Species Identification of Caviar Based on Multiple DNA Barcoding
Abstract
1. Introduction
2. Results
2.1. Preliminary Morphological Identification
2.2. DNA Concentration and Quality
2.3. PCR Specificity
2.4. Plant-Derived Ingredients Identifying
2.5. Sturgeon-Derived Ingredients Identifying
2.6. Phylogenetic Tree Construction and Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Morphological Identification
4.3. DNA Extraction
4.4. Primers and PCR Amplifications
4.5. Specificity Test for Designed Primers
4.6. Sequencing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tavakoli, S.; Luo, Y.K.; Regenstein, J.M.; Daneshvar, E.; Bhatnagar, A.; Tan, Y.Q.; Hong, H. Sturgeon, caviar, and caviar substitutes:from production, gastronomy, nutrition, and quality change to trade and commercial mimicry. Rev. Fish. Sci. Aquac. 2021, 29, 753–768. [Google Scholar] [CrossRef]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and fish roe products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Fopp-Bayat, D. Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 2020, 13, 119–137. [Google Scholar] [CrossRef]
- Xia, Z.; Lara, T.; Siyun, X.; Jinhong, W.; Xiaoguo, Y.; Jing, W.; Andrea, A. Caviar products sold on Chinese Business to customer (B2C) online platforms: Labelling assessment supported by molecular identification. Food Control 2022, 131, 108370. [Google Scholar]
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon meat and caviar production: Global update 2017. J. Appl. 2019, 35, 257–266. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Tawfik, S.; Shafik, N.; Khattab, A.R. Caviar and fish roe substitutes: Current status of their nutritive value, bio-chemical diversity, authenticity and quality control methods with future perspectives. Trends Food Sci. Technol. 2021, 110, 405–417. [Google Scholar] [CrossRef]
- Binsi, P.K.; Nayak, N.; Sarkar, P.C.; Sahu, U.; Lalitha, K.V.; Ninan, G.; Ravishankar, C.N. Conversion of carp roe mass to caviar substitutes: Stabilization with oregano extract. LWT 2019, 108, 446–455. [Google Scholar] [CrossRef]
- Monika, M.; Krzysztof, S.; Renata, P. Snail eggs as a raw material for the production of a caviar substitute. J. Vet. Res. 2020, 64, 543–547. [Google Scholar]
- Małgorzata, S.; Agnieszka, P.; Maria, D.; Daria, S.; Joanna, K.; Judyta, C. Preparation of beebread caviar from buckwheat honey through immobilization with sodium alginate. Molecules 2020, 25, 4483. [Google Scholar]
- Bronzi, P.; Rosenthal, H. Present and future sturgeon and caviar production and marketing: A global market overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Daan, V.U.; Dina, S. The illegal trade in black caviar. Trends Organ. Crime 2016, 19, 67–87. [Google Scholar]
- Ludwig, A.; Lieckfeldt, D.; Jahrl, J. Mislabeled and counterfeit sturgeon caviar from Bulgaria and Romania. J. Appl. Ichthyol. 2015, 31, 587–591. [Google Scholar] [CrossRef]
- DeSalle, R.; Birstein, V.J. PCR identification of black caviar. Nature 1996, 381, 197–198. [Google Scholar] [CrossRef]
- Birstein, V.J.; Doukakis, P.; Sorkin, B.; DeSalle, R. Population aggregation analysis of three caviar-producing species of sturgeons and implications for the species identification of black caviar. Conserv. Biol. 1998, 12, 766–775. [Google Scholar] [CrossRef]
- Congiu, L.; Fontana, F.; Patarnello, T.; Rossi, R.; Zane, L. The use of AFLP in sturgeon identification. J. Appl. Ichthyol. 2002, 18, 286–289. [Google Scholar] [CrossRef]
- Miuge, N.S.; Barmintseva, A.E.; Rastorguev, S.M.; Miuge, V.N.; Barmintsev, V.A. Polymorphism of the mitochondrial DNA control region in eight sturgeon species and development of a system for DNA-based species identification. Genetika 2008, 44, 913–919. [Google Scholar] [CrossRef]
- Boscari, E.; Barmintseva, A.; Pujolar, J.M.; Doukakis, P.; Mugue, N.; Congiu, L. Species and hybrid identification of sturgeon caviar: A new molecular approach to detect illegal trade. Mol Ecol Resour 2014, 14, 489–498. [Google Scholar] [CrossRef]
- Doukakis, P.; Pikitch, E.K.; Rothschild, A.; DeSalle, R.; Amato, G.; Kolokotronis, S.-O. Testing the effectiveness of an international conservation agreement: Marketplace forensics and CITES caviar trade regulation. PLoS ONE 2012, 7, e40907. [Google Scholar] [CrossRef]
- Pappalardo, A.M.; Petraccioli, A.; Capriglione, T.; Ferrito, V. From fish eggs to fish name: Caviar species discrimination by COIBar-RFLP, an efficient molecular approach to detect fraud in the caviar trade. Molecules 2019, 24, 2468. [Google Scholar] [CrossRef]
- Andreea, D.; Maria, S.; Marilena, M.; Emil, G.S. A multistep DNA-based methodology for accurate authentication of sturgeon species. Foods 2022, 11, 1007. [Google Scholar]
- Jenneckens, I.; Meyer, J.N.; Debus, L.; Pitra, C.; Ludwig, A. Evidence of mitochondrial DNA clones of Siberian sturgeon, Acipenser baerii, within Russian sturgeon, Acipenser gueldenstaedtii, caught in the River Volga. Ecol. Lett. 2000, 3, 503–508. [Google Scholar] [CrossRef]
- Birstein, V.J.; Doukakis, P.; DeSalle, R. Polyphyly of mtDNA lineages in the Russian sturgeon, Acipenser gueldenstaedtii: Forensic and evolutionary implications. Conserv. Genet. 2000, 1, 81–88. [Google Scholar] [CrossRef]
- Aspasia, J.T.; Arati, I. Phylogenetic evidence for a case of misleading rather than mislabeling in caviar in the United Kingdom. J. Forensic Sci. 2015, 60 (Suppl. S1), S248–S253. [Google Scholar]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- CODEX STAN 291-2010; Standard for Sturgeon Caviar. Codex Alimentarius Commission: Geneva, Switzerland, 2010.
- Krieger, J.; Hett, A.K.; Fuerst, P.A.; Artyukhin, E.; Ludwig, A. The molecular phylogeny of the order Acipenseriformes revisited. J. Appl. Ichthyol. 2008, 24, 36–45. [Google Scholar] [CrossRef]
- Dillman, C.B.; Zhuang, P.; Zhang, T.; Zhang, L.-Z.; Mugue, N.; Hilton, E.J. Forensic investigations into a GenBank anomaly: Endangered taxa and the importance of voucher specimens in molecular studies. J. Appl. Ichthyol. 2014, 30, 1300–1309. [Google Scholar] [CrossRef]
- Ludwig, A.; May, B.; Debus, L. Heteroplasmy in the mtDNA control region of sturgeon (Acipenser, Huso and Scaphirhynchus). Genetics 2000, 156, 1933–1947. [Google Scholar] [CrossRef]
- Birstein, V.J.; Hanner, R.; DeSalle, R. Phylogeny of the Acipenseriformes: Cytogenetic and molecular approaches. Environ. Biol. Fishes 1997, 48, 1–4. [Google Scholar] [CrossRef]
- Dai, Z.; Qiao, J.; Yang, S.; Hu, S.; Zuo, J.; Zhu, W.; Huang, C. Species authentication of common meat based on PCR analysis of the mitochondrial COI gene. Appl. Biochem. Biotechnol. 2015, 176, 1770–1780. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.R.; Sharma, B.D.; Gokulakrishnan, P.; Mendiratta, S.K.; Sharma, D. Identification of species origin of meat and meat products on the DNA basis: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1340–1351. [Google Scholar] [CrossRef]
- SN/T 1204-2003; Genetically Modified Ingredients in Plants and Their Processed Products—Real Time Fluorescence PC Qualitative Detection Method. General Administration of quality supervision, inspection and quarantine of the People’s Republic of China: Beijing, China, 2003.
- Jiang, N.; Fan, Y.; Zhou, Y.; Wang, W.; Ma, J.; Zeng, L. Transcriptome analysis of Aeromonas hydrophila infected hybrid sturgeon Huso dauricus×Acipenser schrenckii. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Boscari, E.; Barmintseva, A.; Zhang, S.; Yue, H.; Li, C.; Shedko, S.V.; Lieckfeldt, D.; Ludwig, A.; Wei, Q.W.; Mugue, N.S.; et al. Genetic identification of the caviar-producing Amur and Kaluga sturgeons revealed a high level of concealed hybridization. Food Control 2017, 82, 243–250. [Google Scholar] [CrossRef]
- Baker, A.K.; Vixie, B.; Rasco, B.A.; Ovissipour, M.; Ross, C.F. Development of a lexicon for caviar and its usefulness for determining consumer preference. J. Food Sci. 2014, 79, S2533–S2541. [Google Scholar] [CrossRef] [PubMed]
- T/ZZB 0562—2018; Sturgeon Caviar. Zhejiang Provincial Brand Building Federation: Hangzhou, China, 2018.
- Fajardo, V.; González, I.; Martín, I.; Rojas, M.; Hernández, P.E.; García, T.; Martín, R. Real-time PCR for detection and quantification of red deer (Cervus elaphus), fallow deer (Dama dama), and roe deer (Capreolus capreolus) in meat mixtures. Meat Sci. 2007, 79, 289–298. [Google Scholar] [CrossRef]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Pruitt, K.D.; Sherry, S.T.; Yankie, L.; Karsch-Mizrachi, I. GenBank 2023 update. Nucleic Acids Res. 2023, 51, D141–D144. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The brcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetic analysis using maximum likeli-hood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
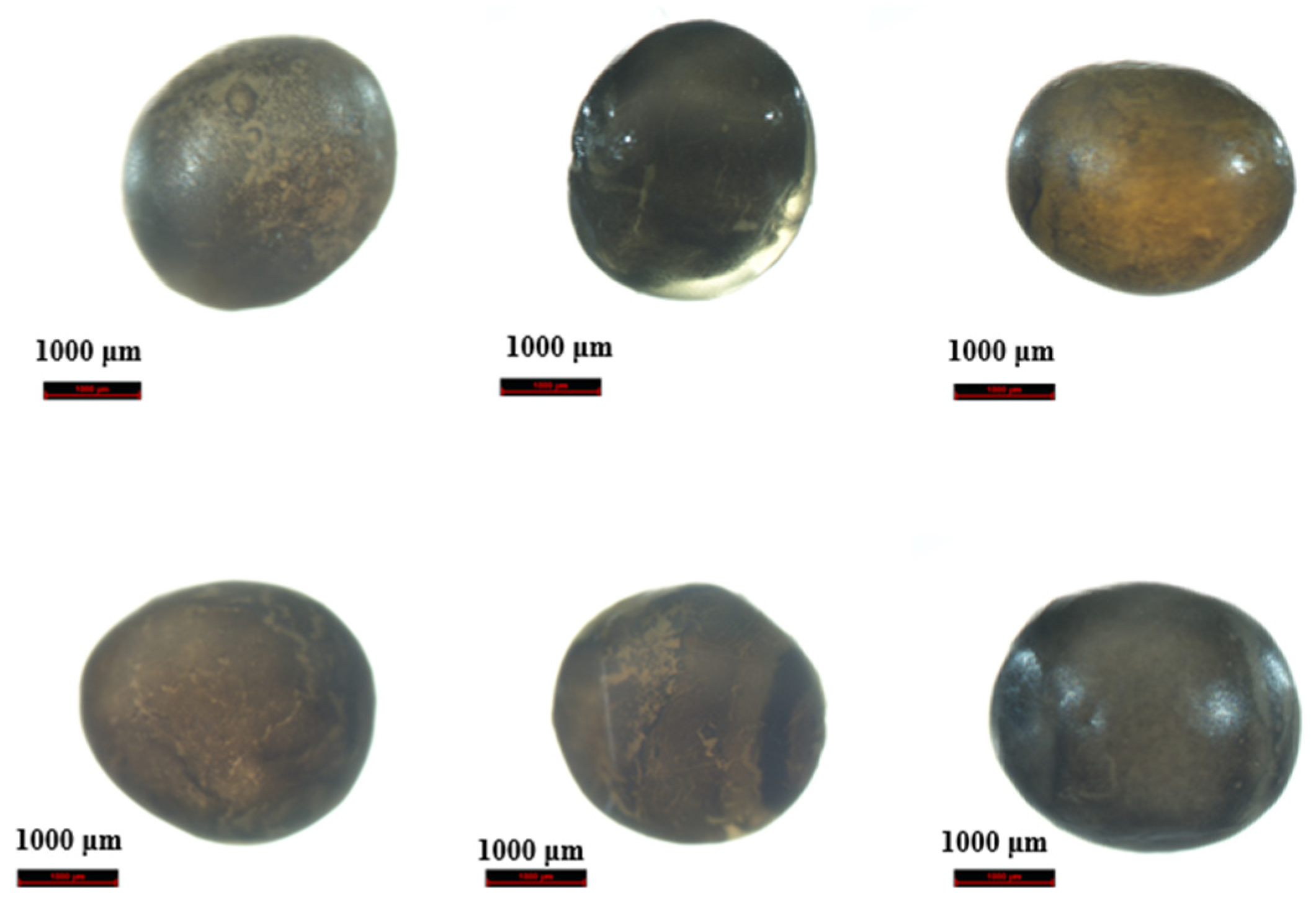
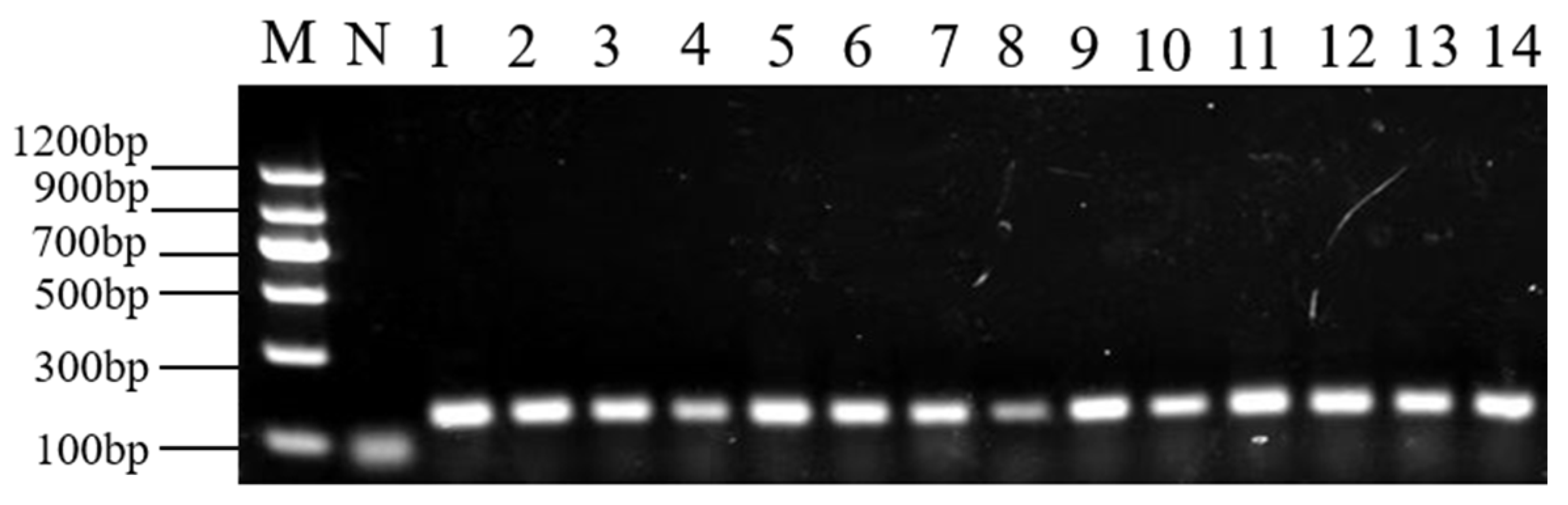
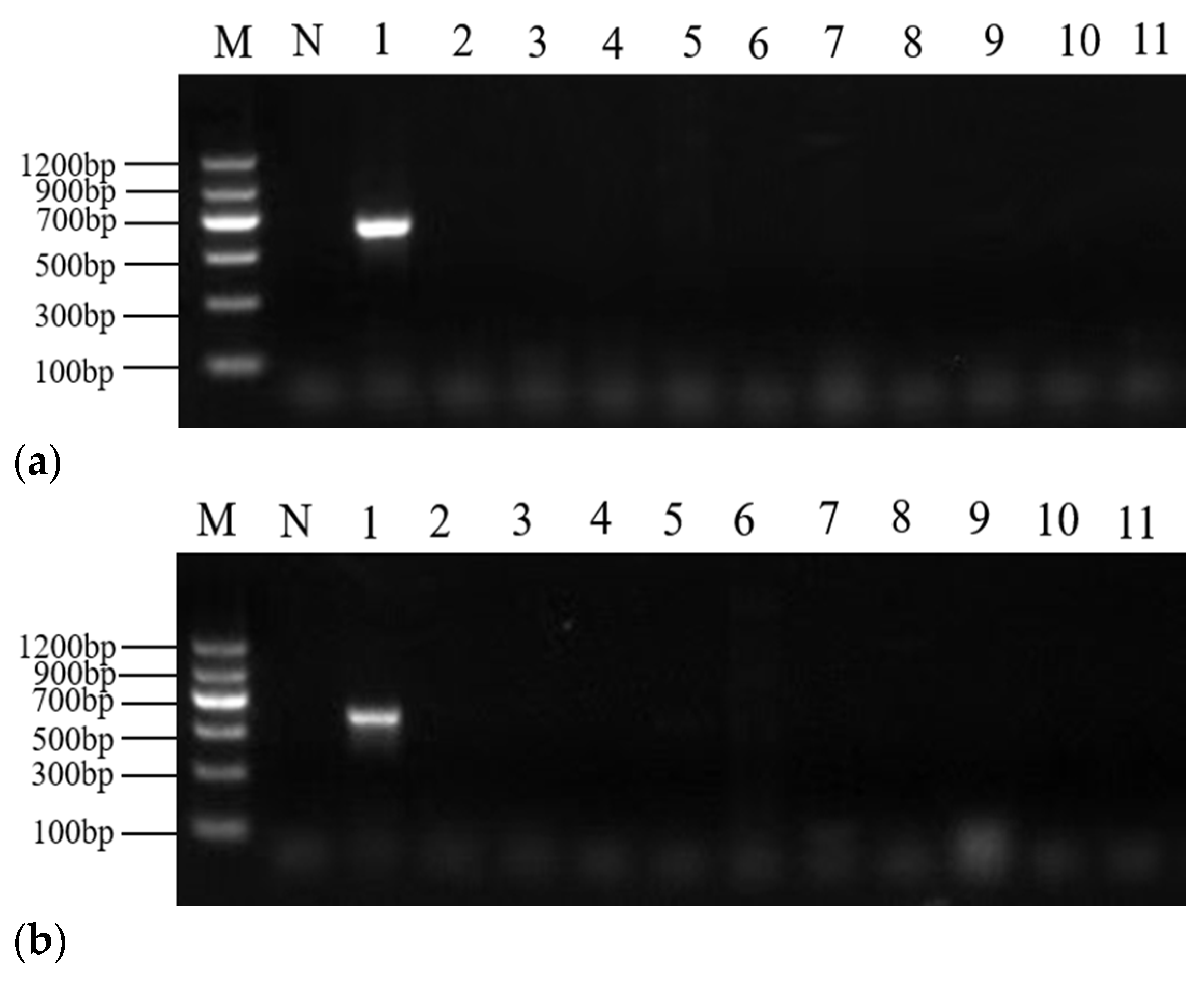


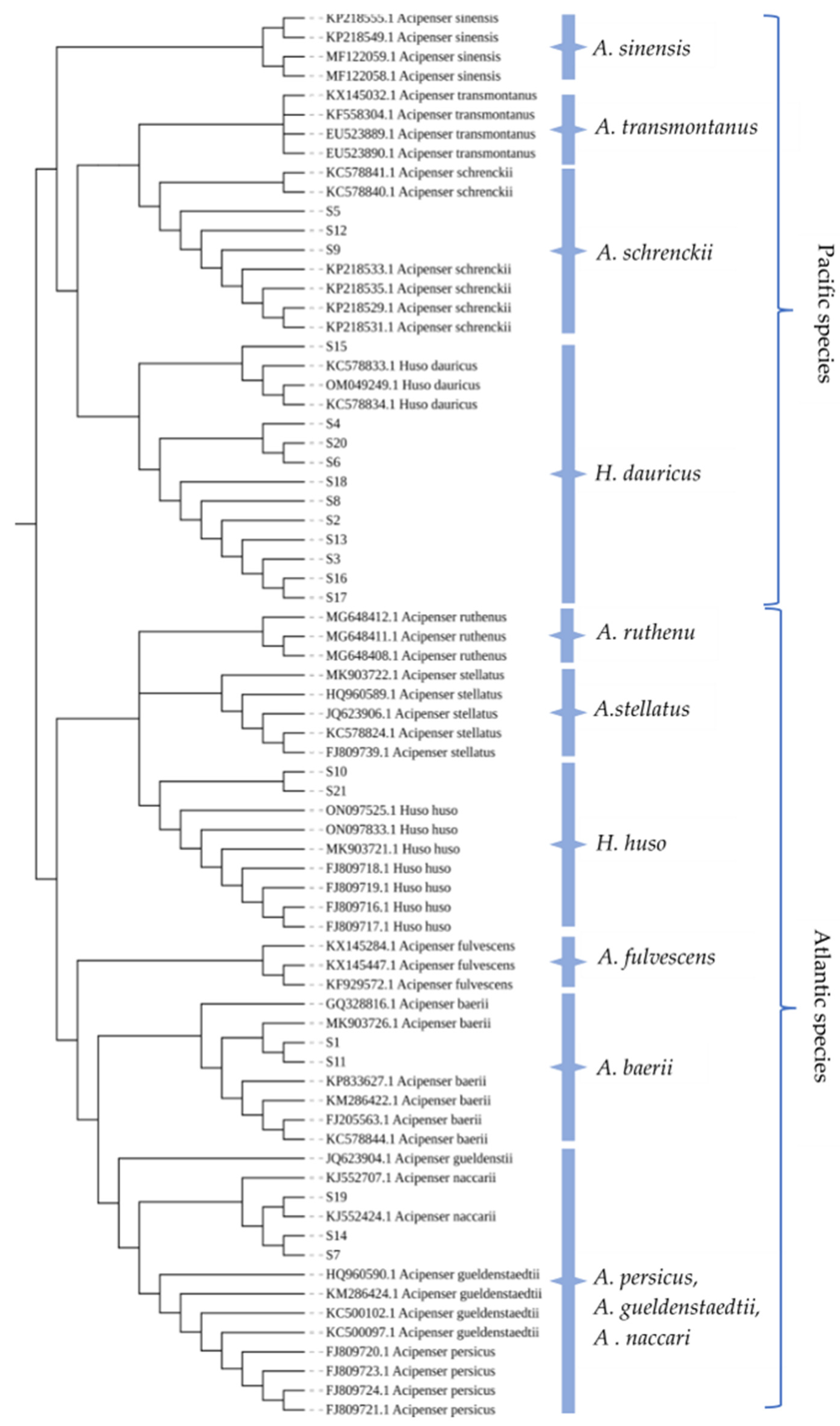

| No. | Labeled Species | Morphological Identification | Molecular Identification (Maternal) |
|---|---|---|---|
| S1 | H. dauricus × A. schrenckii | A. baerii | A. baerii |
| S2 | H. dauricus | H. dauricus | H. dauricus |
| S3 | A. baerii | A. gueldenstaedtii | H. dauricus |
| S4 | A. gueldenstaedtii | / | H. dauricus |
| S5 | A. baerii | / | A. schrenckii |
| S6 | H. dauricus × A. schrenckii | H. dauricus × A. schrenckii | H. dauricus |
| S7 | A. gueldenstaedtii | A. gueldenstaedtii | A. gueldenstaedtii |
| S8 | H. huso | H. dauricus | H. dauricus |
| S9 | A. schrenckii | A. schrenckii | A. schrenckii |
| S10 | H. huso | H. huso | H. huso |
| S11 | A. baerii | A. baerii | A. baerii |
| S12 | A. schrenckii | A. schrenckii | A. schrenckii |
| S13 | H. dauricus × A. schrenckii | / | H. dauricus |
| S14 | A. gueldenstaedtii | A. gueldenstaedtii | A. gueldenstaedtii |
| S15 | H. dauricus | H. dauricus | H. dauricus |
| S16 | A. baerii | H. dauricus × A. schrenckii | H. dauricus |
| S17 | A. schrenckii | H. dauricus | H. dauricus |
| S18 | H. dauricus × A. schrenckii | A. schrenckii | H. dauricus |
| S19 | A. gueldenstaedtii | A. gueldenstaedtii | A. gueldenstaedtii |
| S20 | H. dauricus | H. dauricus | H. dauricus |
| S21 | H. huso | H. huso | H. huso |
| No. | Trade Name | NCBI Database | BLOD Database | |||
|---|---|---|---|---|---|---|
| D-loop Gene (Primer Set III) | COI Gene (Primer Set I) | COI Gene (Primer Set II) | COI Gene (Primer Set I) | COI Gene (Primer Set II) | ||
| S1 * | Highbury Caviar | A. baerii (100%) | A. baerii/A. gueldenstaedtii (100%) | A. baerii/A. gueldenstaedtii (99.83%) | A. baerii (100%) | A. baerii (99.79%) |
| S2 | H. dauricus Caviar | H. dauricus (99.81%) | H. dauricus (99.84%) | H. dauricus (99.58%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S3 * | A. baerii Caviar | H. dauricus (99.81%) | H. dauricus (99.84%) | H. dauricus (99.79%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S4 * | A. gueldenstaedtii Caviar | H. dauricus (99.81%) | H. dauricus (99.69%) | H. dauricus (100%) | H. dauricus (99.85%) | H. dauricus (100%) |
| S5 * | A. baerii Caviar | A. schrenckii (99.80%) | A. schrenckii (100%) | A. schrenckii (99.79%) | A. schrenckii (100%) | A. schrenckii (99.81%) |
| S6 | Highbury Caviar | H. dauricus (99.81%) | H. dauricus (100%) | H. dauricus (100%) | H. dauricus (99.85%) | H. dauricus (100%) |
| S7 | A. gueldenstaedtii Caviar | A. gueldenstaedtii (100%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (100%) | A. gueldenstaedtii/A. persicus/A. naccari (99.83%) A. sinensis (99.83%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (99.79%) | A. gueldenstaedtii/A. naccari(100%) |
| S8 * | H. huso Caviar | H. dauricus (100%) | H. dauricus (99.84%) | H. dauricus (99.79%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S9 | A. schrenckii Caviar | A. schrenckii (99.81%) | A. schrenckii (99.86%) | A. schrenckii (99.58%) | A. schrenckii (99.86%) | A. schrenckii (99.63%) |
| S10 | H. huso Caviar | H. huso (99.78%) | H. huso (99.69%) | H. huso (99.65%) | H. huso (100%) | H. huso (99.65%) |
| S11 | A. baerii Caviar | A. baerii (99.78%) | A. baerii/A. gueldenstaedtii (100%) | A. baerii/A. gueldenstaedtii (99.82%) | A. baerii (100%) | A. baerii (99.79%) |
| S12 | 8-year Caviar | A. schrenckii (99.43%) | A. schrenckii (99.79%) | A. schrenckii (99.79%) | A. schrenckii (99.79%) | A. schrenckii (100%) |
| S13 | 9-year Caviar | H. dauricus (99.81%) | H. dauricus (98%) | H. dauricus (98%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S14 | 10-year Caviar | A. gueldenstaedtii (100%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (99.79%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (99.83%) | A. naccari (99.79%) | A. gueldenstaedtii (99.79%) |
| S15 | 15-year Caviar | H. dauricus (99.62%) | H. dauricus (99.79%) | H. dauricus (99.79%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S16 * | A. baerii Caviar | H. dauricus (99.81%) | H. dauricus (99.79%) | H. dauricus (99.79%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S17 * | A. schrenckii Caviar | H. dauricus (99.81%) | H. dauricus (100%) | H. dauricus (100%) | H. dauricus (100%) | H. dauricus (100%) |
| S18 | Highbury Caviar | H. dauricus (99.81%) | H. dauricus (100%) | H. dauricus (100%) | H. dauricus (100%) | H. dauricus (100%) |
| S19 | A. gueldenstaedtii Caviar | A. gueldenstaedtii (100%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (100%) | A. gueldenstaedtii/A. naccari/A. persicus/A. sinensis (100%) | A. naccari (100%) | A. gueldenstaedtii/A. naccari (100%) |
| S20 | H. dauricus Caviar | H. dauricus (99.81%) | H. dauricus (100%) | H. dauricus (99.79%) | H. dauricus (100%) | H. dauricus (99.79%) |
| S21 | H. huso Caviar | H. huso (99.81%) | H. huso (99.69%) | H. huso (99.65%) | H. huso (100%) | H. huso (99.65%) |
| No. | Caviar | Labeled Species |
|---|---|---|
| S1 | Highbury | H. dauricus ♀ × A. schrenckii♂ |
| S2 | H dauricus Caviar | H. dauricus |
| S3 | A. baerii Caviar | A. baerii |
| S4 | A. gueldenstaedtii Caviar | A. gueldenstaedtii |
| S5 | A. baerii Caviar | A. baerii |
| S6 | Highbury Caviar | H. dauricus ♀ × A. schrenckii♂ |
| S7 | A. gueldenstaedtii Caviar | A. gueldenstaedtii |
| S8 | H. huso Caviar | H. huso |
| S9 | A. schrenckii Caviar | A. schrenckii |
| S10 | H. huso Caviar | H. huso |
| S11 | A. baerii Caviar | A. baerii |
| S12 | 8-year Caviar | A. schrenckii |
| S13 | 9-year Caviar | H. dauricus ♀ × A. schrenckii♂ |
| S14 | 10-year Caviar | A. gueldenstaedtii |
| S15 | 15-year Caviar | H. dauricus |
| S16 | A. baerii Caviar | A. baerii |
| S17 | A. schrenckii Caviar | A. schrenckii |
| S18 | Highbury Caviar | H. dauricus ♀ × A. schrenckii♂ |
| S19 | A. gueldenstaedtii Caviar | A. gueldenstaedtii |
| S20 | H. dauricus Caviar | H. dauricus |
| S21 | H. huso Caviar | H. huso |
| Primer Category | Primer Name | Primer Sequence (from 5′ to 3′) | Product Size (bp) | Annealing Temperature (°C) | Primer References |
|---|---|---|---|---|---|
| 18S rRNA | 18S140F | TCTGCCCTATCAACTTTCGATGG | 140 | 56 | [37] |
| 18S140R | TAATTTGCGCGCCTGCTG | ||||
| Set I (COI) | Fish F2-t1 | TGTAAAACGACGGCCAGT CGACTAATCATAAAGATATCGGCAC | 680 | 54 | [24] |
| Fish R2-t1 | CAGGAAACAGCTATGAC ACTTCAGGGTGACCGAAGAATCAGAA | ||||
| F2-t1 | TGTAAAACGACGGCCAGT CAACCAACCACAAAGACATTGGCAC | ||||
| FR1d-t1 | CAGGAAACAGCTATGAC ACCTCAGGGTGTCC | ||||
| Set II (COI) | Stur-COIF | TGTAAAACGACGGCCAGT ACTGACTRGTSCCCCTAAT | 583 | 56 | Design of this study |
| Stur-COIR | CAGGAAACAGCTATGAC CTATGTARCCAAAAGGTTC | ||||
| Set III (D-loop) | SturDF | TGTAAAACGACGGCCAGT ATGTARTAAGAGCCGAACA | 520 | 54 | Design of this study |
| SturDR | CAGGAAACAGCTATGAC AGTCAGTCCTGCTTTTGG | ||||
| tRNA-Leu | tRNAleu-F | CGAAATCGGTAGACGCTACG | 180 | 60 | [32] |
| tRNAleu-R | TTCCATTGAGTCTCTGCACCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Pan, Y.; Xia, H.; Yu, K.; Yao, Y.; Guan, F. Species Identification of Caviar Based on Multiple DNA Barcoding. Molecules 2023, 28, 5046. https://doi.org/10.3390/molecules28135046
Hu Q, Pan Y, Xia H, Yu K, Yao Y, Guan F. Species Identification of Caviar Based on Multiple DNA Barcoding. Molecules. 2023; 28(13):5046. https://doi.org/10.3390/molecules28135046
Chicago/Turabian StyleHu, Qingqing, Yingqiu Pan, Huili Xia, Kexin Yu, Yian Yao, and Feng Guan. 2023. "Species Identification of Caviar Based on Multiple DNA Barcoding" Molecules 28, no. 13: 5046. https://doi.org/10.3390/molecules28135046
APA StyleHu, Q., Pan, Y., Xia, H., Yu, K., Yao, Y., & Guan, F. (2023). Species Identification of Caviar Based on Multiple DNA Barcoding. Molecules, 28(13), 5046. https://doi.org/10.3390/molecules28135046





