Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean)
Abstract
1. Introduction
2. Results
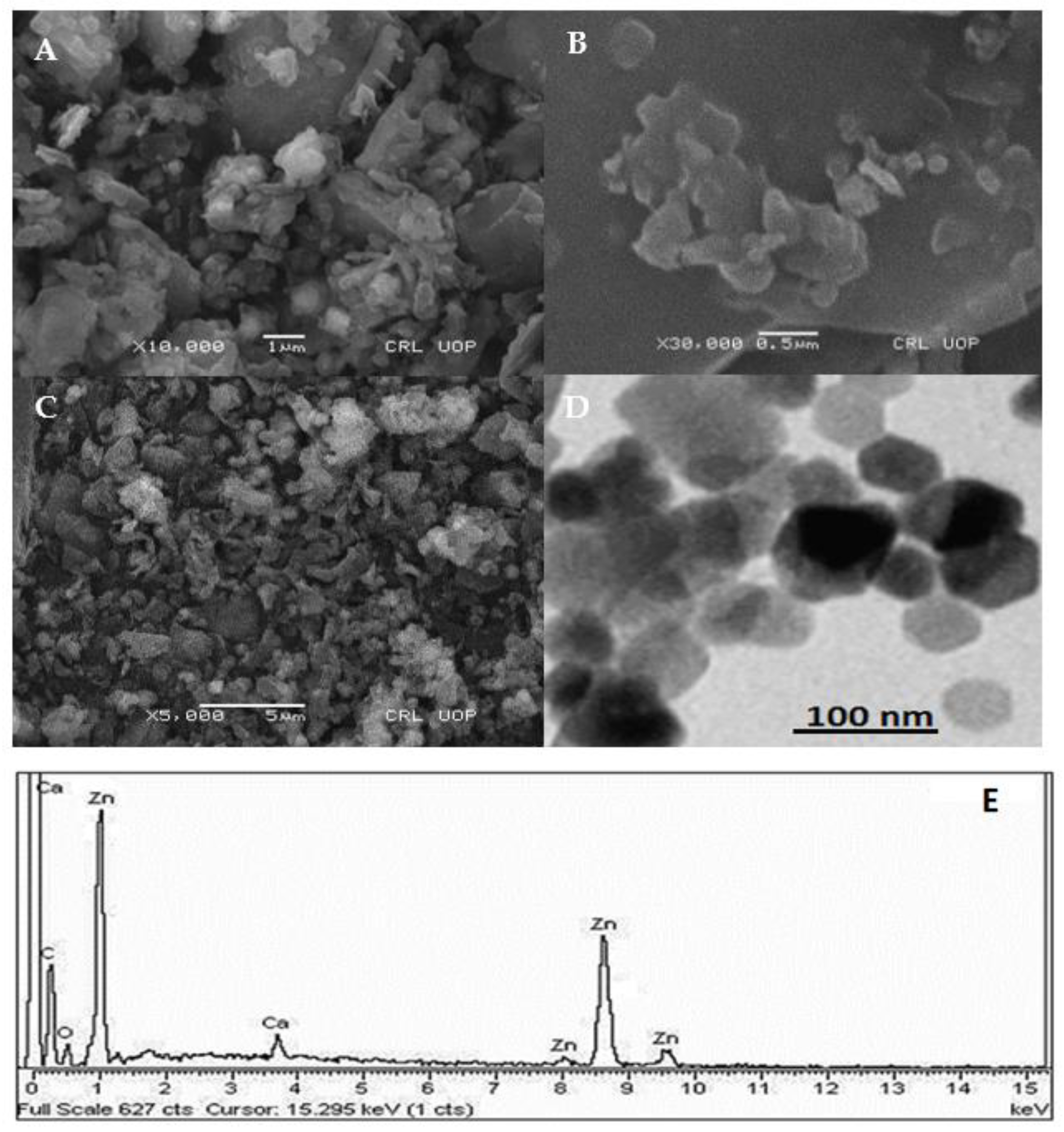
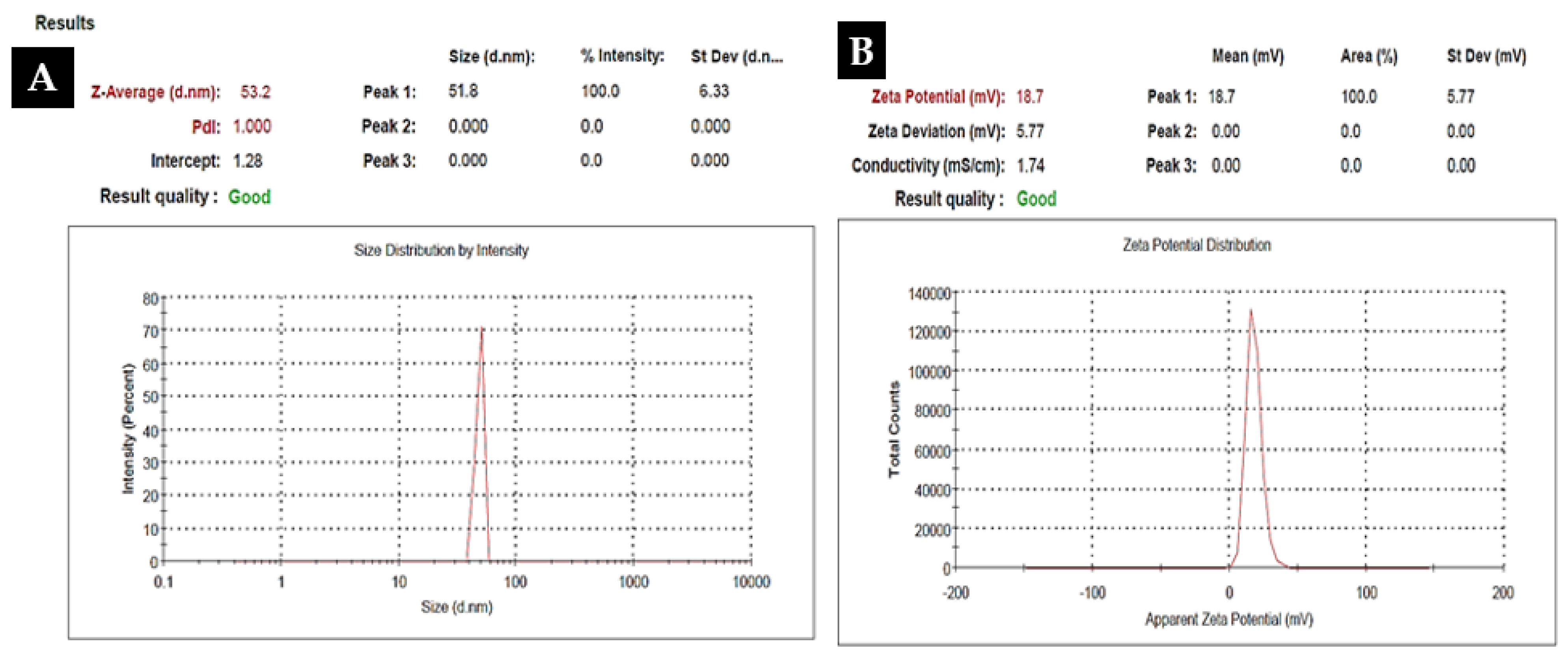
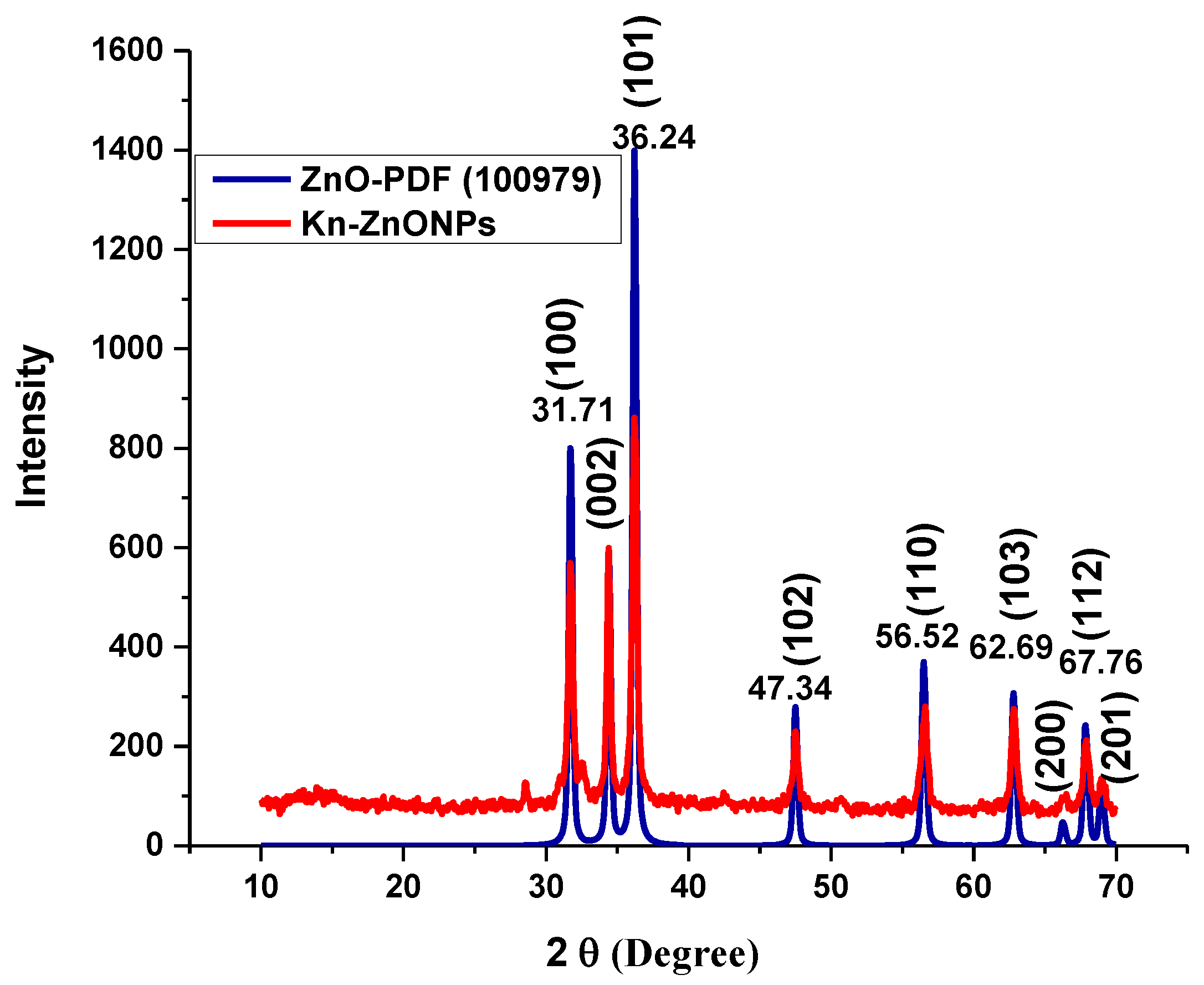
2.1. Characterization of Kn-ZnONPs
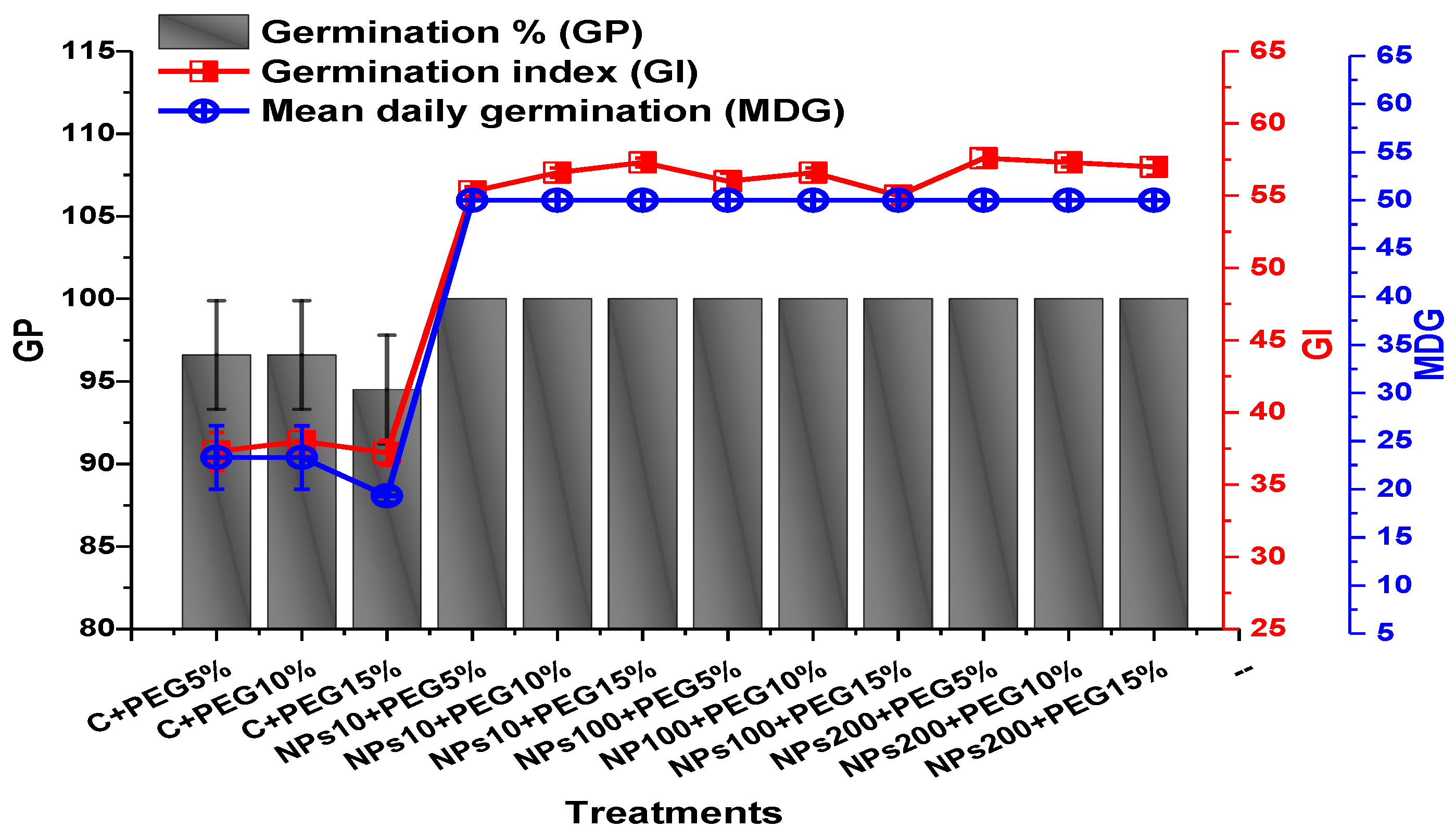
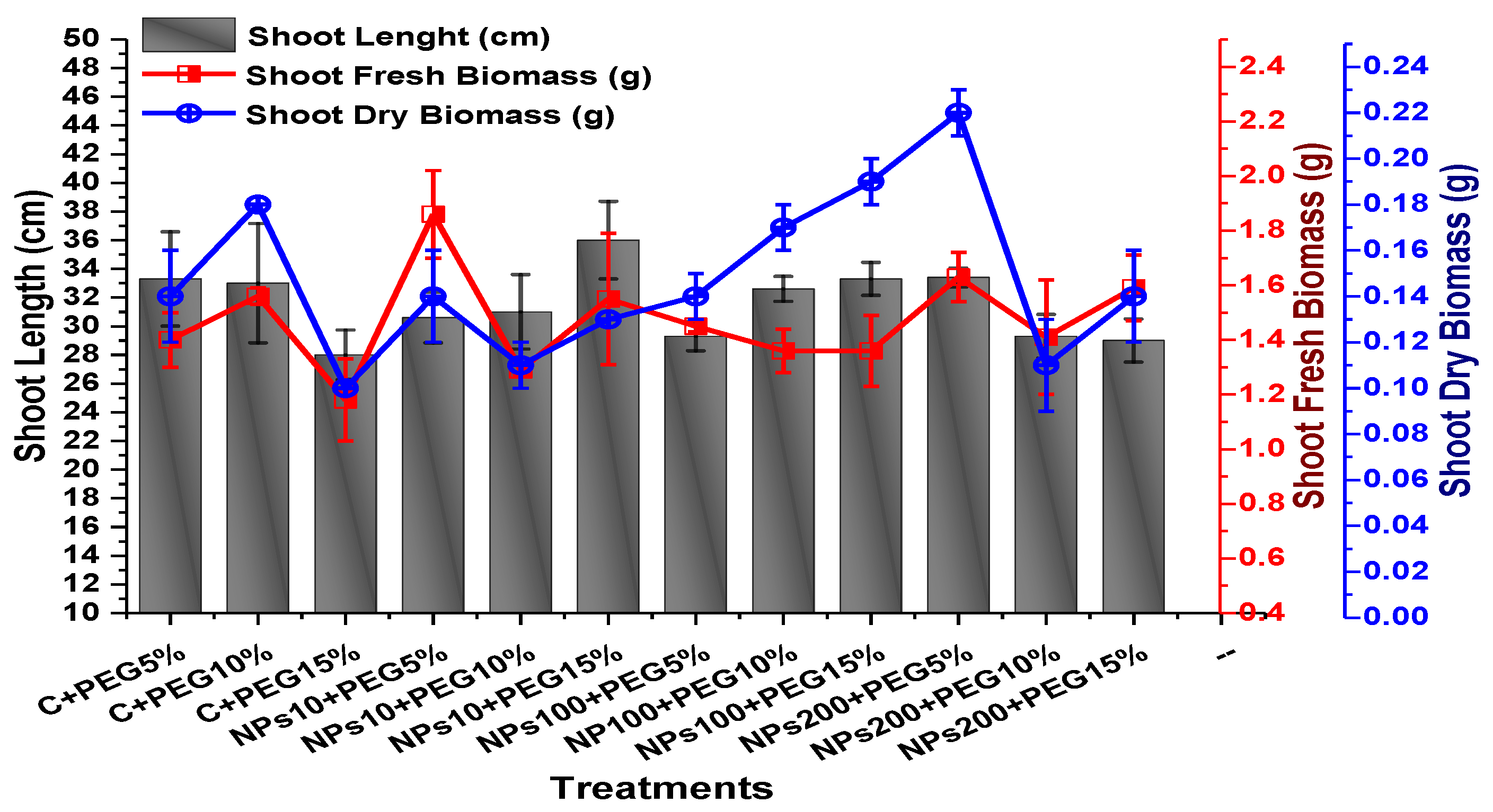
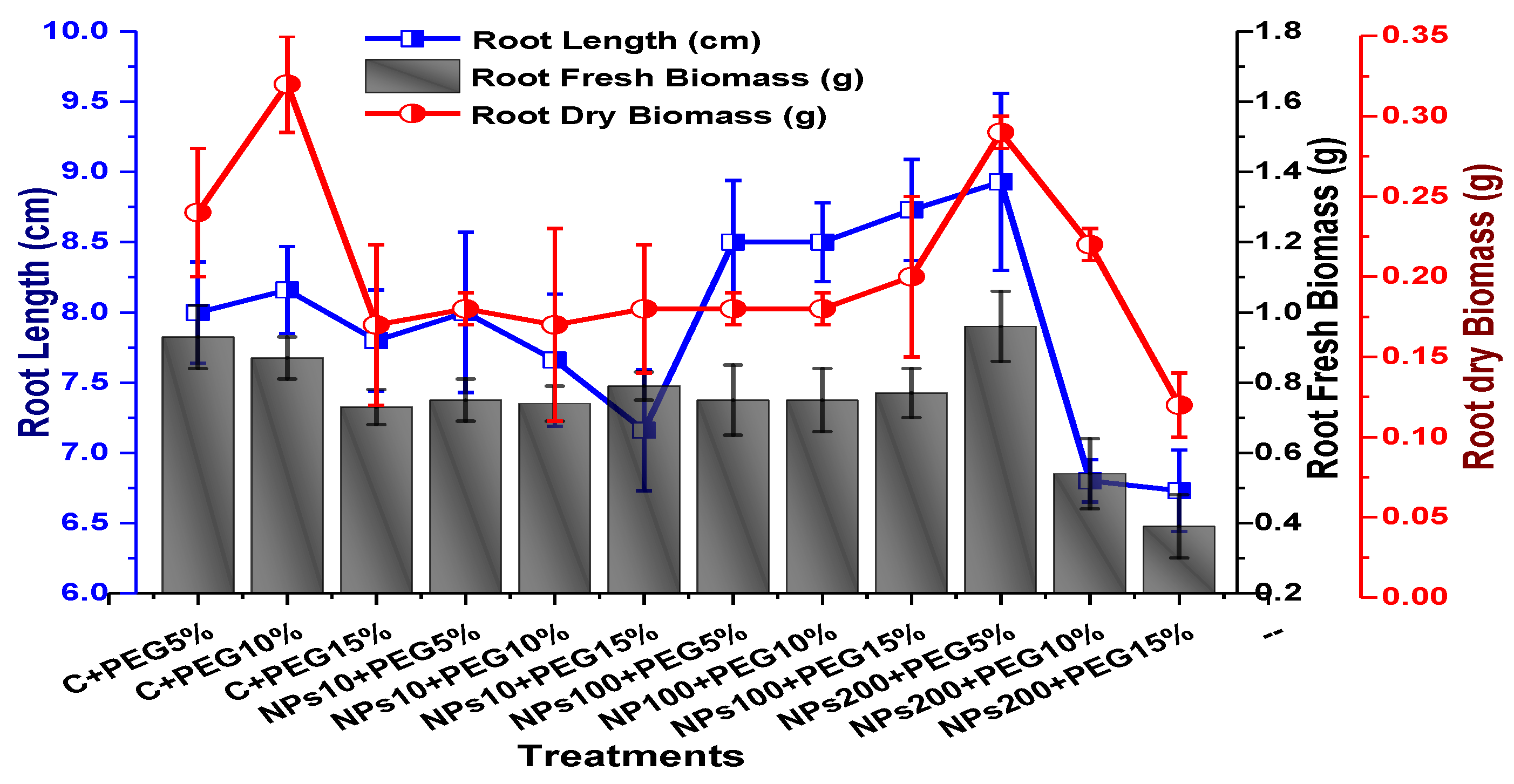
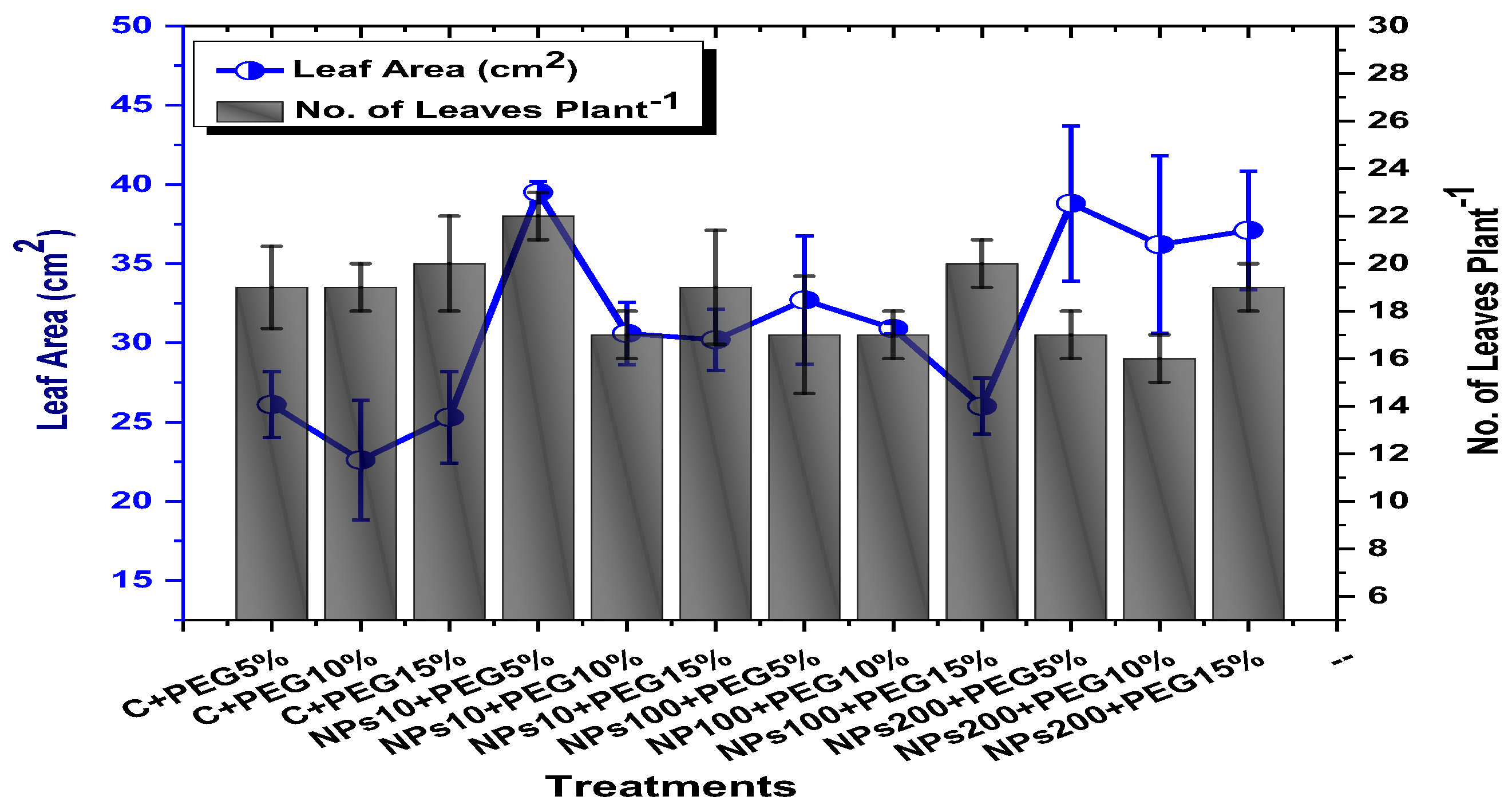
2.2. Effect of Kn-ZnONPs on Seed Germination and Agronomic Profile
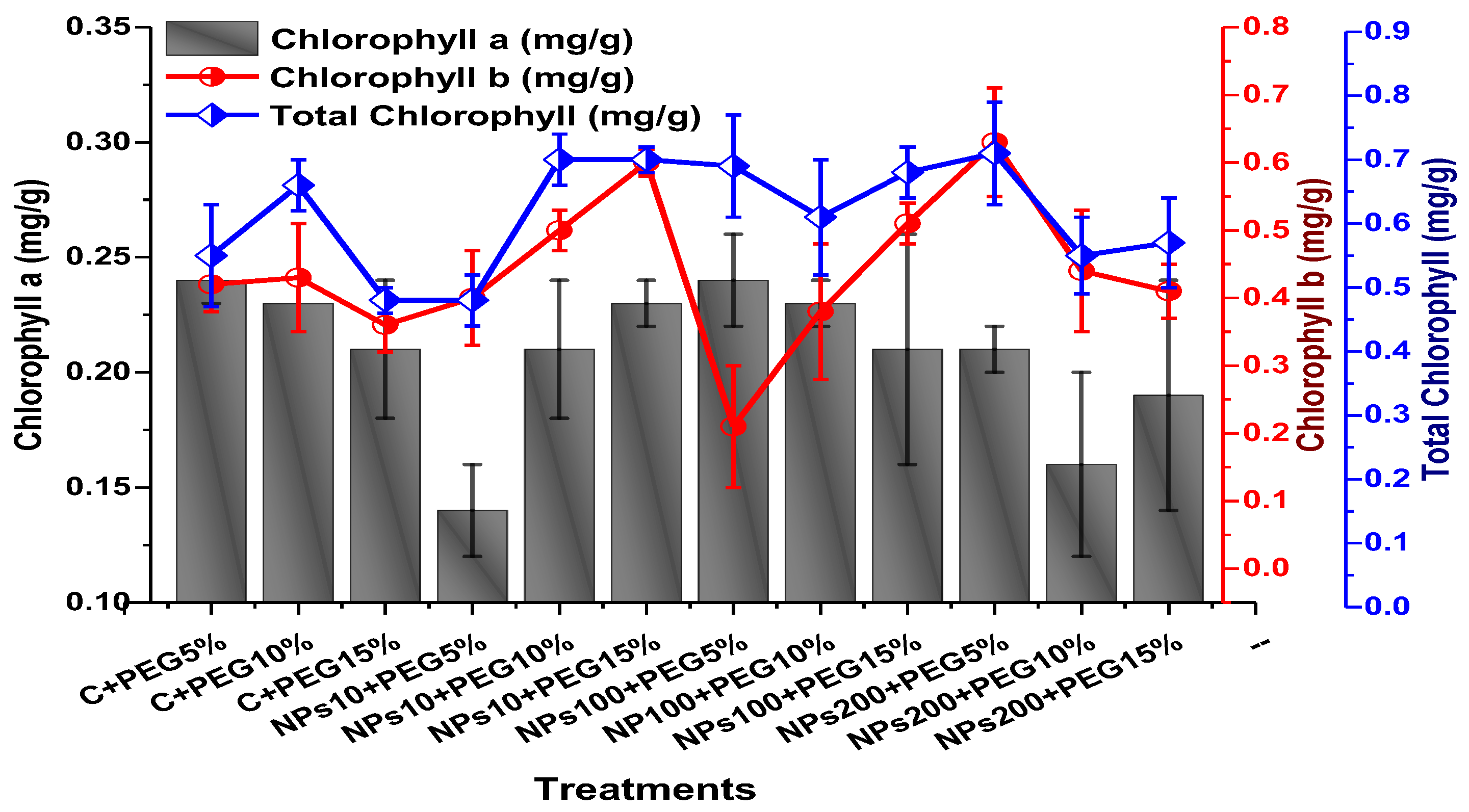
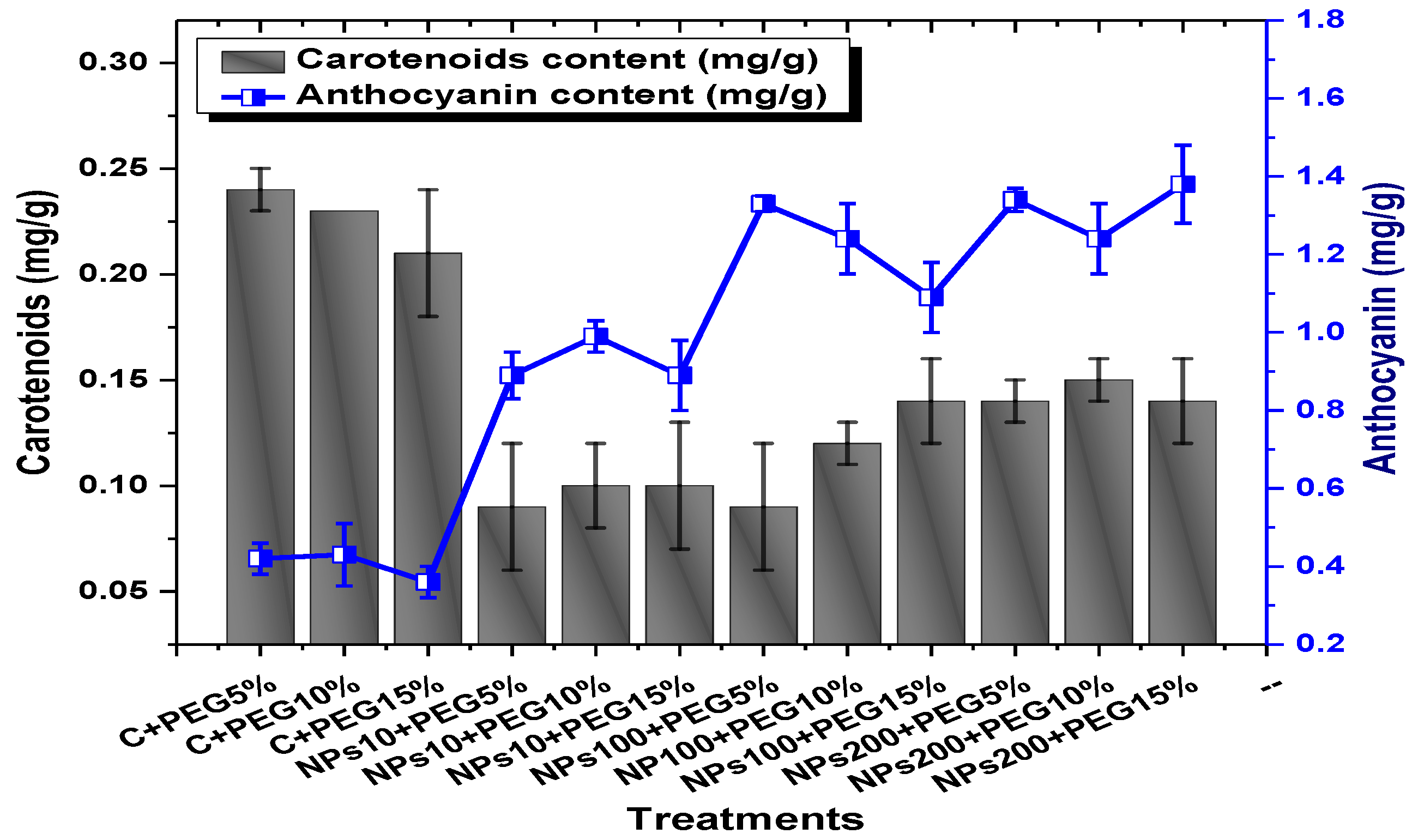
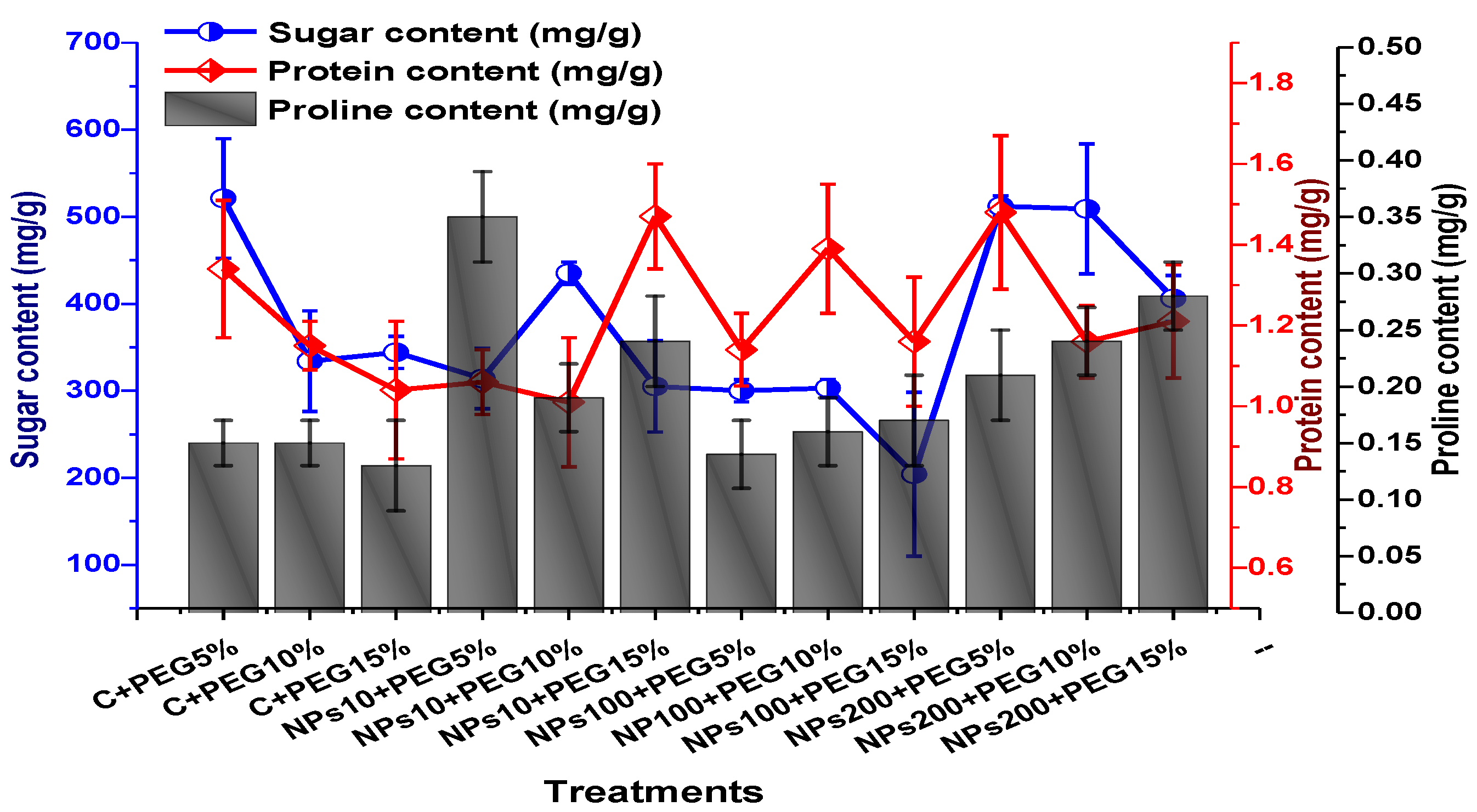
2.3. Effect of Kn-ZnONPs on Photosynthetic Pigments and Osmoprotectants Assimilates
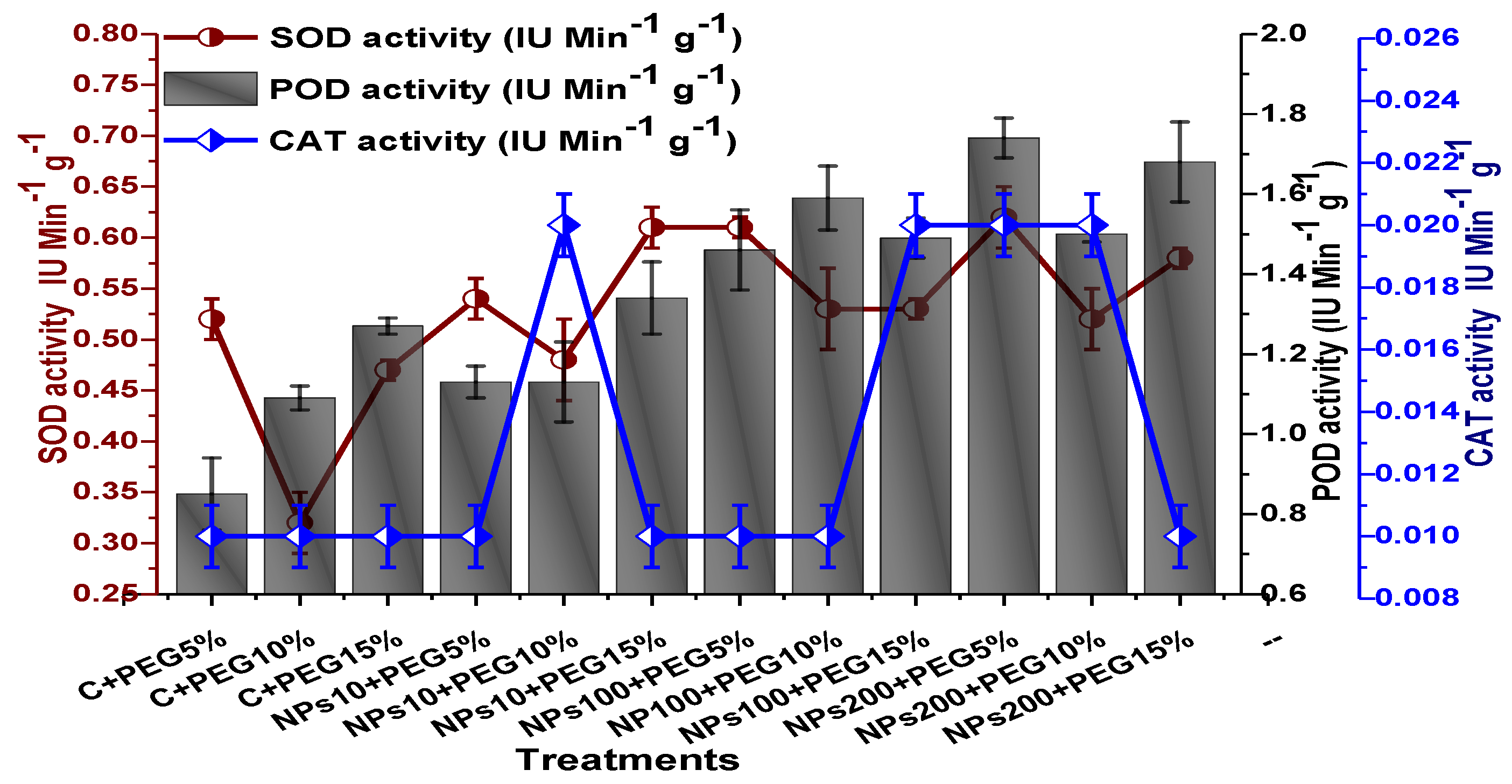
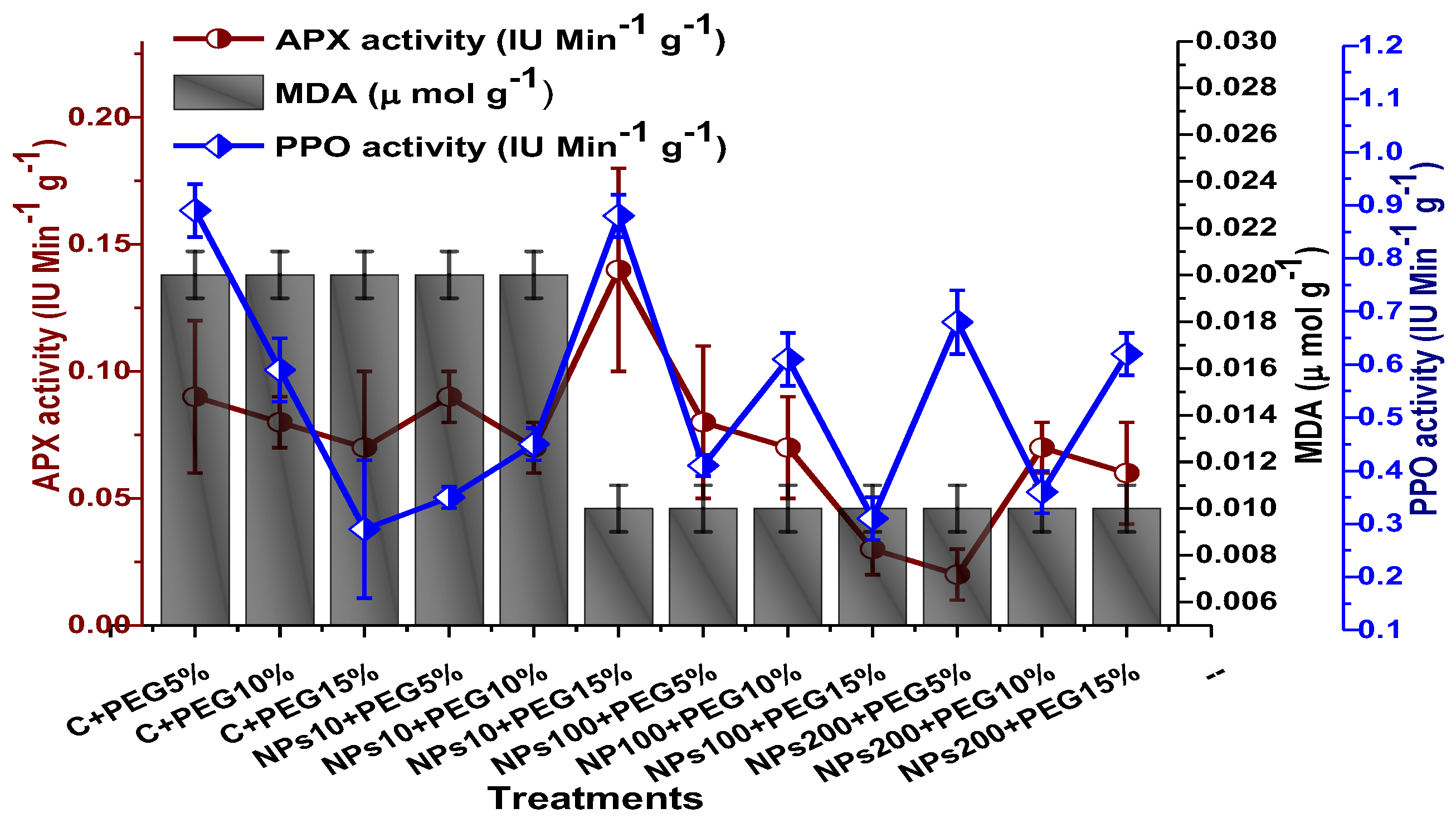
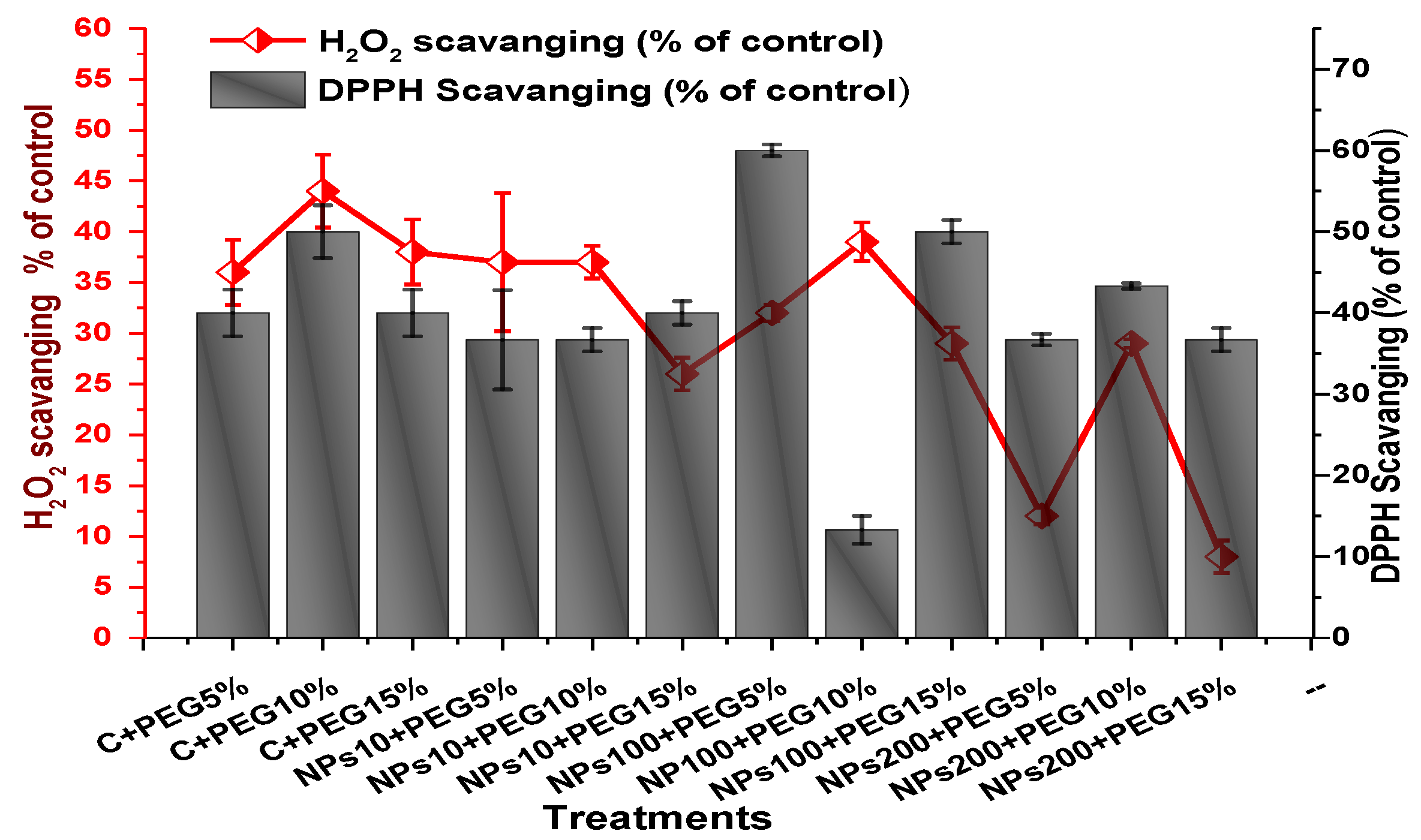
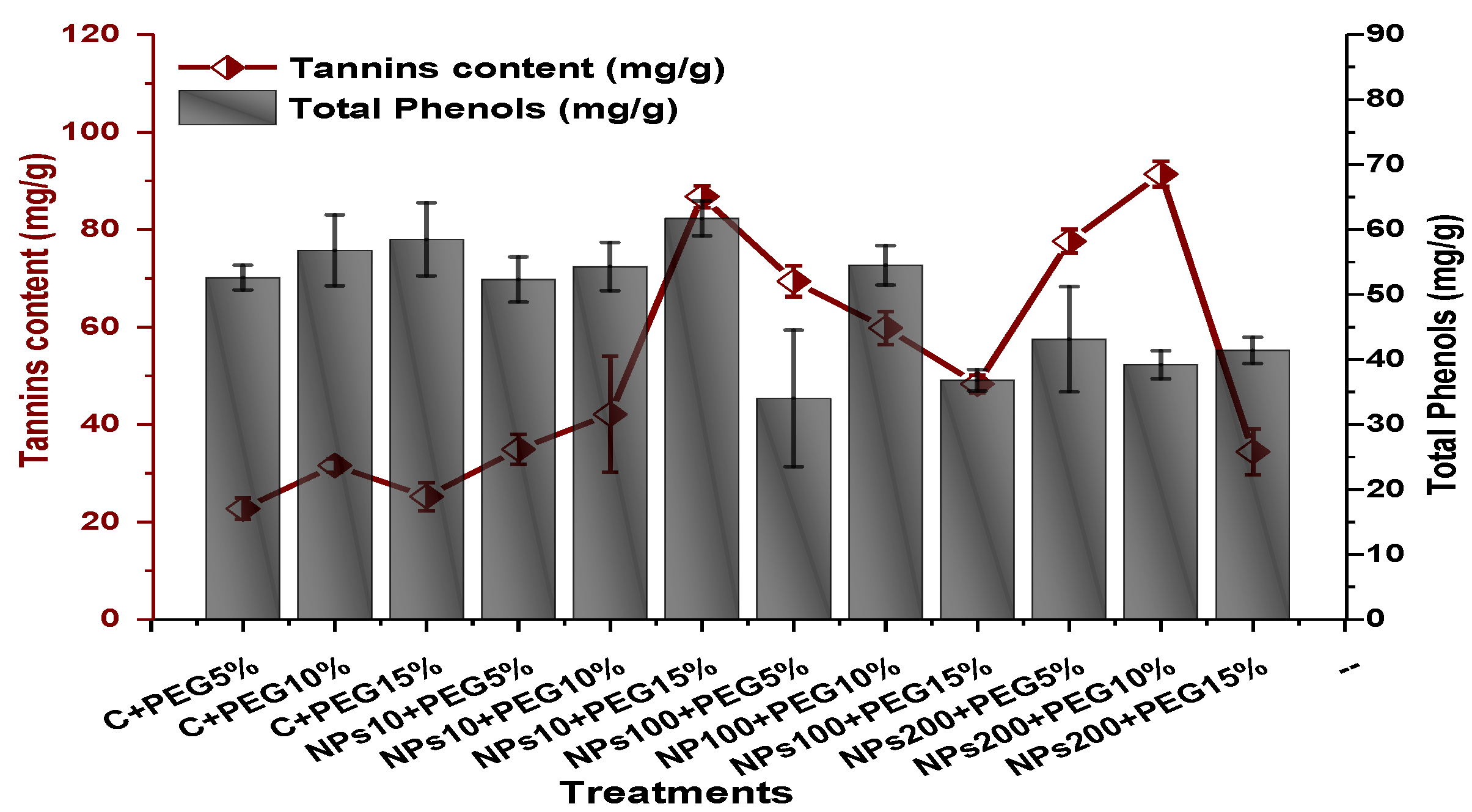
2.4. Effect of Kn-ZnONPs on Antioxidant Biosystem
3. Discussion
4. Materials and Methods
4.1. Synthesis of Kn-ZnONPs and Characterization
4.2. Laboratory Experimental Detail
4.3. Field Experiment Detail
4.4. Measurement of Biochemical Parameters
4.4.1. Measurement of Proline Protein and Soluble Sugar Contents
4.4.2. Measurement of Photosynthetic Pigments
4.4.3. Measurement of Antioxidant Enzymes (SOD, POD, APX, and CAT)
4.4.4. Measurement of Lipid Peroxidation and PPO
4.4.5. Measurement of Total Phenolic and Tannin Contents
4.4.6. Measurement of Hydrogen Peroxide (H2O2) and DPPH Scavenging Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. Climate Change and Food Security: Risks and Responses; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Available online: https://www.fao.org/3/i5188e/I5188E.pdf (accessed on 21 June 2023).
- Mishra, A.K.; Mishra, R.K.; Kumar, A. Assessment of meteorological drought impact on crop yield in India. Nat. Hazards 2020, 102, 2125–2142. [Google Scholar]
- Baloch, M.A.; Umrani, M.A.; Baloch, M.A.; Rajper, M.A. Wheat Productivity under Water Stress: A Review. J. Agric. Ecol. Res. Int. 2021, 24, 8–15. [Google Scholar]
- Jamil, M.; Ahmed, T.; Ashraf, U.; Ali, A.; Malik, S.A. Drought risk assessment and management in Pakistan: A review. Int. J. Disaster Risk Reduct. 2020, 50, 101805. [Google Scholar]
- Khurshid, N.; Javed, M.A.; Raza, G. Climate change: A threat to agricultural productivity in Pakistan. Environ. Dev. Sustain. 2019, 21, 1715–1734. [Google Scholar]
- Government of Pakistan. Pakistan Economic Survey 2019–2020; Ministry of Finance: Islamabad, Pakistan, 2020. [Google Scholar]
- Tariq, A.; Nasir, M.; Zaman, Q.U. Impact of drought on agriculture in Pakistan: A review. Int. J. Agric. Biol. Eng. 2021, 14, 27–38. [Google Scholar]
- Asian Development Bank. Drought Risk Assessment and Management: A Framework for the Indus Basin in Pakistan; Asian Development Bank: Manila, Phillipines, 2017. [Google Scholar]
- Arunanondcha, J.; Suriya-arunroj, D.; Tang, T. Environmental changes and their impacts on agriculture and society. Procedia Manuf. 2018, 20, 276–282. [Google Scholar]
- Raza, M.A.; Abbas, M.; Akram, M.W.; Ali, Q. Impact of Climate Change on Agriculture: A Review. J. Anim. Plant Sci. 2019, 29, 986–992. [Google Scholar]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, L.; Ghani, M.I.; Peng, Q.; Fan, R.; Hu, X.; Chen, X. Effects of Drought Stress Induced by Hypertonic Polyethylene Glycol (PEG-6000) on Passiflora edulis Sims Physiological Properties. Plants 2023, 12, 2296. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar]
- Iqbal, J.; Abbasi, B.A.; Munir, A.; Uddin, S.; Kanwal, S.; Mahmood, T. Facile green synthesis approach for the production of chromium oxide nanoparticles and their different in vitro biological activities. Microsc. Res. Tech. 2020, 83, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Ahmad, R.; Zia, L.; Kanwal, S.; Mahmood, T.; Chen, J.T. Bioactivities of Geranium wallichianum leaf extracts conjugated with zinc oxide nanoparticles. Biomolecules 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 6, 3097. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Ahmad, S.; Mirza, B. Antioxidant and cytotoxic activity and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. 2012, 11, 241. [Google Scholar] [PubMed]
- Thema, F.; Manikandan, E.; Dhlamini, N.; Maaza, M. Green synthesis of ZnO nanoparticles via Agatbosma betulina natural extract. Matter. Let. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Ahmad, W.; Nadeem, M.; Giglioli-Guicorc’h, N.; Hano, C. Biogenic zinc oxide nanoparticles-enhanced biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar] [CrossRef]
- Saitoh, L.; Babu, R.R.; Kannappan, S.; Kojima, K.; Mizutani, T.; Ochiai, S. Performance of spray deposited poly [N-9”-hepta-decanyl-2,7-carbazole-alt-5,5-(40,70-di-2-thienyl-20,10,30-benzothiadiazole)] [6,6]-phenyl-C61-butyric acid methyl ester blend active layer based bulk heterojunction organic solar cell devices. Thin. Solid Films. 2012, 520, 3111–3117. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Al-Roumi, I.A.; Al-Hamdani, Q.M. Effect of cutting stage and treatment with gibberellin and kinetin on the growth and yield of local black barley (Hordeum disticum L.). Res. J. Coll. Basic Educ. 2012, 12, 739–756. [Google Scholar]
- Al-Nuaimi, H.T.A. Effect of Salinity of İrrigation Water and Spraying with Gibberellin and Potassium on Growth and Yield of Wheat. Ph.D. Thesis, College of Agriculture, University of Baghdad, Baghdad, Iraq, 2015. [Google Scholar]
- Yaronskay, E.B.; Gritskevich, E.R.; Trukhanovets, N.L.; Averina, N.G. Effect of kinetin on early stages of chlorophyll biosynthesis in streptomycin-treated barley seedlings. Russ. J. Plant Physiol. 2007, 54, 388–395. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, A.; Chaudhary, A.; Mishra, A.; Rawat, S.; B., B.Y.; Shami, V.; Kaushik, P. Response of Wheat Genotypes to Drought Stress Stimulated by PEG. Stresses 2022, 2, 26–51. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed Biofortification by Engineered Nanomaterials: A Pathway To Alleviate Malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef]
- Ul Haq, T.; Ullah, R.; Khan, M.N.; Nazish, M.; Almutairi, S.M.; Rasheed, R.A. Seed Priming with Glutamic-Acid-Functionalized Iron Nanoparticles Modulating Response of Vigna radiata (L.) R. Wilczek (Mung Bean) to Induce Osmotic Stress. Micromachines 2023, 14, 736. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokine. In The Arabidopsis Book; American Society of Plants Biologist: Rockville, MD, USA, 2014; p. 12. [Google Scholar]
- Austin, R.B. Crop traits and the potential yield of wheat. J. Agric. Sci. Camb. 1982, 98, 447–453. [Google Scholar] [CrossRef]
- Miliauskiene, J.; Brazaityte, A.; Sutuliene, R.; Urbutis, M.; Tuckute, S. ZnO Nanoparticle Size-Dependent Effects on Swiss Chard Growth and Nutritional Quality. Agriculture 2022, 12, 1905. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Pandey, B.K.; Shahi, A.K.; Shah, J.; Kotnala, R.K.; Gopa, R. Giant ferromagnetism in Li doped ZnO nanoparticles at room temperature. J. Alloys Compd. 2020, 823, 153710. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Pazurkiewicz-kocot, K.; Andrzej, K.; Haduch, K. The effect of kinetin on the chlorophyll pigments content in leaves of Zea mays L. seedlings and accumulation of some metal ions. Inż. Ochr. Śr. 2011, 14, 397–409. [Google Scholar]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Zhang, L.; Pan, X.; Hu, X. Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene Potentiates Sulfur-Mediated Reversal of Cadmium Inhibited Photosynthetic Responses in Mustard. Front. Plant Sci. 2016, 7, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Ahanger, M.B.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef]
- Bowler, T.; Torres, F.I.A.; Terner, J.; Pittman, R.N.; Proffitt, E.; Ward, K.R. Measurement of hemoglobin oxygen saturation using Raman microscopy and 532-nm excitation. J. Appl. Physiol. 2016, 104, 1809–1817. [Google Scholar]
- De Moura, F.B.; Vieira, M.R.d.S.; SimÕEs, A.d.N.; Ferreira-Silva, S.L.; de Souza, C.A.V.; de Souza, E.S.; da Rocha, A.T.; da Silva, L.F.; Junior, M.A. Physiological Effect of Kinetin on the Photosynthetic Apparatus and Antioxidant Enzymes Activities During Production of Anthurium. Hortic. Plant. J. 2018, 4, 182–192. [Google Scholar] [CrossRef]
- Mehri, S. Effect of gibberellic acid foliar and kinetin on the antioxidant catalase anzymes and peroxidase in maize under drought stress. Cumhur. Üniversitesi Fen-Edeb. Fakültesi Fen Bilim. Derg. 2015, 36, 595–603. [Google Scholar]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Mustafavi, S.H.; Badi, H.N.; Sekara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 2018, 40, 102. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Gnanasangeetha, D.; Thambavani, S.D. Biogenic Production of Zinc Oxide Nanoparticle using Acalypha indica. J. Chem. Biol. Phys. Sci. 2013, 4, 238–246. [Google Scholar]
- Rostami, F.; Ehsanpour, A.A. Application of silver thiosulfate (STS) on silver accumulation and protein pattern of potato (Solanum tubersum L.) under in vitro culture. Ann. Appl. Biol. 2009, 38, 46–54. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 2016, 28, 350–356. [Google Scholar] [CrossRef]
- Gholami, M.; Rahemi, M.; Kholdebarin, B.; Rastegar, S. Use of rapid screening methods for detecting drought tolerance cultivars of Figure (Ficus carica L.). Sci. Hortic. 2012, 148, 109–117. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2012, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Saxena, S.C.; Salvi, P.; Kamble, N.U.; Joshi, P.K.; Majee, M.; Arora, S. Ectopic overexpression of cytosolic ascorbate peroxidase gene (Apx1) improves salinity stress tolerance in Brassica juncea by strengthening antioxidative defence mechanism. Acta Physiol. Plant. 2020, 42, 45. [Google Scholar] [CrossRef]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of total phenolic content, in-vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, B. Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protocol 2016, 6, 1913. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Calorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanow, I.; Edreva, A. oxidative stress and some antioxidants system in acid rain treated bean plants; protective role of exogenous polyamine. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Ullah, R.; Bibi, S.; Khan, M.N.; Al Mohaimeed, A.M.; Naz, Q.; Kamal, A. Application of Bio-Inspired Gold Nanoparticles as Advanced Nanomaterial in Halt Nociceptive Pathway and Hepatotoxicity via Triggering Antioxidation System. Catalysts 2023, 13, 786. [Google Scholar] [CrossRef]
- Ma, J.; Ali, S.; Saleem, M.H.; Mumtaz, S.; Yasin, G.; Ali, B.; Al-Ghamdi, A.A.; Elshikh, M.S.; Vodnar, D.C.; Marc, R.A. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 1, 983156. [Google Scholar] [CrossRef]
- Shah, W.; Ullah, S.; Ali, S.; Idrees, M.; Khan, M.N.; Ali, K.; Khan, A.; Ali, M.; Younas, F. Effect of exogenous alpha-tocopherol on physio-biochemical attributes and agronomic performance of lentil (Lens culinaris Medik.) under drought stress. PLoS ONE 2021, 16, e0248200. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajmal, M.; Ullah, R.; Muhammad, Z.; Khan, M.N.; Kakar, H.A.; Kaplan, A.; Okla, M.K.; Saleh, I.A.; Kamal, A.; Abdullah, A.; et al. Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean). Molecules 2023, 28, 5059. https://doi.org/10.3390/molecules28135059
Ajmal M, Ullah R, Muhammad Z, Khan MN, Kakar HA, Kaplan A, Okla MK, Saleh IA, Kamal A, Abdullah A, et al. Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean). Molecules. 2023; 28(13):5059. https://doi.org/10.3390/molecules28135059
Chicago/Turabian StyleAjmal, Maham, Rehman Ullah, Zahir Muhammad, Muhammad Nauman Khan, Hussain Ahmad Kakar, Alevcan Kaplan, Mohammad K. Okla, Ibrahim A. Saleh, Asif Kamal, Abdullah Abdullah, and et al. 2023. "Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean)" Molecules 28, no. 13: 5059. https://doi.org/10.3390/molecules28135059
APA StyleAjmal, M., Ullah, R., Muhammad, Z., Khan, M. N., Kakar, H. A., Kaplan, A., Okla, M. K., Saleh, I. A., Kamal, A., Abdullah, A., & Abdul Razak, S. (2023). Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean). Molecules, 28(13), 5059. https://doi.org/10.3390/molecules28135059






