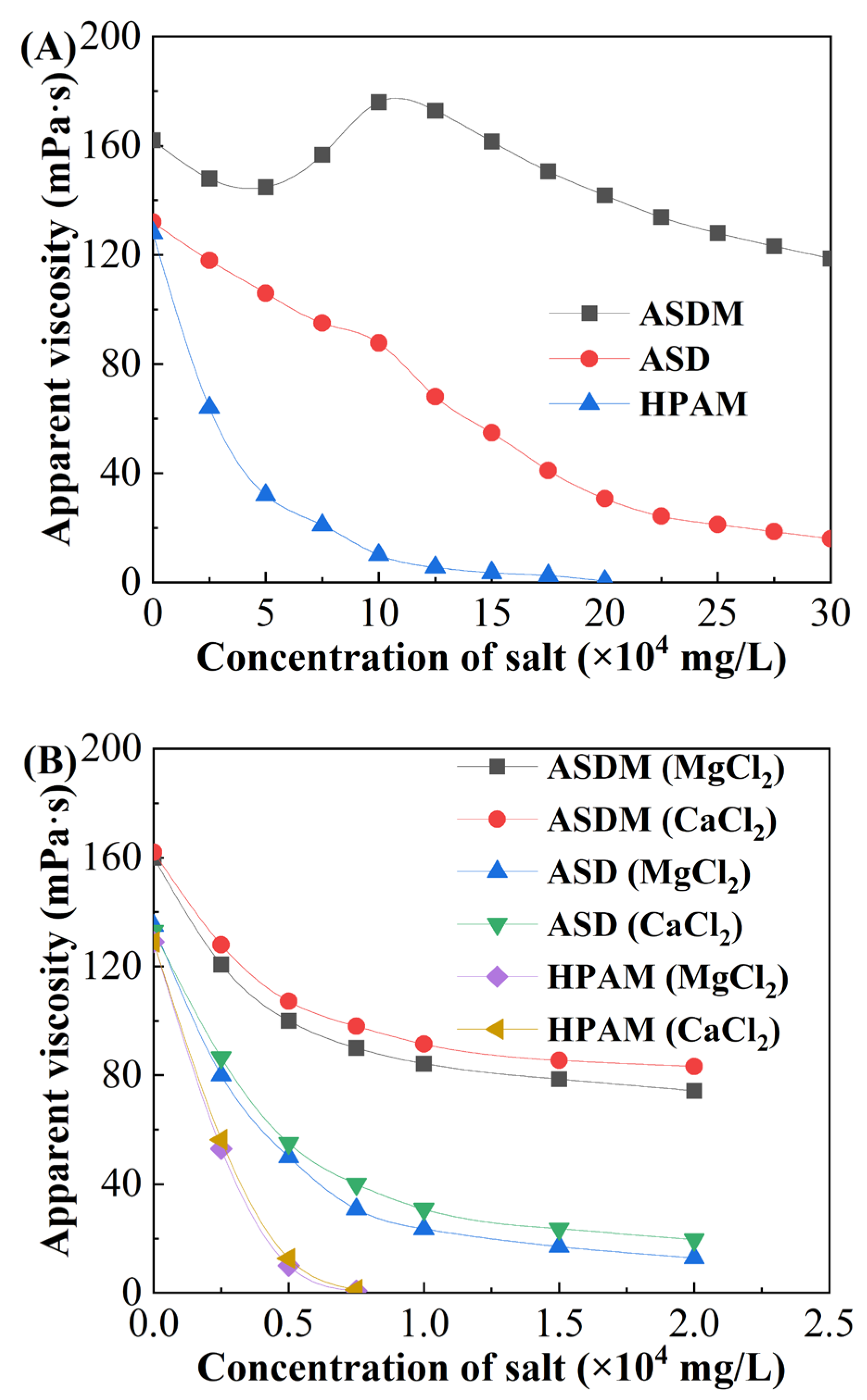
2.4. Salt Resistance
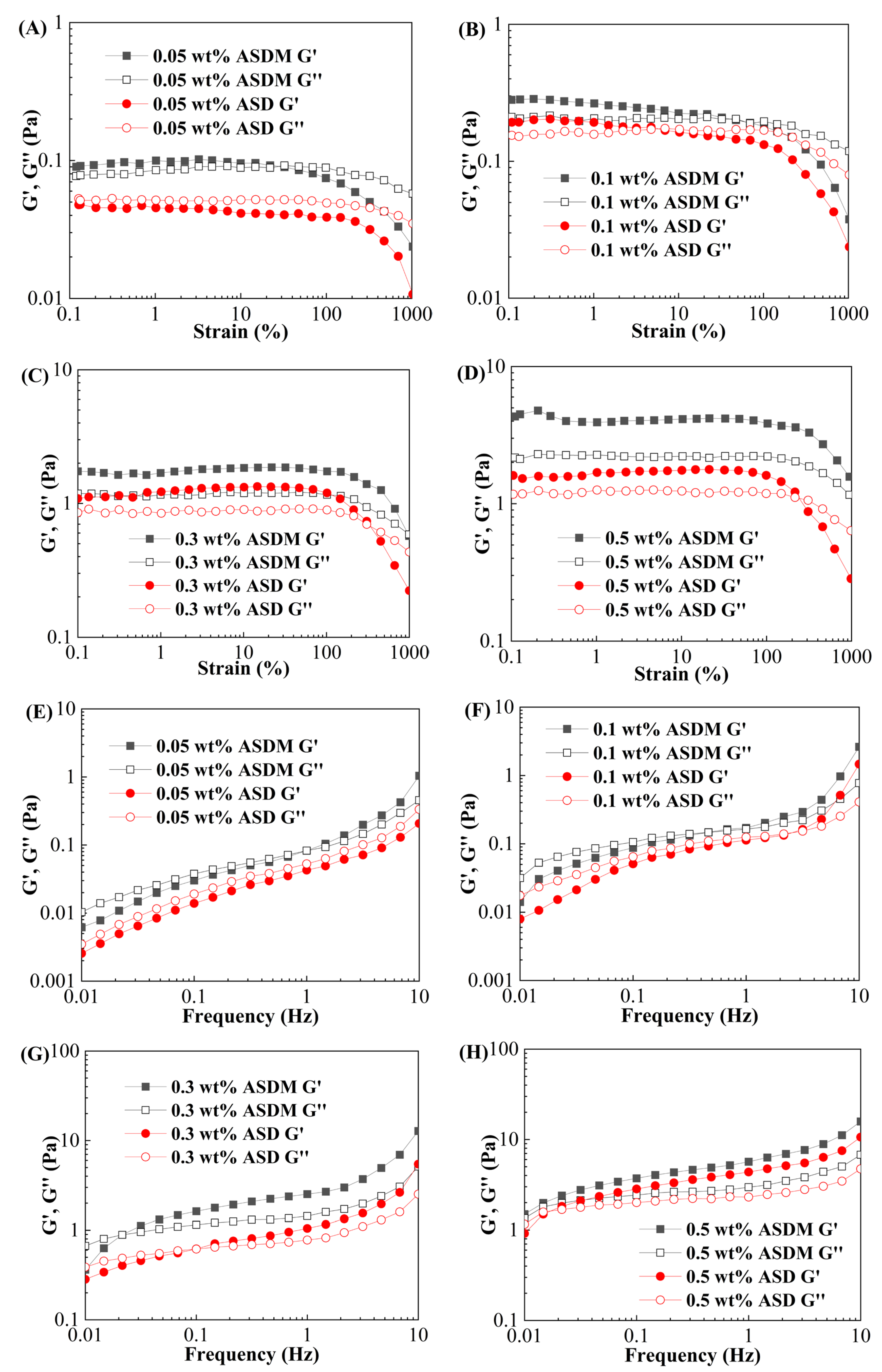
2.5. Viscoelasticity
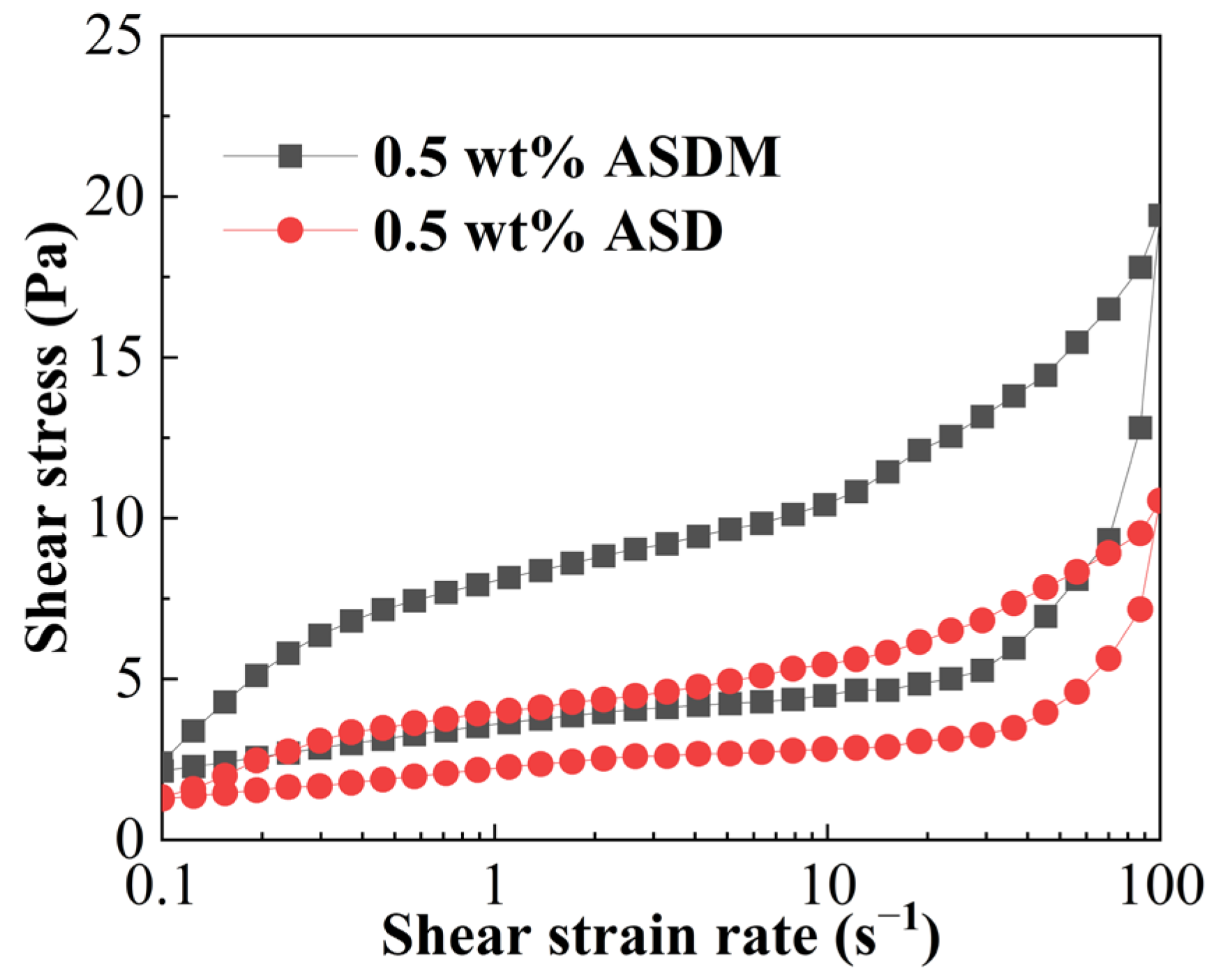
2.6. Thixotropy
2.7. Shear Recovery
2.8. Sand-Carrying Performance
2.9. Temperature and Shear Resistance Performance
2.10. Microscopic Morphology and Mechanism Analysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis
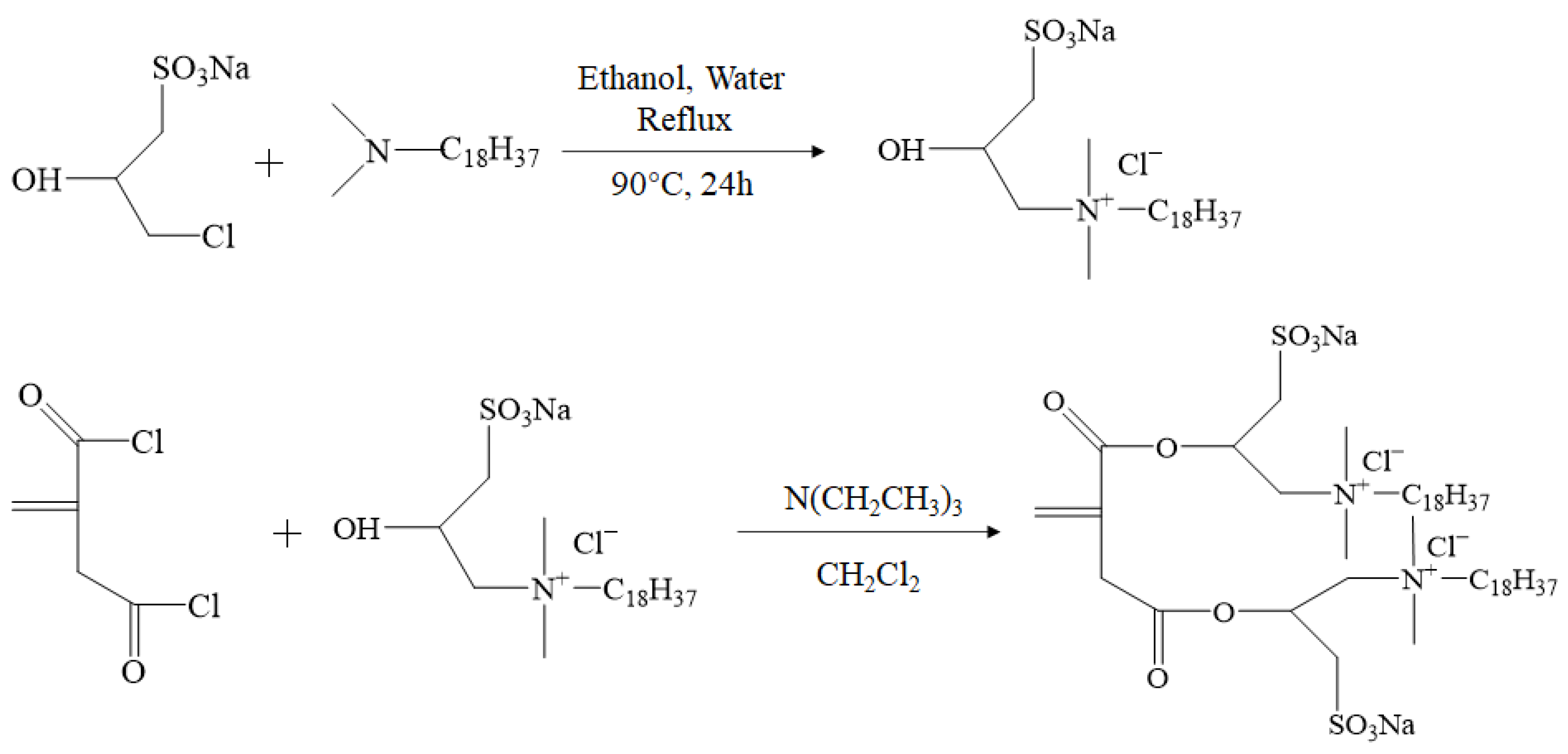
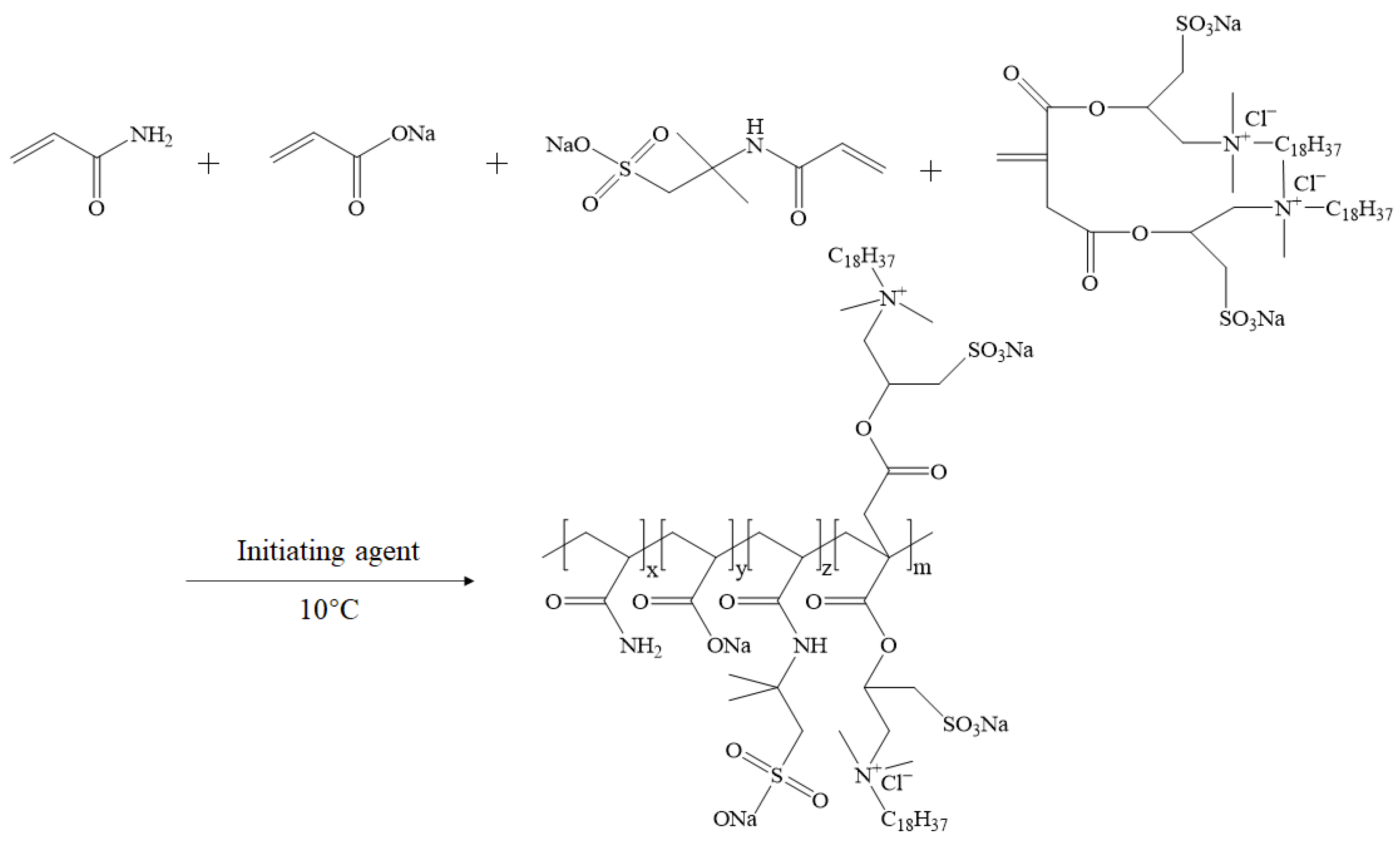
3.2.1. Preparation of Double-Tail Hydrophobic Monomer (DHM)
3.2.2. Synthesis of Thickeners ASD and ASDM
3.3. Structural Characterization
3.4. Critical Association Concentration
3.5. Dissolution Rate
3.6. Apparent Viscosity
3.7. Viscoelasticity
3.8. Thixotropy
3.9. Shear Recovery Performance
3.10. Sand-Carrying Performance
3.11. Temperature and Shear Resistance Performance
3.12. Scanning Electron Microscopy
4. Conclusions
- (1)
- ASDM could be quickly diluted in water within 6 min, only two-thirds of the time required for dissolving ASD. ASDM exhibited salt-thickening performance, and the apparent viscosity of 0.5 wt% ASDM reached 175.9 mPa·s in 100,000 mg/L brine, 100.6% higher than that of ASD.
- (2)
- ASDM possessed better properties of thickening ability, viscoelasticity, thixotropy, sand-carrying and temperature and shear resistance than ASD due to the synergistic effect of hydrophobic association of DHM and linear entanglement of NPS.
- (3)
- The shear recovery rate of 0.5 wt% ASDM and 0.5 wt% ASD reached 99.6% and 94.5%; both ASDM and ASD had good shear viscosity recovery properties because of the unique reversibility of their associative structures.
- (4)
- The reasons why ASDM owned excellent temperature, salt and shear resistance properties were that a strong compact spatial network structure of ASDM was formed between molecules, providing a dual structure of hydrophobic association and linear entanglement. The bridging effect of the long double-tail hydrophobic chains DHM on the polymer backbone was similar to the support of a bridge abutment on the bridge deck, resulting in increased thermal stability and shear resistance of the polymer chains. The oxyethylene groups of NPS complexed with metal salt ions, and the lone pair of electrons of the oxygen atom on the oxyethylene groups were filled into the empty orbitals by the salt ions, increasing salt resistance and enhancing the polarity of the groups.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, G.; Chen, S.; Meng, X.; Wang, L.; Sun, X.; Wang, M.; Sun, H.; Zhang, H.; Qin, J.; Zhu, D. Development of antifreeze fracturing fluid systems for tight petroleum reservoir stimulations. Energy Fuels 2021, 35, 12119–12131. [Google Scholar] [CrossRef]
- Wei, J.; Jia, W.; Zuo, L.; Chen, H.; Feng, Y. Turbulent drag reduction with an ultra-high-molecular-weight water-soluble polymer in slick-water hydrofracking. Molecules 2022, 27, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Z.; Liu, Y.; Zhang, H.; Qin, J.; Zhao, Q.; Wang, G.; Shi, C.; Su, Z. Carbon nanofiber-enhanced HPAM/PEI gels for conformance control in petroleum reservoirs with high temperatures. Energy Fuels 2022, 36, 12606–12616. [Google Scholar] [CrossRef]
- Kotb, A.; Almubarak, T.; Nasr-El-Din, H.A. Surfactant and friction reducer interaction in high salinity slickwater fracturing. In Proceeding of the SPE International Hydraulic Fracturing Technology Conference and Exhibition, Muscat, Oman, 11 January 2022. [Google Scholar]
- Da, Q.A.; Yao, C.J.; Zhang, X.; Wang, X.P.; Qu, X.H.; Lei, G.L. Investigation on microscopic invasion characteristics and retention mechanism of fracturing fluid in fractured porous media. Pet. Sci. 2022, 19, 1745–1756. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Jiang, H.; Zhou, F. A novel multifunction fracturing fluid compounding of nano-emulsion and viscous slickwater for unconventional gas and oil. Arab. J. Chem. 2022, 15, 103749. [Google Scholar] [CrossRef]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of smart viscoelastic surfactants and its applications in fracturing fluid: A review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Huang, Z.; Mao, J.; Cun, M.; Yang, X.; Lin, C.; Zhang, Y.; Zhang, H.; Mao, J.; Wang, Q.; Zhang, Q.; et al. Polyhydroxy cationic viscoelastic surfactant for clean fracturing fluids: Study on the salt tolerance and the effect of salt on the high temperature stability of wormlike micelles. J. Mol. Liquid. 2022, 366, 120354. [Google Scholar] [CrossRef]
- Liu, G.; Lai, X.; Cheng, X.; Lei, Y.; Wang, L.; Wen, X.; Fan, M.; Gao, J.; Liu, Y.; Ma, Q. Reactive carbamate surfactants: Synthesis, characterization, and improved rheological properties of hydrophobically modified polyacrylamide containing surfactant structures. J. Appl. Polym. Sci. 2023, 140, 53529. [Google Scholar] [CrossRef]
- Poppel, B. Fighting the Fear: Overcoming preconceived notions of low polymer cross-linked gels and high viscosity polyacrylamides in unconventional fracturing. In Proceedings of the SPE Hydraulic Fracturing Technology Conference and Exhibition, Woodlands, TX, USA, 28 February 2020. [Google Scholar]
- Wang, L.; Wang, D.; Shen, Y.; Lai, X.; Guo, X. Study on properties of hydrophobic associating polymer as drag reduction agent for fracturing fluid. J. Polym. Res. 2016, 23, 235. [Google Scholar] [CrossRef]
- Huang, Z.; Li, P.; Rao, Z.; He, F.; Quan, H.; Li, Z. Preparation and performance evaluation of hydrophobically associating polymer AHAPAM. Oilfield Chem. 2018, 35, 289–295. [Google Scholar]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Progress Poly. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Du, Y.; Zhu, Y.; Ji, Y.; Xu, H.; Zhang, H.; Yuan, S. Effect of salt-resistant monomers on viscosity of modified polymers based on the hydrolyzed poly-acrylamide (HPAM): A molecular dynamics study. J. Mol. Liquid. 2021, 325, 115161. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Lv, K.; Wen, Q.; Bai, Y.; Liu, J.; Huang, X.; Wang, J.; Jin, J.; Li, J. Preparation and performance evaluation of a variable viscosity friction reducer suspension with high salt tolerance and low damage. Energy Fuels 2023, 37, 993–1005. [Google Scholar] [CrossRef]
- Du, A.; Mao, J.; Zhang, H.; Xu, T.; Yang, X.; Lin, C.; Zhang, Y.; Liu, J. A novel hydrophobically associating polymer based on Twin-tailed amphiphilic Monomer: Experimental study and molecular dynamics simulation. J. Mol. Liquid. 2021, 341, 117293. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Zheng, W.; Li, X.; Wang, F.; Li, X.; Zhang, D.; Turtabayev, S.; Kang, W. Research on synthesis and salt thickening behavior of a binary copolymer amphiphilic polymer. J. Pet. Sci. Eng. 2021, 204, 108713. [Google Scholar] [CrossRef]
- Zhang, T.C.; Ge, J.J.; Wu, H.; Guo, H.B.; Jiao, B.L.; Qian, Z. Effect of AMPS (2-acrylamido-2-methylpropane sulfonic acid) content on the properties of polymer gels. Pet. Sci. 2022, 19, 697–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Xu, T.; Du, A.; Zhang, Z.; Zhang, W.; Ma, S. Preparation of a novel fracturing fluid system with excellent elasticity and low friction. Polymers 2019, 11, 1539. [Google Scholar] [CrossRef]
- Yang, D.; Yang, B.; Jiang, Z.; Zhang, H.; Zhong, Y.; Shao, Y. Study on mechanism of a simple method to regulate salt tolerance of hydrophobically associating water-soluble polymers. Colloids Surf. A 2022, 638, 128266. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Lv, K.; Wen, Q.; Bai, Y.; Wang, J.; Liu, J.; Huang, X.; Jin, J.; Li, J. Preparation and evaluation of acryloyl morpholine modified emulsion fracturing fluid thickener with high temperature resistance and salt resistance. J. Appl. Polym. Sci. 2023, 140, 53338. [Google Scholar] [CrossRef]
- Shi, S.; Sun, J.; Lv, K.; Liu, J.; Bai, Y.; Wang, J.; Huang, X.; Jin, J.; Li, J. Comparative Studies on Thickeners as Hydraulic Fracturing Fluids: Suspension versus Powder. Gels 2022, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Jiang, F.; He, Y.; Wei, B.; Tang, Y. Synthesis of a novel comb micro-block hydrophobically associating copolymer for Ca2+/Mg2+ resistance. RSC Adv. 2016, 6, 43634–43637. [Google Scholar] [CrossRef]
- Ma, X.; Mu, H.; Hu, Y.; Yang, S. Synthesis and properties of hydrophobically associating polymer fracturing fluid. Colloids Surf. A 2021, 626, 127013. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Guo, J.; Lai, J.; Wang, D.; He, L.; Qin, Y. A study of relation between suspension behavior and microstructure and viscoelastic property of guar gum fracturing fluid. J. Pet. Sci. Eng. 2014, 124, 432–435. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, S.; Wang, G.; Wu, B.; Zhang, Y. Coalbed methane reservoir stimulation using guar-based fracturing fluid: A review. J. Nat. Gas Sci. Eng. 2019, 66, 107–125. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Liao, B.; Ding, X.; Zheng, Z.; Cheng, X.; Pang, H.; Peng, Y. Polyelectrolyte self-assembly approach to smart nanocontainers. Polym. Bull. 2006, 56, 305–311. [Google Scholar] [CrossRef]
- Berezovska, I.; Kapilov, K.; Dhavalikar, P.; Cosgriff-Hernandez, E.; Silverstein, M.S. Reactive surfactants for achieving open-cell polyHIPE foams from pickering emulsions. Macromol. Mater. Eng. 2021, 306, 2000825. [Google Scholar] [CrossRef]
- Akamatsu, M.; Ogura, K.; Tsuchiya, K.; Sakai, K.; Abe, M.; Sakai, H. Phase behavior and polymerization of the ternary polymerizable cationic gemini surfactant/fatty alcohol/water system. Langmuir 2020, 36, 986–990. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Rokicki, G. Reactive surfactants-chemistry and applications Part I. Polymerizable surfactants. Polimery 2016, 61, 747–757. [Google Scholar] [CrossRef]
- Han, W.J.; Lee, J.H.; Choi, H.J. Poly (diphenylamine)/polyaniline core/shell composite nanospheres synthesized using a reactive surfactant and their electrorheology. Polymer 2020, 188, 122161. [Google Scholar] [CrossRef]
- Meli, M.V.; Badia, A.; Grütter, P.; Lennox, R.B. Self-assembled masks for the transfer of nanometer-scale patterns into surfaces: Characterization by AFM and LFM. Nano Lett. 2002, 2, 131–135. [Google Scholar] [CrossRef]
- Băran, A.; Aricov, L.; Stîngă, G.; Iovescu, A.; Leontieş, A.R.; Jerca, V.V. The effect of C12E6 nonionic surfactant on the solubilization of Eosin Y in unmodified-and hydrophobically modified poly (Acrylic acid) solutions. J. Mol. Liquid. 2022, 346, 117103. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.H.; Urban, V.S.; Littrell, K.C.; Thiyagarajan, P.; Yu, L. Syntheses of amphiphilic diblock copolymers containing a conjugated block and their self-assembling properties. J. Am. Chem. Soc. 2000, 122, 6855–6861. [Google Scholar] [CrossRef]
- Mao, J.; Cao, H.; Zhang, H.; Du, A.; Xue, J.; Lin, C.; Yang, X.; Wang, Q.; Mao, J.; Chen, A. Design of salt-responsive low-viscosity and high-elasticity hydrophobic association polymers and study of association structure changes under high-salt conditions. Colloids Surf. A 2022, 650, 129512. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Yang, X.; Lin, C.; Zhang, H.; Xu, T.; Wang, Q. Synthesis of a hydrophobic association polymer with an inner salt structure for fracture fluid with ultra-high-salinity water. Colloids Surf. A 2022, 636, 128062. [Google Scholar] [CrossRef]
- Fan, M.; Wang, L.; Li, J.; He, P.; Lai, X.; Gao, J.; Liu, G.; Wen, X. Preparation of supramolecular viscoelastic polymers with shear, temperature, and salt resistance/sensitivity by amphiphilic functional monomer modification. Polym. Test. 2022, 116, 107799. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Liao, Y.; Xu, T.; Zhang, H.; Du, A.; Yang, X.; Lin, C.; Mao, J. Evaluation of temperature resistance of non-chemical crosslinked double-tailed hydrophobically associating polymer fracturing fluid. React. Funct. Polym. 2022, 175, 105268. [Google Scholar] [CrossRef]
- Adams, D.J.; Kitchen, C.; Adams, S.; Furzeland, S.; Atkins, D.; Schuetz, P.; Fernyhoug, C.; Tzokova, N.; Ryan, A.; Butler, M.F. On the mechanism of formation of vesicles from poly (ethylene oxide)-block-poly (caprolactone) copolymers. Soft Matter. 2009, 5, 3086–3096. [Google Scholar] [CrossRef]
- Fan, M.; Lai, X.; Tang, M.; Li, J.; Wang, L.; Gao, J. Preparation and properties of a clean, low-damage waterproof locking damage multifunctional integrated water-based fracturing fluid. J. Appl. Polym. Sci. 2022, 139, 53207. [Google Scholar] [CrossRef]
- Härtel, A.; Janssen, M.; Samin, S.; van Roij, R. Fundamental measure theory for the electric double layer: Implications for blue-energy harvesting and water desalination. J. Phys. Condens. Matter. 2015, 27, 194129. [Google Scholar] [CrossRef]
- Barnes, H.A. Thixotropy—A review. J. Non-Newton Fluid Mech. 1997, 70, 1–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Xu, T.; Lin, C.; Mao, J.; Tan, H.; Zhang, Z.; Yang, B.; et al. Preparation of a hydrophobic-associating polymer with ultra-high salt resistance using synergistic effect. Polymers 2019, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, S.; Chen, Y.; Chen, H.; Wang, M.; Tan, Y. Salt endurable and shear resistant polymer systems based on dynamically reversible acyl hydrazone bond. J. Mol. Liquid. 2022, 346, 117083. [Google Scholar] [CrossRef]





| Fracturing Fluid | Temperature (°C) | Setting Velocity (mm/s) |
|---|---|---|
| 0.3 wt% ASDM | 25 | 0.028 |
| 0.3 wt% ASD | 25 | 0.053 |
| 0.5 wt% ASDM | 90 | 0.082 |
| 0.5 wt% ASD | 90 | 0.147 |
| 0.8 wt% ASD | 120 | 0.153 |
| 1.0 wt% ASD | 140 | 0.228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Sun, J.; Lv, K.; Liu, J.; Bai, Y.; Wang, J.; Huang, X.; Jin, J.; Li, J. Fracturing Fluid Polymer Thickener with Superior Temperature, Salt and Shear Resistance Properties from the Synergistic Effect of Double-Tail Hydrophobic Monomer and Nonionic Polymerizable Surfactant. Molecules 2023, 28, 5104. https://doi.org/10.3390/molecules28135104
Shi S, Sun J, Lv K, Liu J, Bai Y, Wang J, Huang X, Jin J, Li J. Fracturing Fluid Polymer Thickener with Superior Temperature, Salt and Shear Resistance Properties from the Synergistic Effect of Double-Tail Hydrophobic Monomer and Nonionic Polymerizable Surfactant. Molecules. 2023; 28(13):5104. https://doi.org/10.3390/molecules28135104
Chicago/Turabian StyleShi, Shenglong, Jinsheng Sun, Kaihe Lv, Jingping Liu, Yingrui Bai, Jintang Wang, Xianbin Huang, Jiafeng Jin, and Jian Li. 2023. "Fracturing Fluid Polymer Thickener with Superior Temperature, Salt and Shear Resistance Properties from the Synergistic Effect of Double-Tail Hydrophobic Monomer and Nonionic Polymerizable Surfactant" Molecules 28, no. 13: 5104. https://doi.org/10.3390/molecules28135104
APA StyleShi, S., Sun, J., Lv, K., Liu, J., Bai, Y., Wang, J., Huang, X., Jin, J., & Li, J. (2023). Fracturing Fluid Polymer Thickener with Superior Temperature, Salt and Shear Resistance Properties from the Synergistic Effect of Double-Tail Hydrophobic Monomer and Nonionic Polymerizable Surfactant. Molecules, 28(13), 5104. https://doi.org/10.3390/molecules28135104










