Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior
Abstract
:1. Introduction
2. Results and Discussion
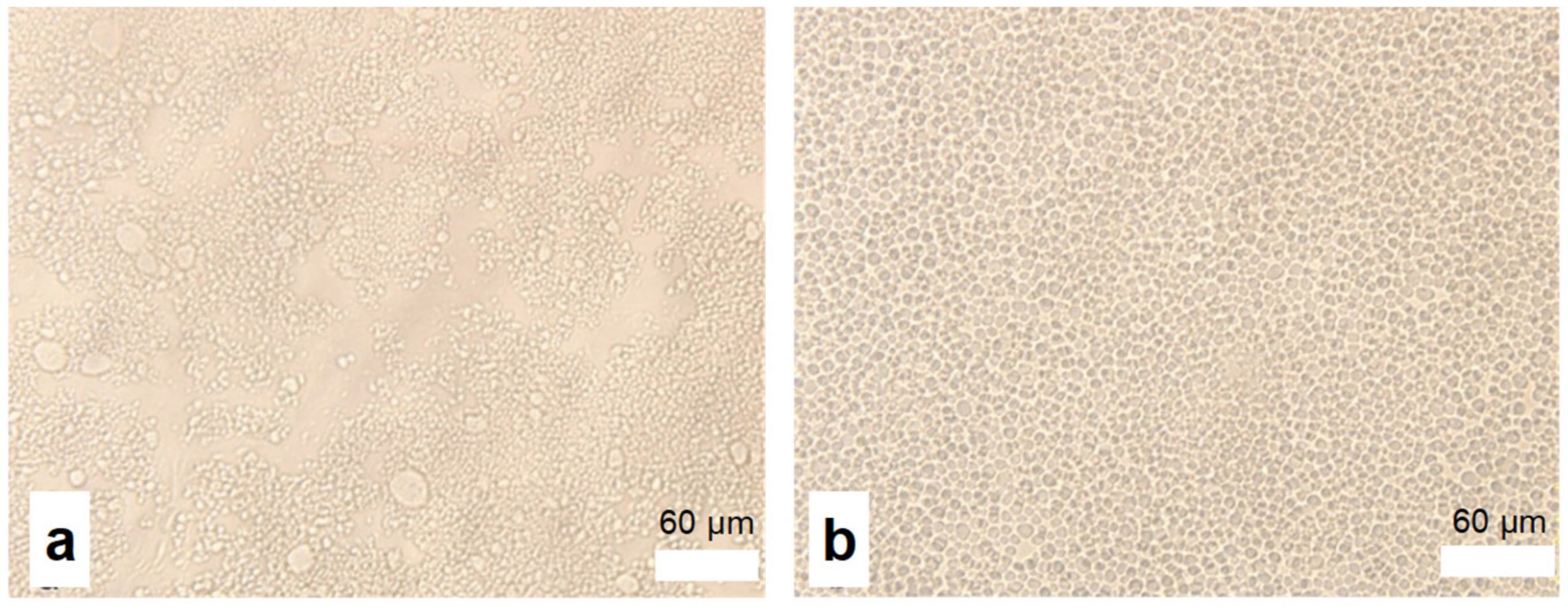
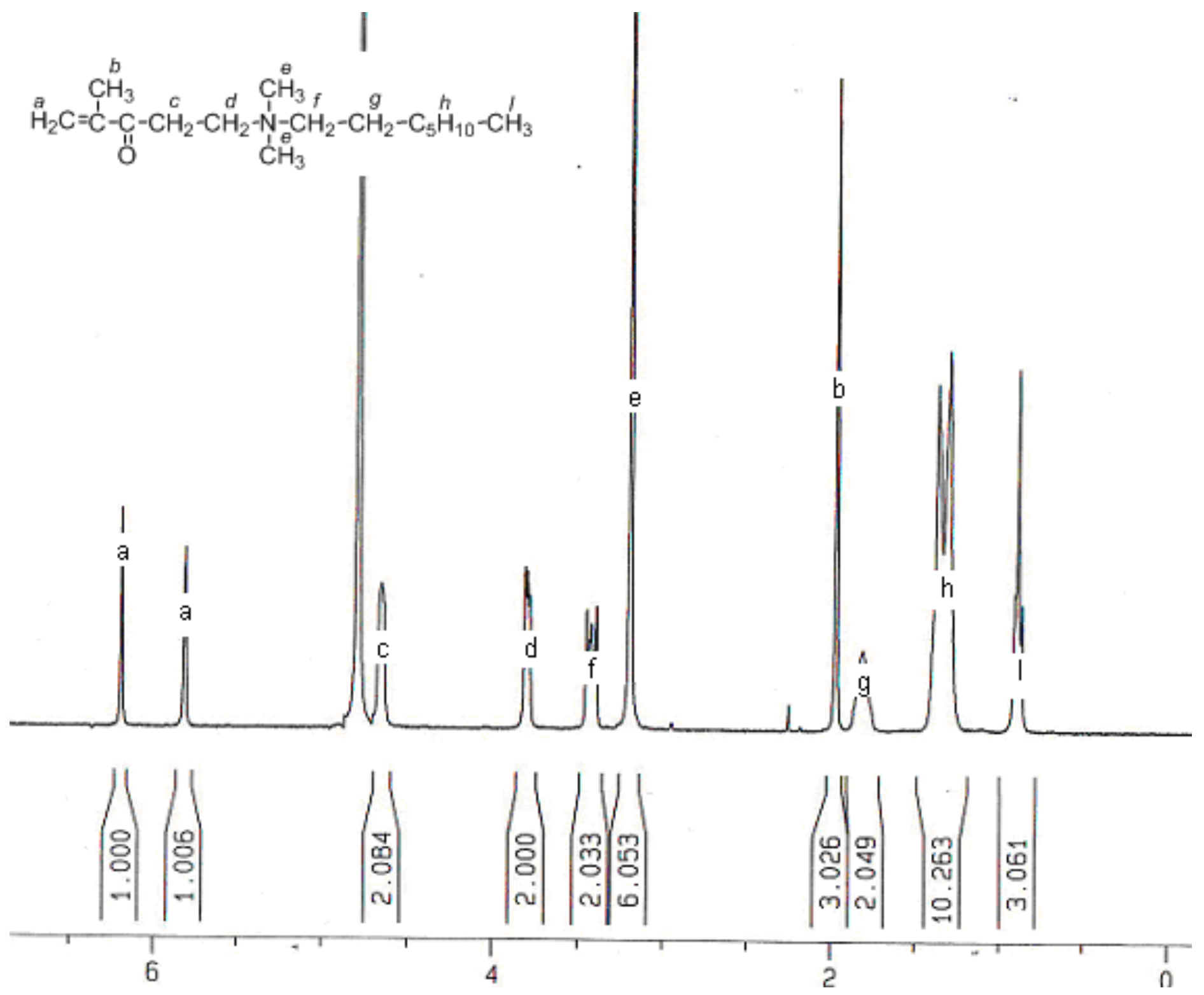
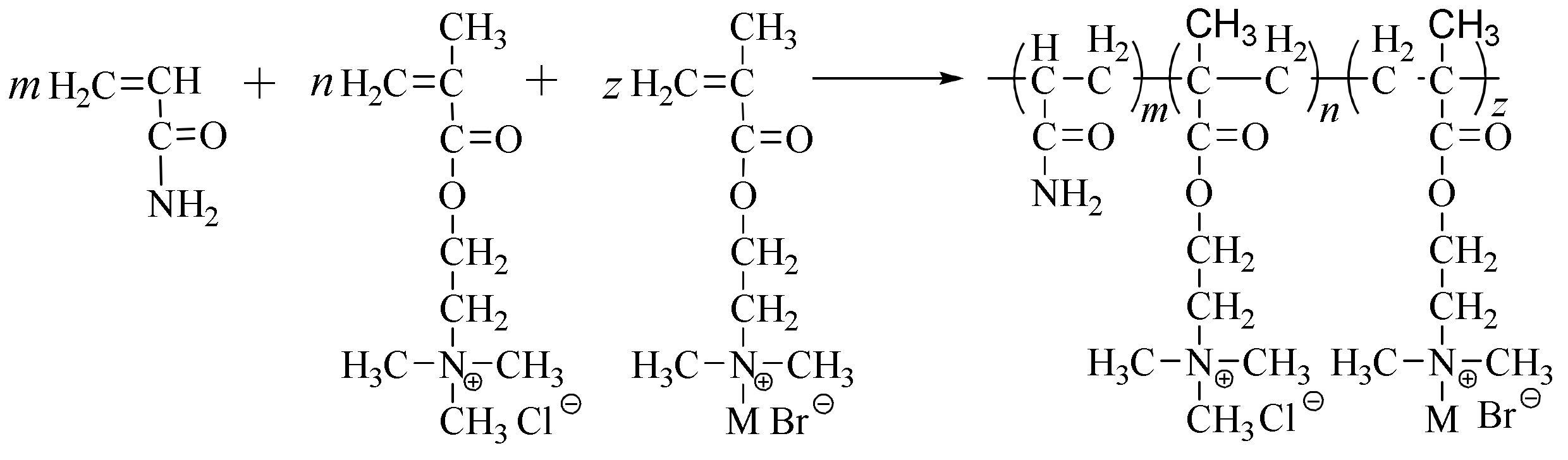
2.1. Synthesis and Characterization
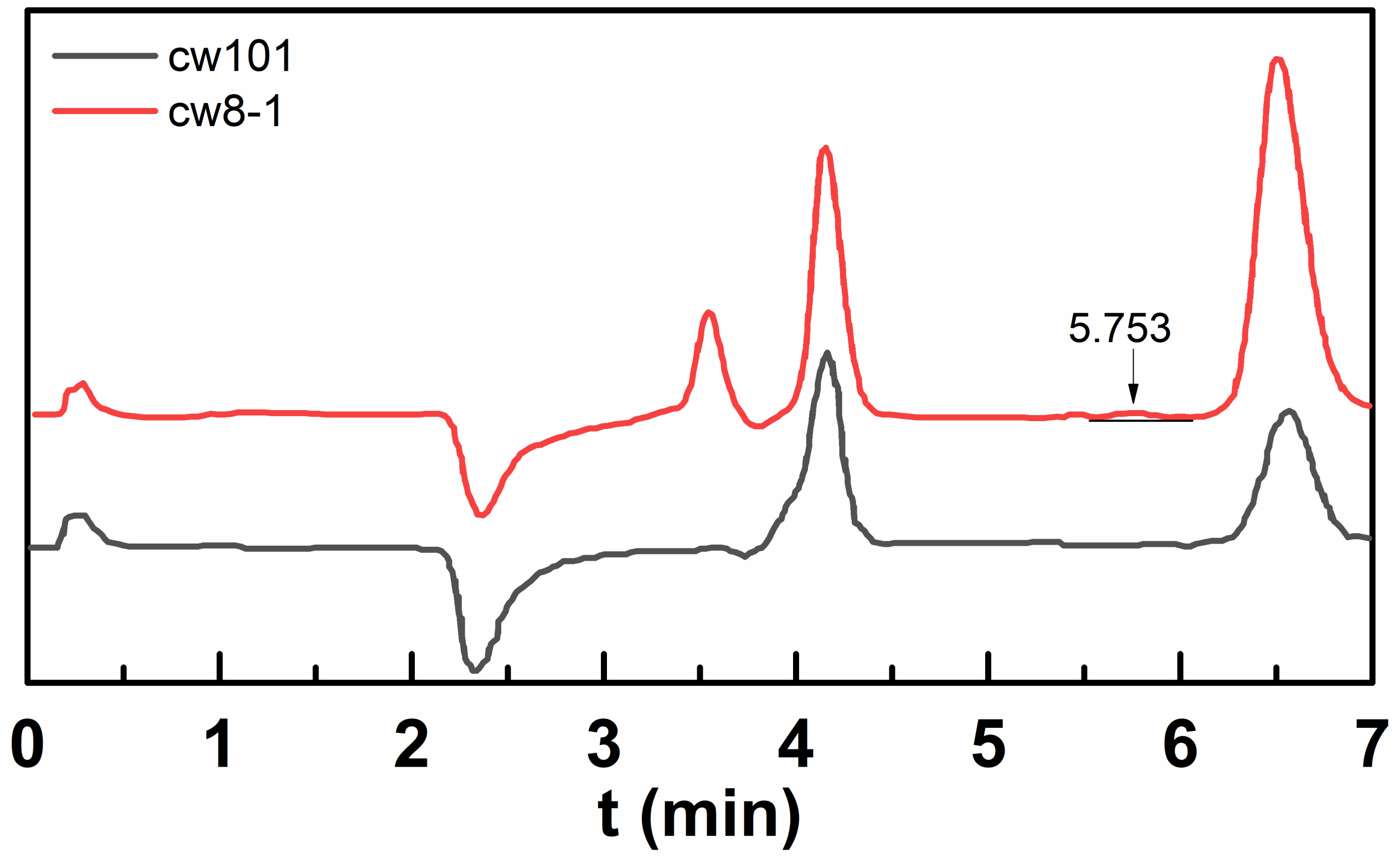
2.2. Compositional Analysis
2.2.1. IC Analysis
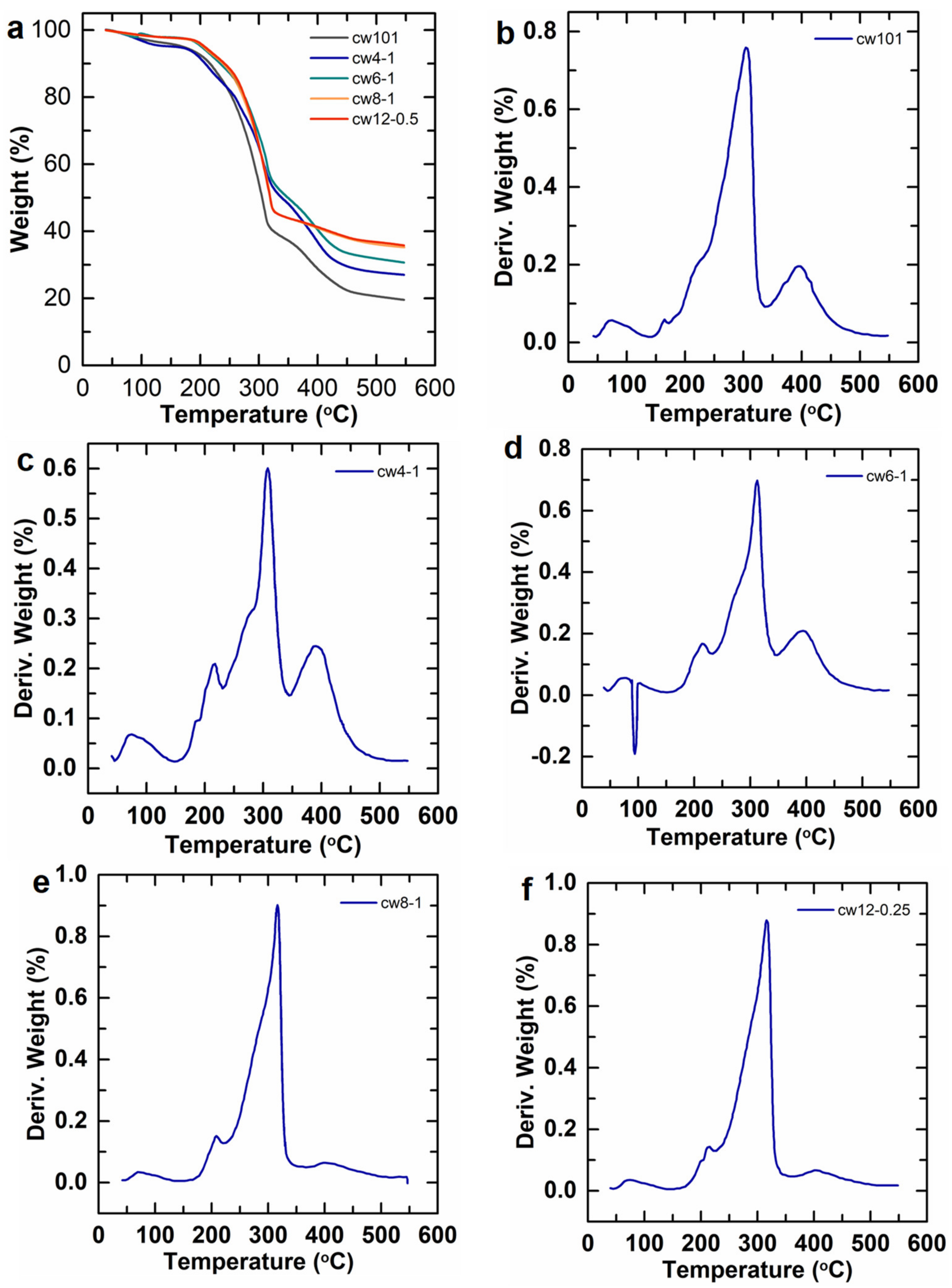
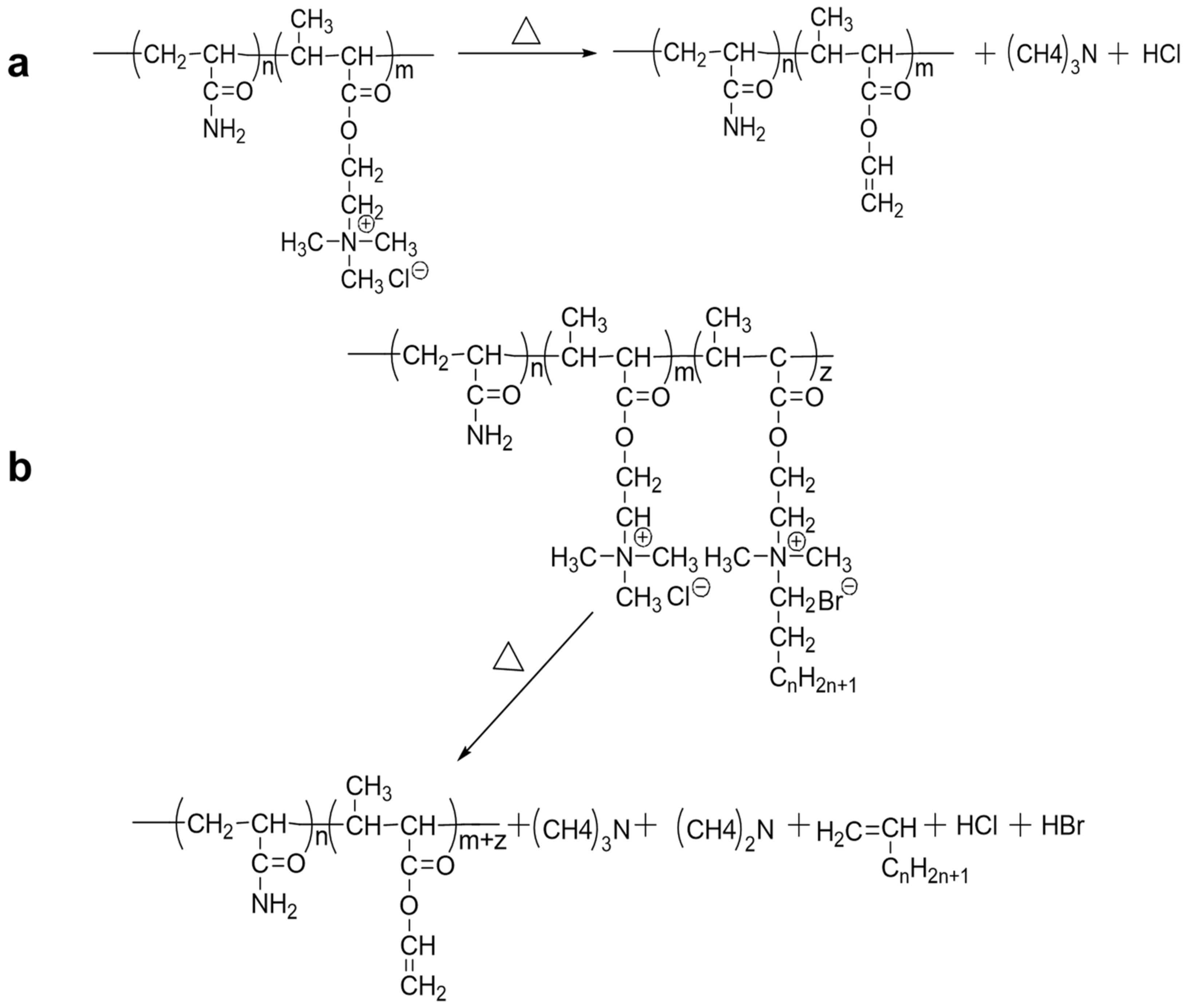
2.2.2. TG and DTG Analysis
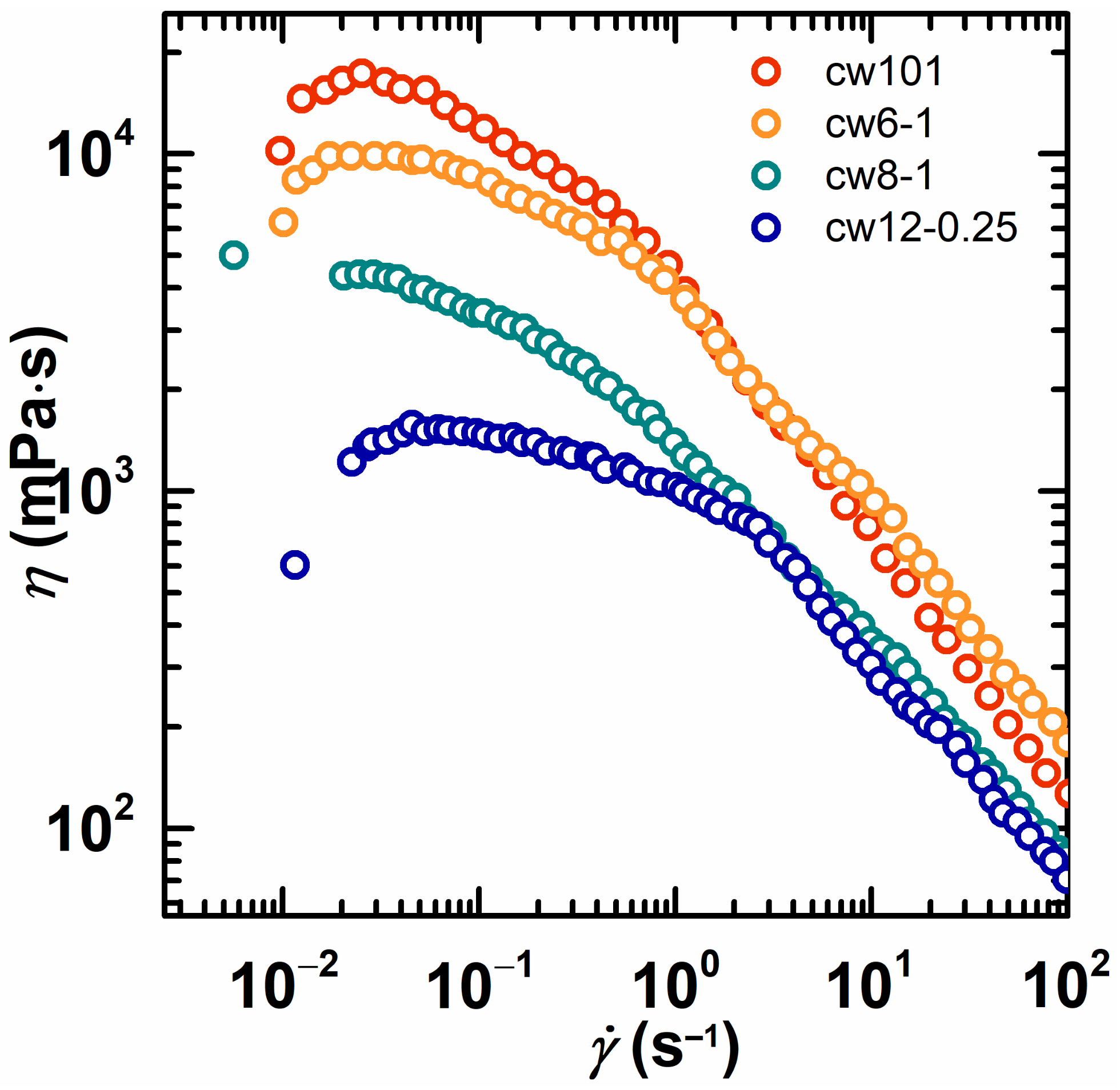
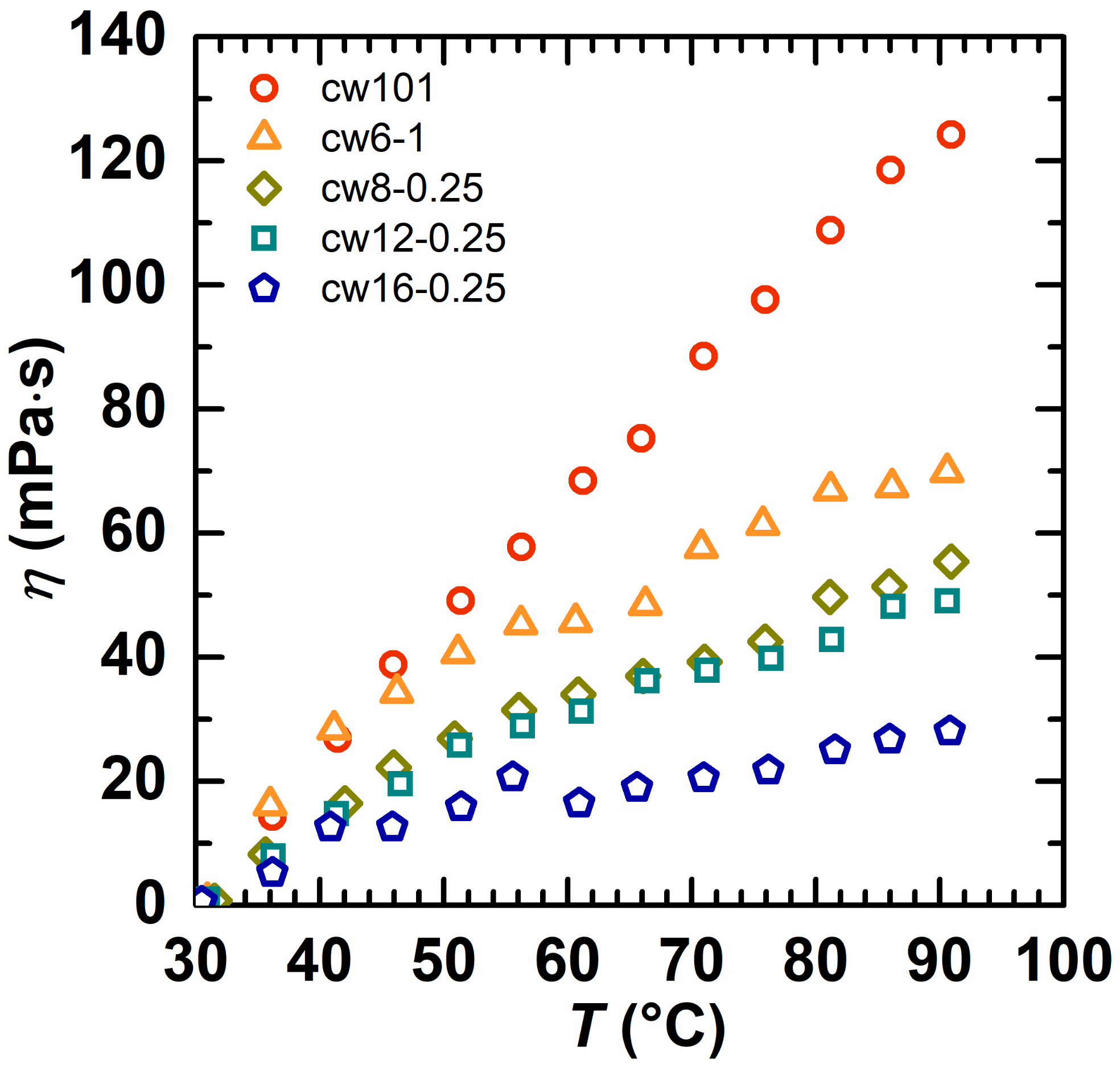
2.3. Rheological Behavior
3. Experimental Section
3.1. Materials
3.2. Synthesis Hydrophobic Monomers
3.3. Dispersion Polymerization of HAPAM
3.4. Ion Change Chromatography (IC), Thermogravimetric Analysis (TG) and Differential Thermal Gravity (DTG)
3.5. Measurement of Intrinsic Viscosity
3.6. Observation of the Particles in Polymer Dispersion
3.7. Determination of HAPAM Solubility in Water
3.8. Rheological Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Glass, J.E. (Ed.) Water-Soluble Polymers: Beauty with Performance; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1986. [Google Scholar]
- Mccormick, C.L.; Bock, J.; Schulz, D.N. Water-Soluble Polymers. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bikales, N.M., Overberg, C.G., Menge, G., Eds.; Wiley-Interscience: New York, NY, USA, 1989. [Google Scholar]
- Zhu, R.; Feng, Y.; Luo, P. Net contribution of hydrophobic association to the thickening power of hydrophobically modified polyelectrolytes prepared by micellar polymerization. Macromolecules 2020, 53, 1326–1337. [Google Scholar] [CrossRef]
- SchuIz, D.N.; Glass, J.E. (Eds.) Polymers as Rheology Modifiers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 462. [Google Scholar]
- Xie, B.; Ma, J.; Wang, Y.; Tchameni, A.P.; Luo, M.; Wen, J. Enhanced hydrophobically modified polyacrylamide gel for lost circulation treatment in high temperature drilling. J. Mol. Liq. 2021, 325, 115155. [Google Scholar] [CrossRef]
- Tian, H.; Quan, H.; Huang, Z. Investigation on rheological properties and thickening mechanism of a novel thickener based on hydrophobically associating water-soluble polymer during the acid rock reaction. J. Pet. Sci. Eng. 2020, 188, 106895. [Google Scholar] [CrossRef]
- Feng, Y.; Billon, L.; Grassl, B.; Khoukh, A.; François, J. Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. 1. Synthesis and characterization. Polymer 2002, 43, 2055–2064. [Google Scholar] [CrossRef]
- Cram, S.L.; Brown, H.R.; Spinks, G.M.; Hourdet, D.; Creton, C. Hydrophobically modified dimethylacrylamide synthesis and rheological behavior. Macromolecules 2005, 38, 2981–2989. [Google Scholar] [CrossRef]
- Volpert, E.; Selb, J.; Candau, F. Influence of the hydrophobe structure on composition, microstructure, and rheology in associating polyacrylamides prepared by micellar copolymerization. Macromolecules 1996, 29, 1452–1463. [Google Scholar] [CrossRef]
- Wu, S.; Shanks, R.A. Synthesis and characterisation of hydrophobic modified polyacrylamide. Polym. Int. 2004, 53, 1821–1830. [Google Scholar] [CrossRef]
- Candau, F.; Selb, J. Hydrophobically-modified polyacrylamides prepared by micellar polymerization. Adv. Colloid Interface Sci. 1999, 79, 149–172. [Google Scholar] [CrossRef]
- Lara-Ceniceros, A.C.; Rivera-Vallejo, C.; Jimènez-Regalado, E.J. Synthesis, characterization and rheological properties of three different associative polymers obtained by micellar polymerization. Polym. Bull. 2007, 58, 425–433. [Google Scholar] [CrossRef]
- Volpert, E.; Selb, J.; Candau, F. Associating behaviour of polyacrylamides hydrophobically modified with dihexylacrylamide. Polymer 1998, 39, 1025–1033. [Google Scholar] [CrossRef]
- Pabon, M.; Corpart, J.M.; Selb, J.; Candau, J. Synthesis in inverse emulsion and associating behavior of hydrophobically modified polyacrylamides. J. Appl. Polym. Sci. 2004, 91, 916–924. [Google Scholar] [CrossRef]
- Hill, A.; Candau, F.; Selb, J. Aqueous solution properties of hybrophobically associating copolymers. In Trends in Colloid and Interface Science V; Progress in Colloid & Polymer Science; Steinkopff: Heidelberg, Germany, 1991; Volume 84, pp. 61–65. [Google Scholar]
- Deguchi, S.; Lindman, B. Novel approach for the synthesis of hydrophobe modified polyacrylamide. Direct N-alkylation of polyacrylamide in dimethyl sulfoxide. Polymer 1999, 40, 7163–7165. [Google Scholar] [CrossRef]
- Magny, B.; Lafuma, F.; Iliopoulos, I. Determination of microstructure of hydrophobically modified water-soluble polymers by C-13 NMR. Polymer 1992, 33, 3151–3154. [Google Scholar] [CrossRef]
- Mccomick, C.L.; Johnson, C.B. Polymers in Aqueous Solusion: Performance through Association; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1989; Volume 223, p. 437. [Google Scholar]
- Pabon, M.; Corpart, J.M.; Selb, J.; Candau, J. Synthesis in inverse emulsion and properties of water-soluble associating polymers. J. Appl. Polym. Sci. 2004, 84, 1418–1430. [Google Scholar] [CrossRef]
- Lu, H.S.; Feng, Y.J. Synthesis, characterization and kinetics of hydrophobically associating polyacrylamide. e-Polymers 2007, 7, 1–13. [Google Scholar]
- Hosoda, Y.; Ueshima, T.; Ishihara, S.; Imamura, K. Contemporary Topics in Polymer Science; Bailey, W.J., Tsuruta, T., Eds.; Plenum Press: New York, NY, USA, 1980; Volume 4, p. 575. [Google Scholar]
- Jaeger, W.; Hahn, M.; Lieske, A.; Zimmermann, A.; Brand, F. Polymerization of water soluble cationic vinyl monomers. Macromol. Symp. 1996, 111, 95–106. [Google Scholar] [CrossRef]
- Zimmermann, A.; Jaeger, W.; Reichert, K.H. Polyelectrolytes—Dispersion polymerization of a water soluble cationic vinyl monomer. Polym. News 1997, 22, 390. [Google Scholar]
- Hildebrandt, V.; Reichert, K.H. On the kinetics of the stabilized precipitation polymerization of a cationic monomer in aqueous sodium chloride solutions. Angew. Makromol. Chem. 1997, 245, 165–181. [Google Scholar] [CrossRef]
- Song, B.K.; Cho, M.S.; Yoon, K.J.; Lee, D.C. Dispersion polymerization of acrylamide with quaternary ammonium cationic comonomer in aqueous solution. J. Appl. Polym. Sci. 2003, 87, 1101–1108. [Google Scholar] [CrossRef]
- Chen, D.N.; Liu, X.G.; Yue, Y.M.; Zhang, W.D.; Wang, P.X. Dispersion copolymerization of acrylamide with quaternary ammonium cationic monomer in aqueous salts solution. Eur. Polym. J. 2006, 42, 1284–1297. [Google Scholar] [CrossRef]
- Wu, Y.M.; Chen, Q.F.; Xu, J.; Bi, J.M. Aqueous. dispersion polymerization of acrylamide with quaternary ammonium cationic comonomer. J. Appl. Polym. Sci. 2008, 108, 134–139. [Google Scholar] [CrossRef]
- Crowe, C.D.; Hendrickson-Stives, A.K.; Kuhn, S.L.; Jackson, J.B.; Keating, C.D. Designing and 3D Printing an Improved Method of Measuring Contact Angle in the Middle School Classroom. J. Chem. Educ. 2021, 98, 1997–2004. [Google Scholar] [CrossRef]
- Kabir, H.; Garg, N. Machine learning enabled orthogonal camera goniometry for accurate and robust contact angle measurements. Sci. Rep. 2023, 13, 1497. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some Aspects of the Properties and Degradation of Polyacrylamides. Chem. Rev. 2002, 102, 3067–3083. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, J.D.; Kasperski, K.L. Thermogravimetric study of polyacrylamide with evolved gas analysis. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1807–1823. [Google Scholar] [CrossRef]
- Leung, W.M.; Axelson, D.E.; Van Dyke, J.D. Thermal degradation of polyacrylamide and poly(acrylamide-co-acrylate). J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1825–1846. [Google Scholar] [CrossRef]
- Vilcu, D.; Irinei, F.; Ionescu-Bujor, J.; Olteanu, M.; Demetrescu, I. Kinetic parameters obtained from TG and DTG curves of acrylamide-maleic anhydride copolymers. J. Therm. Anal. Calorim. 1985, 30, 495–502. [Google Scholar] [CrossRef]
- Vilcu, R.; Irinei, F.; Ionescu-Bujor, J.; Olteanu, O.; Demetrescu, I. Thermal stability of copolymer acrylamide–maleic anhydride. J. Appl. Polym. Sci. 1987, 33, 2431–2437. [Google Scholar] [CrossRef]
- Cunha, L.D.; Coutinho, F.M.B.; Teixira, V.G.; De Fesus, E.F.O.; Comes, A.S. Surface modification of styrene-divinylbenzene copolymers by polyacrylamide grafting via gamma irradiation. Polym. Bull. 2008, 61, 319–330. [Google Scholar] [CrossRef]
- Chang, Y.H.; McCormick, C.L. Water-soluble copolymers. 49. Effect of the distribution of the hydrophobic cationic monomer dimethyldodecyl (2-acrylamidoethyl) ammonium bromide on the solution behavior of associating acrylamide copolymers. Macromolecules 1993, 26, 6121–6126. [Google Scholar] [CrossRef]
- Vrahopoulou, E.P.; McHugh, A.J. A consideration of the Yamamoto network theory with non-Gaussian chain segments. J. Rheol. 1987, 31, 371–384. [Google Scholar] [CrossRef]
- Jenkins, R.D.; Silebi, C.A.; El-Aasser, M.S. Steady-Shear and Linear-Viscoelastic Material Properties of Model Associative Polymer-Solutions; Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 18, p. 343. [Google Scholar]
- Johnson, K.M.; Fevola, M.J.; McCormick, C.L. Hydrophobically modified acrylamide-based polybetaines. I. synthesis, characterization, and stimuli-responsive solution behavior. J. Appl. Polym. Sci. 2004, 92, 647–657. [Google Scholar] [CrossRef]
- Candau, F.; Leong, Y.S.; Pouyet, G.; Caudau, S.J. Inverse microemulsion polymerization of acrylamide: Characterization of the water-in-oil microemulsions and the final mircro-latexes. J. Colloid Interface Sci. 1984, 101, 167–183. [Google Scholar] [CrossRef]




| Simple Code a | Monomers | Intrinsic Viscosity [η] (mL/g) | |||
|---|---|---|---|---|---|
| AM (wt%) | DMC (wt%) | Hydrophobic Monomer | |||
| Code | Content (mol%) b | ||||
| CW4-1 | 5.00 | 5.00 | DM-4 | 1.00 | 757 |
| CW6-1 | 5.00 | 5.00 | DM-6 | 1.00 | 590 |
| CW8-0.25 | 5.00 | 5.00 | DM-8 | 0.25 | 1020 |
| CW8-0.5 | 5.00 | 5.00 | DM-8 | 0.50 | 871 |
| CW8-1 | 5.00 | 5.00 | DM-8 | 1.00 | 702 |
| CW8-2 | 5.00 | 5.00 | DM-8 | 2.00 | 531 |
| CW12-0.25 | 5.00 | 5.00 | DM-12 | 0.25 | 638 |
| CW16-0.25 | 5.00 | 5.00 | DM-16 | 0.25 | 542 |
| CW101 | 10.00 | 10.00 | — | — | 1231 |
| CW206 | 10.00 | 10.00 | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Zhang, S.; Zhang, Y.; Yin, H.; Feng, Y. Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior. Molecules 2023, 28, 2698. https://doi.org/10.3390/molecules28062698
Lv Y, Zhang S, Zhang Y, Yin H, Feng Y. Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior. Molecules. 2023; 28(6):2698. https://doi.org/10.3390/molecules28062698
Chicago/Turabian StyleLv, Yongli, Sheng Zhang, Yunshan Zhang, Hongyao Yin, and Yujun Feng. 2023. "Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior" Molecules 28, no. 6: 2698. https://doi.org/10.3390/molecules28062698
APA StyleLv, Y., Zhang, S., Zhang, Y., Yin, H., & Feng, Y. (2023). Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior. Molecules, 28(6), 2698. https://doi.org/10.3390/molecules28062698






