Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Pot Experiment
3.2. Saturation of Water with Ozone
3.3. Measurement of O3 Concentration in H2O
3.3.1. Iodometric Method
3.3.2. Spectrophotometric Method
3.4. Measurement of Dissolved Anions in H2O
3.5. Antioxidant Potential, Polyphenol and Vitamin C Contents
3.6. Polyphenolic Compounds Profiles Analysis
3.7. SOD, CAT, PPO and PAL Activity
3.8. ROS Level Analysis
3.9. Relative Chlorophyll Content in Leaves (SPAD)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Song, J.S.; Lee, M.J.; Ra, J.E.; Lee, K.S.; Eom, S.; Ham, H.M.; Kim, H.J.; Kim, S.B.; Lim, J. Growth and bioactive phytochemicals in barley (Hordeum vulgare L.) sprouts affected by atmospheric pressure plasma during seed germination. J. Phys. D Appl. Phys. 2020, 53, 314002. [Google Scholar] [CrossRef]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant activity of flavonoids isolated from young green barley leaves toward biological lipid samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Sharma, P.; Goudar, G.; Longvah, T.; Gour, V.S.; Kothari, S.L.; Wani, I.A. Fate of Polyphenols and Antioxidant Activity of Barley during Processing. Food Rev. Int. 2022, 38, 163–198. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Kapusta, I.; Królikowski, K.; Balawejder, M. Induction of Biosynthesis Antioxidant Molecules in Young Barley Plants by Trioxygen. Molecules 2022, 27, 7195. [Google Scholar] [CrossRef]
- Březinová Belcredi, N.; Ehrenbergerová, J.; Fiedlerová, V.; Běláková, S.; Vaculová, K. Antioxidant Vitamins in Barley Green Biomass. J. Agric. Food Chem. 2010, 58, 11755–11761. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Naidu, R. Impact of water and fertilizer management on arsenic bioaccumulation and speciation in rice plants grown under greenhouse conditions. Chemosphere 2019, 214, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Matlok, N.; Piechowiak, T.; Gorzelany, J.; Zardzewiały, M.; Balawejder, M. Effect of Ozone Fumigation on Physiological Processes and Bioactive Compounds of Red-Veined Sorrel (Rumex sanguineus ssp. sanguineus). Agronomy 2020, 10, 1726. [Google Scholar] [CrossRef]
- Polyzos, N.; Paschoalinotto, B.; Compocholi, M.; Dias, M.I.; Barros, L.; Petropoulos, S.A. The Effects of Fertilization Regime on the Growth Parameters and Bioactive Properties of Pot-Grown Cichorium spinosum L. Plants. Biol. Life Sci. Forum 2022, 16, 6. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Effects of Ozone Treatment on Microbial Status and the Contents of Selected Bioactive Compounds in Origanum majorana L. Plants. Plants 2020, 9, 1637. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Pieniążek, M.; Antos, P.; Witek, G.; Szostek, M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability 2019, 11, 5287. [Google Scholar] [CrossRef]
- Darwish, T.; Atallah, T.; Hajhasan, S.; Chranek, A. Management of nitrogen by fertigation of potato in Lebanon. Nutr. Cycl. Agroecosyst. 2003, 67, 1–11. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The Modification of Substrate in the Soilless Cultivation of Raspberries (Rubus idaeus L.) as a Factor Stimulating the Biosynthesis of Selected Bioactive Compounds in Fruits. Molecules 2023, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, Bioactive Compounds, Antioxidant Capacity, and Enzymes of Raspberries at Different Maturity Stages, Effects of Organic vs. Conventional Fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The effects of ozone treatments on the agro-physiological parameters of tomato plants and the soil microbial community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Espin, A.; Garcia-Caparros, P.; Llanderal, A.; Colunje, J.; Moreira, J.F.; Lao, M.T. Physiological and Nutritional Responses to Ozone Application in Tomato Seedling Plants. Agriculture 2023, 13, 60. [Google Scholar] [CrossRef]
- Mahmoud Yousif, M.N.; Abdelhameed, R.M.; Abu-Hashem, A.A.; Yousif, N.M. Synthesis and Biological Activity of Chromene Derivatives, chromeno [2, 3-d] [1, 3] oxazine derivatives, and chromeno [2, 3-d] pyrimidine derivatives. Egypt. J. Chem. 2023, 66, 113–120. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Al-Hussain, S.A.; Zaki, M.E.A. Synthesis of Novel Benzodifuranyl; 1,3,5-Triazines; 1,3,5-Oxadiazepines; and Thiazolopyrimidines Derived from Visnaginone and Khellinone as Anti-Inflammatory and Analgesic Agents. Molecules 2020, 25, 220. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A. Synthesis of New Furothiazolo Pyrimido Quinazolinones from Visnagenone or Khellinone and Antimicrobial Activity. Molecules 2018, 23, 2793. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Nitric Oxide and Hydrogen Peroxide Signaling in Higher Plants; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-11128-1. [Google Scholar] [CrossRef]
- Stan, H.J.; Schicker, S. Effect of repetitive ozone treatment on bean plants—Stress ethylene production and leaf necrosis. Atmos. Environ. 1982, 16, 2267–2270. [Google Scholar] [CrossRef]
- Cavendish, H. Experiments on air. Philos. Trans. 1785, 75, 372. [Google Scholar]
- Xiao, C.L.; Subbarao, K.V. Effects of irrigation and Verticillium dahliae on cauliflower root and shoot growth dynamics. Phytopathology 2000, 90, 995–1004. [Google Scholar] [CrossRef]
- Arbogast, M.; Powelson, M.L.; Cappaert, M.R.; Watrud, L.S. Response of six potato cultivars to amount of applied water and Verticillium dahliae. Phytopathoogy 1999, 89, 782–788. [Google Scholar] [CrossRef]
- Rodríguez, E.; García-Garrido, J.M.; García, P.A.; Campos, M. Agricultural factors affecting Verticillium wilt in olive orchards in Spain. Eur. J. Plant Pathol. 2008, 122, 287–295. [Google Scholar] [CrossRef]
- Pasqua, V.; Paciolla, C.; Sasanelli, N.; De Leonardis, S.; Melillo, M.T. Ozonated water reduces susceptibility in tomato plants to Meloidogyne incognita by the modulation of the antioxidant system. Mol. Plant Pathol. 2017, 18, 529–539. [Google Scholar] [CrossRef]
- Fujiwara, K.; Fujii, T. Effects of spraying ozonated water on the severity of powdery mildew infection on cucumber leaves. Ozone Sci. Eng. 2002, 24, 463–469. [Google Scholar] [CrossRef]
- Fujiwara, K.; Fujii, T.; Park, J.S. Comparison of foliar spray efficacy ofelectrolytically ozonated water and acidic electrolyzed oxidizing water for control-ling powdery mildew infection on cucumber leaves. Ozone Sci. Eng. 2009, 31, 10–14. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 9. [Google Scholar] [CrossRef]
- Gutiérrez, D.R.; Lemos, L.; Rodríguez, S.D.C. Effect of UV-C and Ozone on the Bioactive Compounds and Antioxidant Capacity of Minimally Processed Rocket (Eruca Sativa Mill.). Int. J. New Technol. Res. 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Markiewicz-Żukowska, R.; Nowakowski, P.; Naliwajko, S.K.; Moskwa, J.; Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Mielcarek, K.; Soroczyńska, J.; et al. Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies. Antioxidants 2021, 10, 1402. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Brauch, D.; Porzel, A.; Schumann, E.; Pillen, K.; Mock, H.P. Changes in isovitexin-O-glycosylation during the development of young barley plants. Phytochemistry 2018, 148, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-κB and MAPK on LPS-induced RAW 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, Ö.; Dursun, E.; Bulent, Y.; Okur, B. Yield and quality of garden cress affected by different nitrogen sources and growing period. Afr. J. Agric. Res. 2011, 6, 608–617. [Google Scholar]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Lau, V.; Mattson, N. Effects of Hydrogen Peroxide on Organically Fertilized Hydroponic Lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 106. [Google Scholar] [CrossRef]
- Jason’s Guide to Organic and Hydroponic Gardening. Available online: https://www.jasons-indoor-guide-to-organic-and-hydroponics-gardening.com/using-hydrogen-peroxide.html (accessed on 23 January 2019).
- Noda, T.; Iimure, K.; Okamoto, S.; Saito, A. Expression analysis of polyphenol oxidase isozymes by active staining method and tissue browning of head lettuce (Lactuca sativa L.). Biosci. Biotechnol. Biochem. 2017, 81, 1484–1488. [Google Scholar] [CrossRef]
- Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Physiological Response of Maize Plants (Zea mays L.) to the Use of the Potassium Quercetin Derivative. Int. J. Mol. Sci. 2021, 22, 7384. [Google Scholar] [CrossRef]
- Pellinen, R.; Palva, T.; Kangasjärvi, J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999, 20, 349–356. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud., A.A.C.; Fotopoulos., V. Biostimulants for the Regulation of Reactive Oxygen Species Metabo-lism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Arena, C.; Vitale, E.; Rahimi, A.; Mirzapour, M.; Nasar, J.; Kisaka, O.; Sow, S.; Ranjan, S.; Gitari, H. Impact of Different Fertilizer Sources under Supplemental Irrigation and Rainfed Conditions on Eco-Physiological Responses and Yield Characteristics of Dragon’s Head (Lallemantia iberica). Plants 2023, 12, 1693. [Google Scholar] [CrossRef] [PubMed]
- Matlok, N.; Szostek, M.; Antos, P.; Gajdek, G.; Gorzelany, J.; Bobrecka-Jamro, D.; Balawejder, M. Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Appl. Sci. 2020, 10, 2579. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Blicharz-Kania, A.; Andrejko, D.; Kluza, F.; Rydzak, L.; Kobus, Z. Assessment of the Potential Use of Young Barley Shoots and Leaves for the Production of Green Juices. Sustainability 2019, 11, 3960. [Google Scholar] [CrossRef]
- Yuan, D.; Xie, S.; Ding, C.; Lin, F.; He, Y.; Wang, Z.; Cen, K. The Benefits of Small Quantities of Nitrogen in the Oxygen Feed to Ozone Generators. Ozone Sci. Eng. 2018, 40, 313–320. [Google Scholar] [CrossRef]
- Sentman, D.D.; Stenbaek-Nielsen, H.C.; McHarg, M.G.; Morrill, J.S. Plasma chemistry of sprite streamers. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Osawaa, N.; Tsuji, T.; Ogiso, R.; Yoshioka, Y. Effect of nitrogen addition to ozone generation characteristics by diffuse and filamentary dielectric barrier discharges at atmospheric pressure. Eur. Phys. J. Appl. Phys. 2017, 78, 20804. [Google Scholar] [CrossRef]
- Wood, E.C.; Herndon, S.C.; Onasch, T.B.; Kroll, J.H.; Canagaratna, M.R.; Kolb, C.E.; Worsnop, D.R.; Neuman, J.A.; Seila, R.; Zavala, M.; et al. A case study of ozone production, nitrogen oxides, and the radical budget in Mexico City. Atmos. Chem. Phys. 2009, 9, 2499–2517. [Google Scholar] [CrossRef]
- Dreschoff, G.; Boyarchuk, K.A.; Jungner, H.; Kocharov, G.E.; Koudriavtsev, I.V.; Mursula, K. Nitrate Generation in the Earth’s Atmosphere by Cosmic Rays. In Proceedings of the 26th International Cosmic Ray Conference, Salt Lake City, UT, USA, 17–25 August 1999; Kieda, D., Salamon, M., Dingus, B., Eds.; Under the auspices of the International Union of Pure and Applied Physics (IUPAP). Volume 4, pp. 318–321. [Google Scholar]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen fertilisation decreases the yield of bioactive compounds in Carlina acaulis L. grown in the field. Ind. Crop. Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Janik, I.; Bartels, D.M.; Jonah, C.D. Hydroxyl radical self-recombination reaction and absorption spectrum in water up to 350 degrees C. J. Phys. Chem. A 2007, 111, 1835–1843. [Google Scholar] [CrossRef]
- Bulusu, R.K.M.; Wandell, R.J.; Gallan, R.O.; Locke, B.R. Nitric oxide scavenging of hydroxyl radicals in a nanosecond pulsed plasma discharge gas–liquid reactor. J. Phys. D Appl. Phys. 2019, 52, 504002. [Google Scholar] [CrossRef]
- Kasper, J.M.; Clausen, C.A.; Cooper, C.D. Control of Nitrogen Oxide Emissions by Hydrogen Peroxide-Enhanced Gas-Phase Oxidation of Nitric Oxide. J Air Waste Manag. Assoc. 1996, 46, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Matłok, N.; Piechowiak, T.; Królikowski, K.; Balawejder, M. Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating. Agriculture 2022, 12, 662. [Google Scholar] [CrossRef]
- Józefczyk, R.; Antos, P.; Pieniążek, M.; Balawejder, M. Procedure for detoxication of linuron contaminated soil based on ozonation and fluidization process. Arch. Environ. Prot. 2022, 48, 3. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of Organic and Mineral Fertilization on Yield and Selected Quality Parameters for Dried Herbs of Two Varieties of Oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. AFSci 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Matłok, N.; Kapusta, I.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Characterisation of Some Phytochemicals Extracted from Black Elder (Sambucus nigra L.) Flowers Subjected to Ozone Treatment. Molecules 2021, 26, 5548. [Google Scholar] [CrossRef]
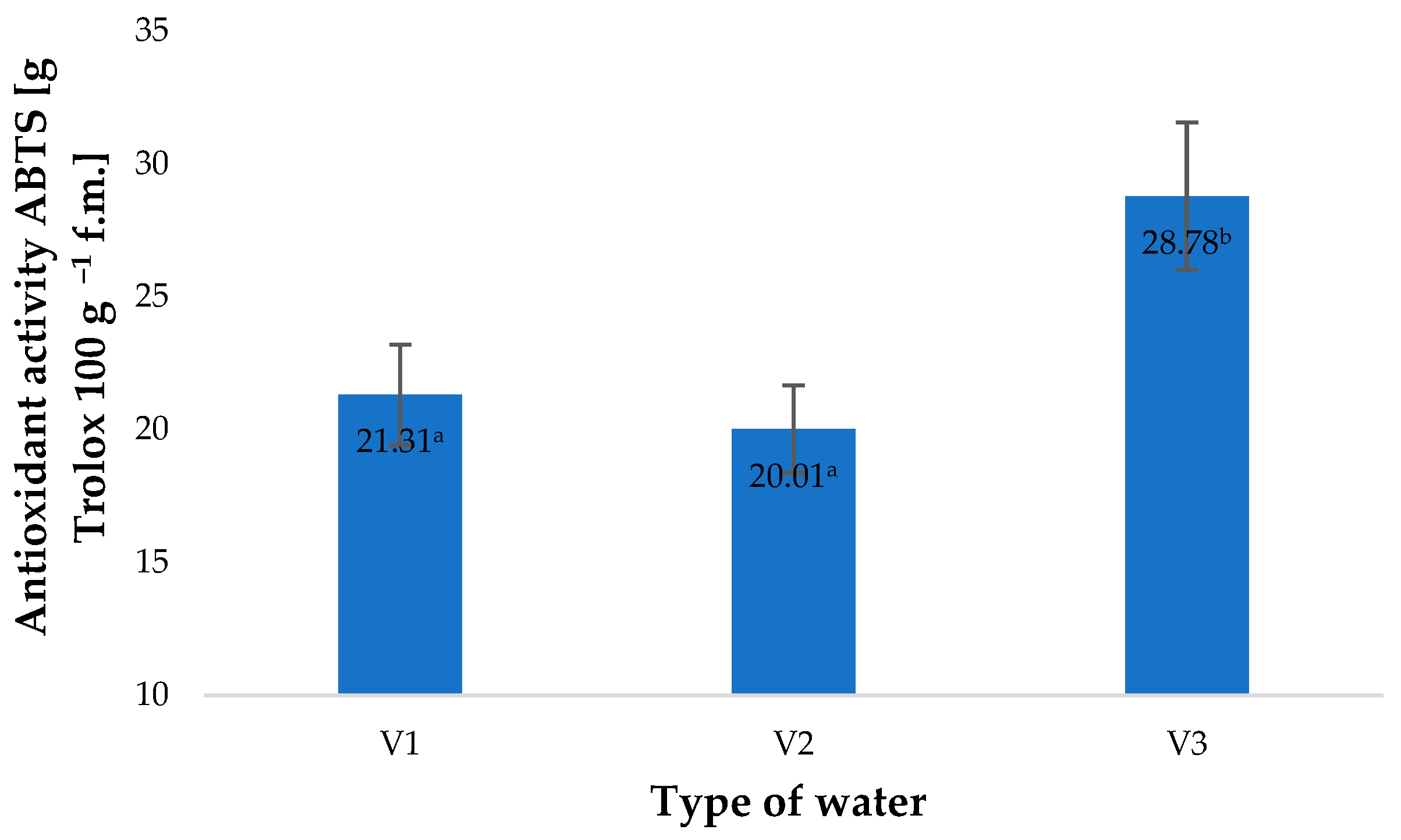
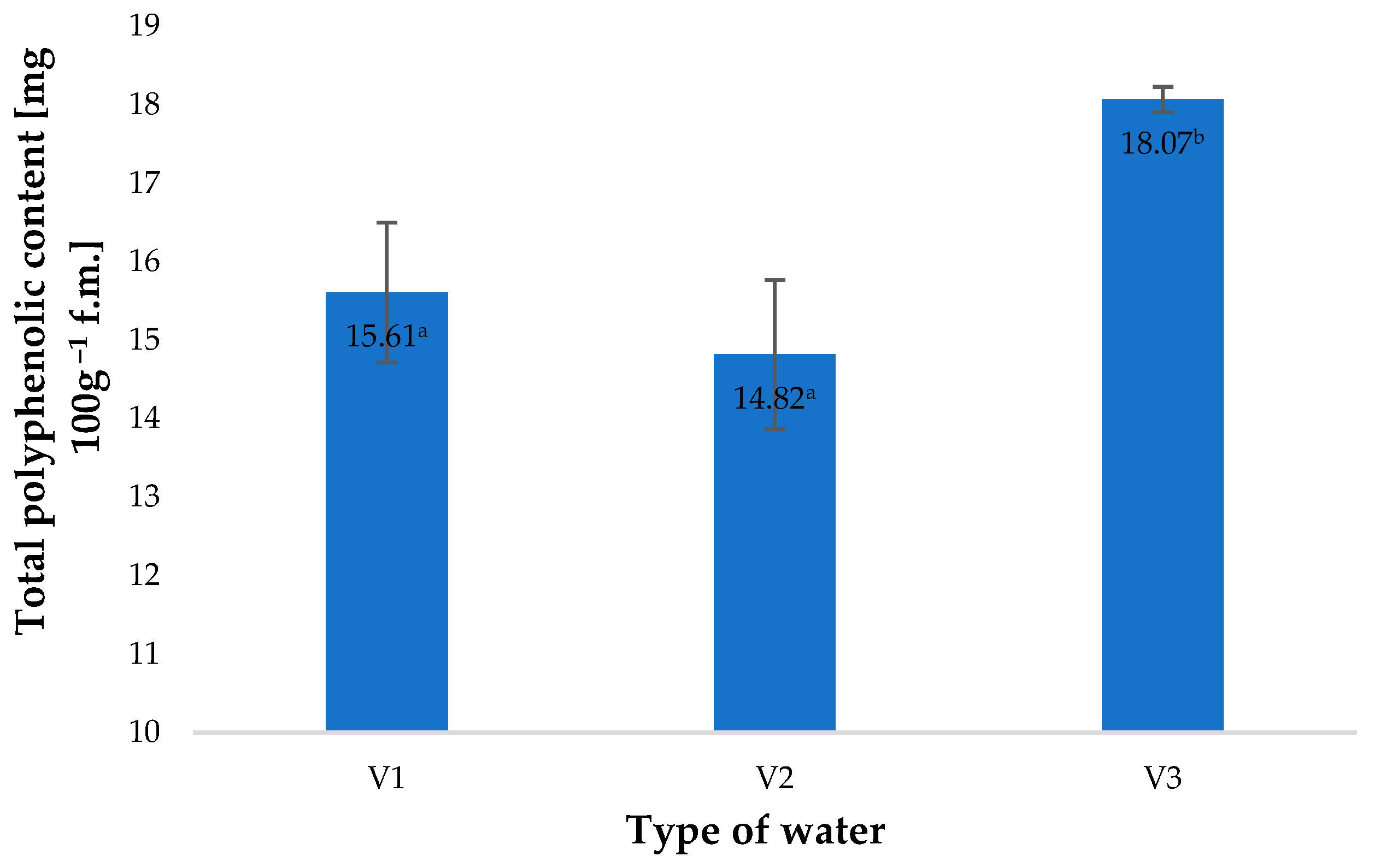

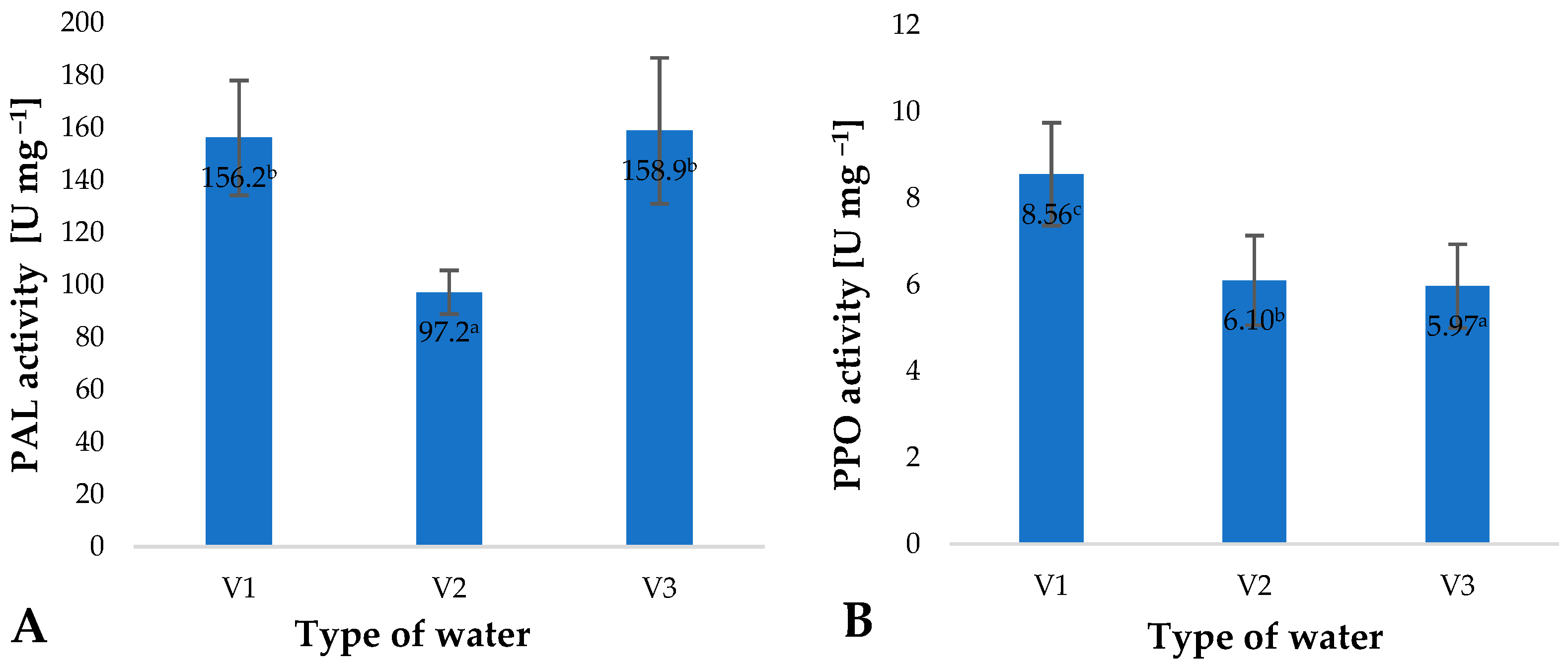
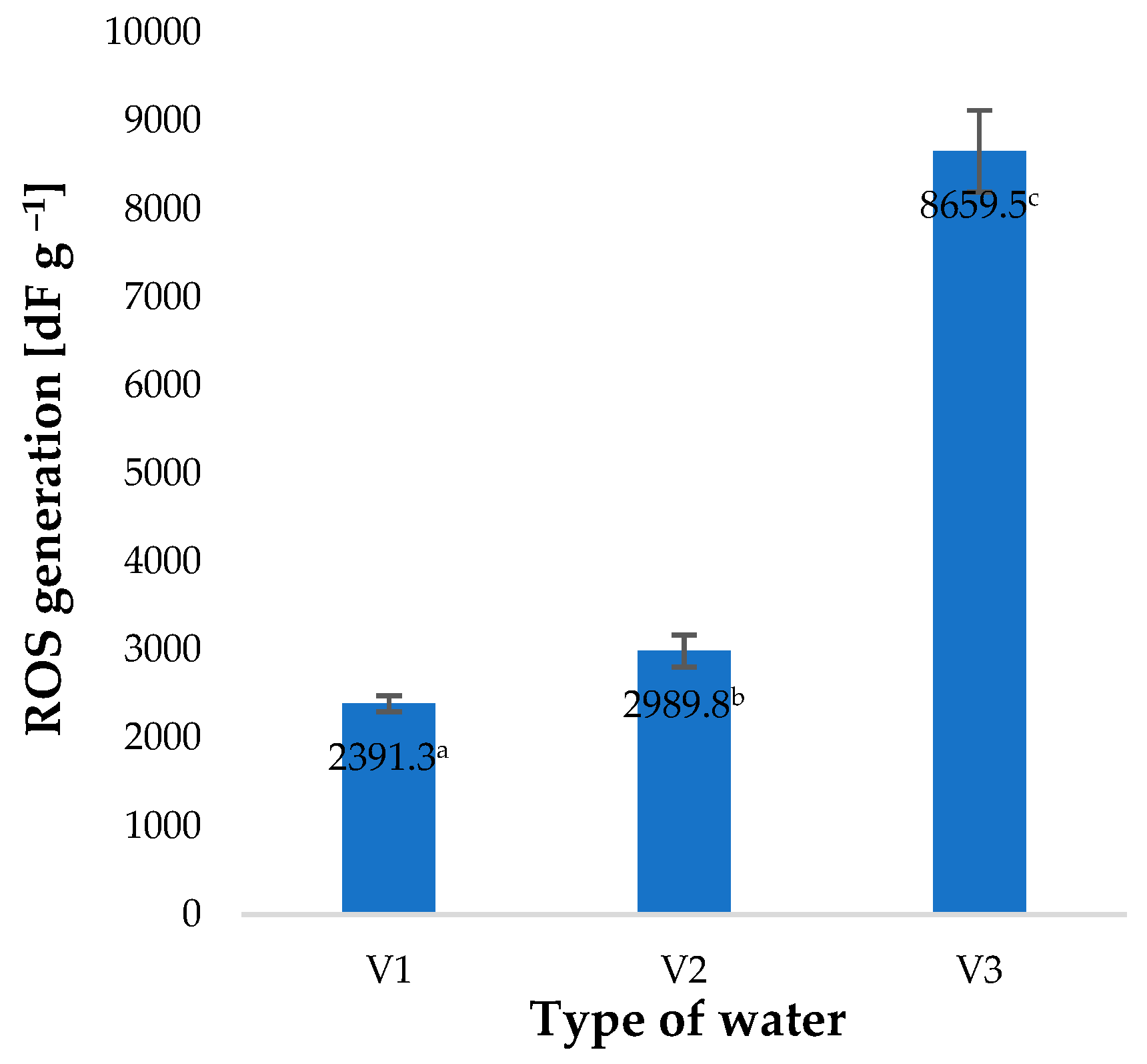
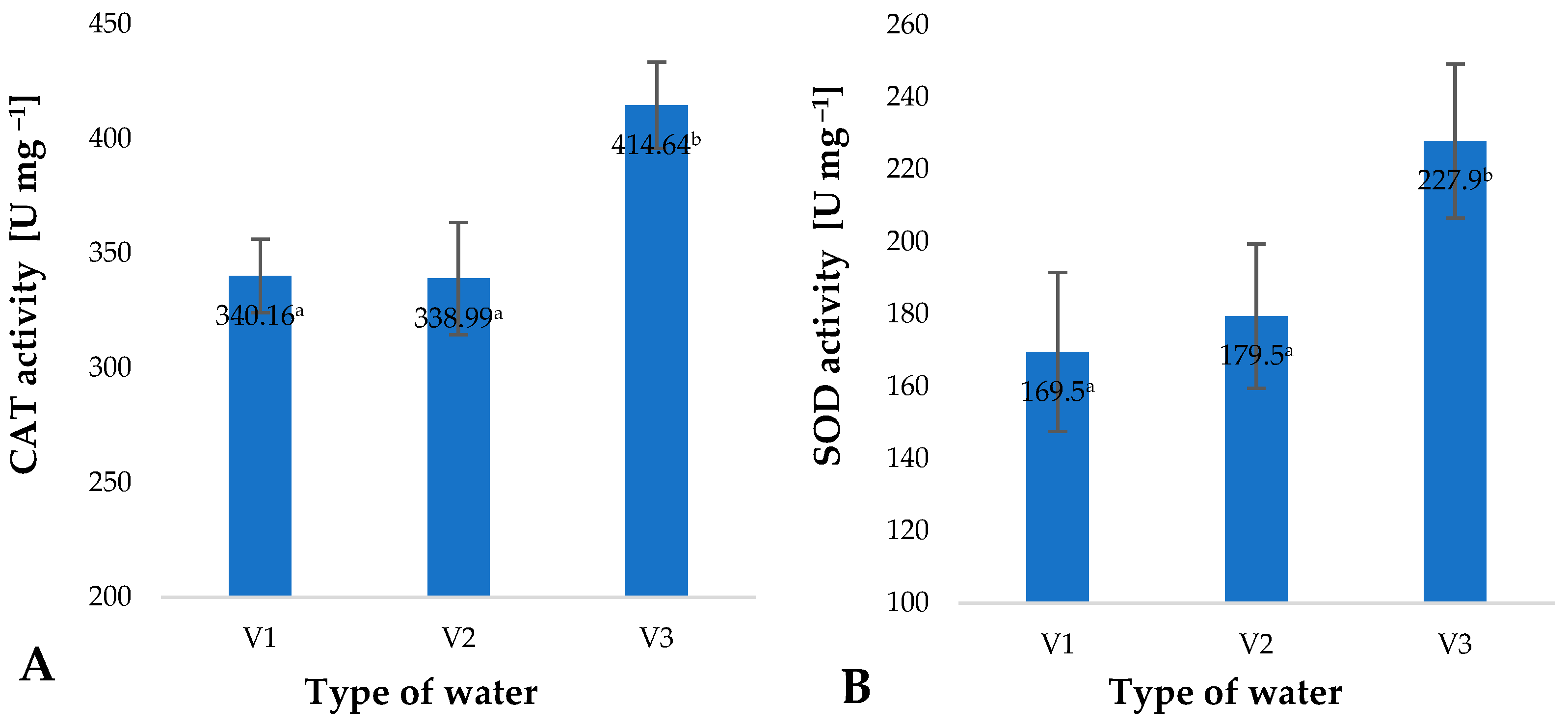
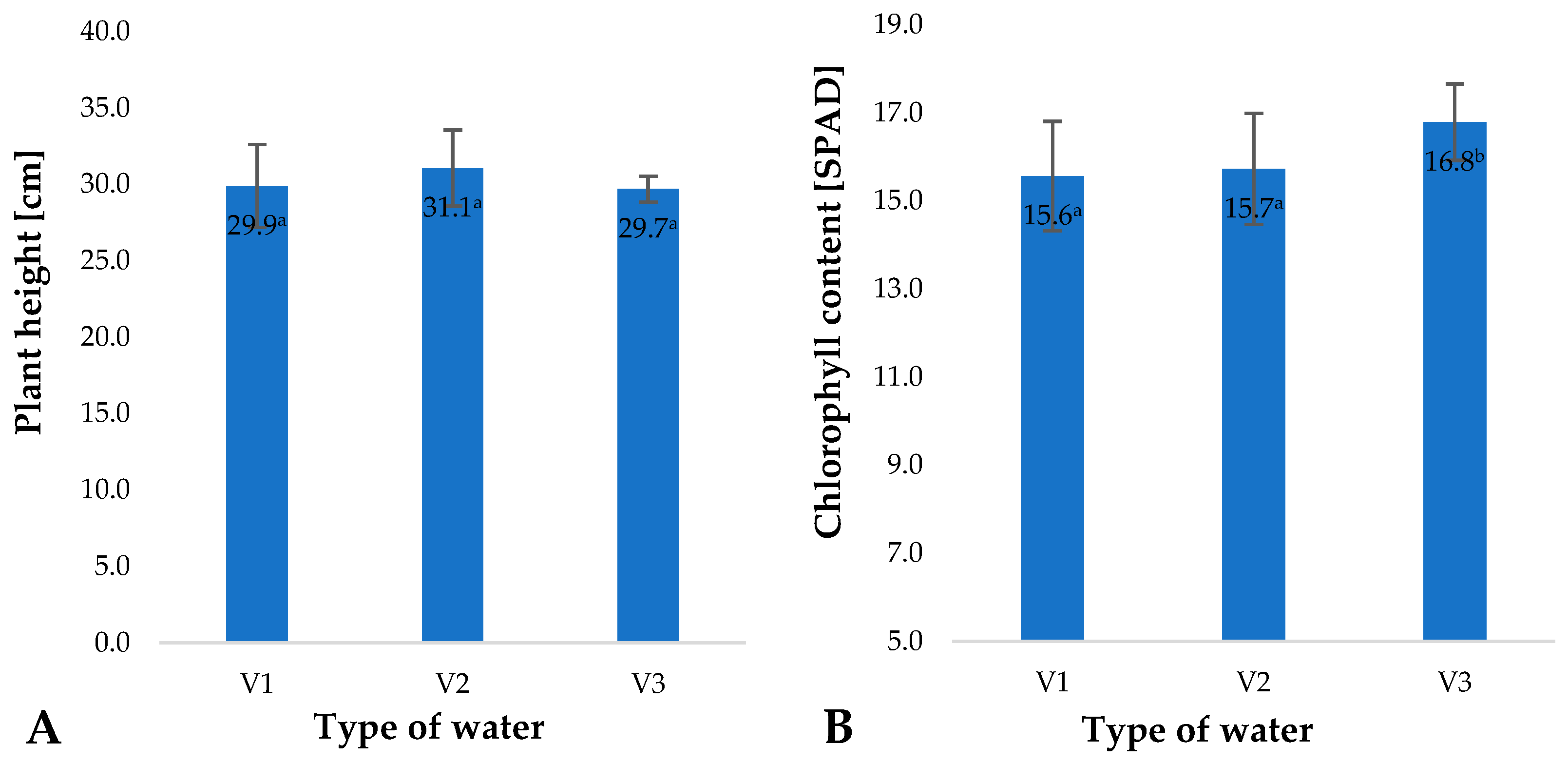
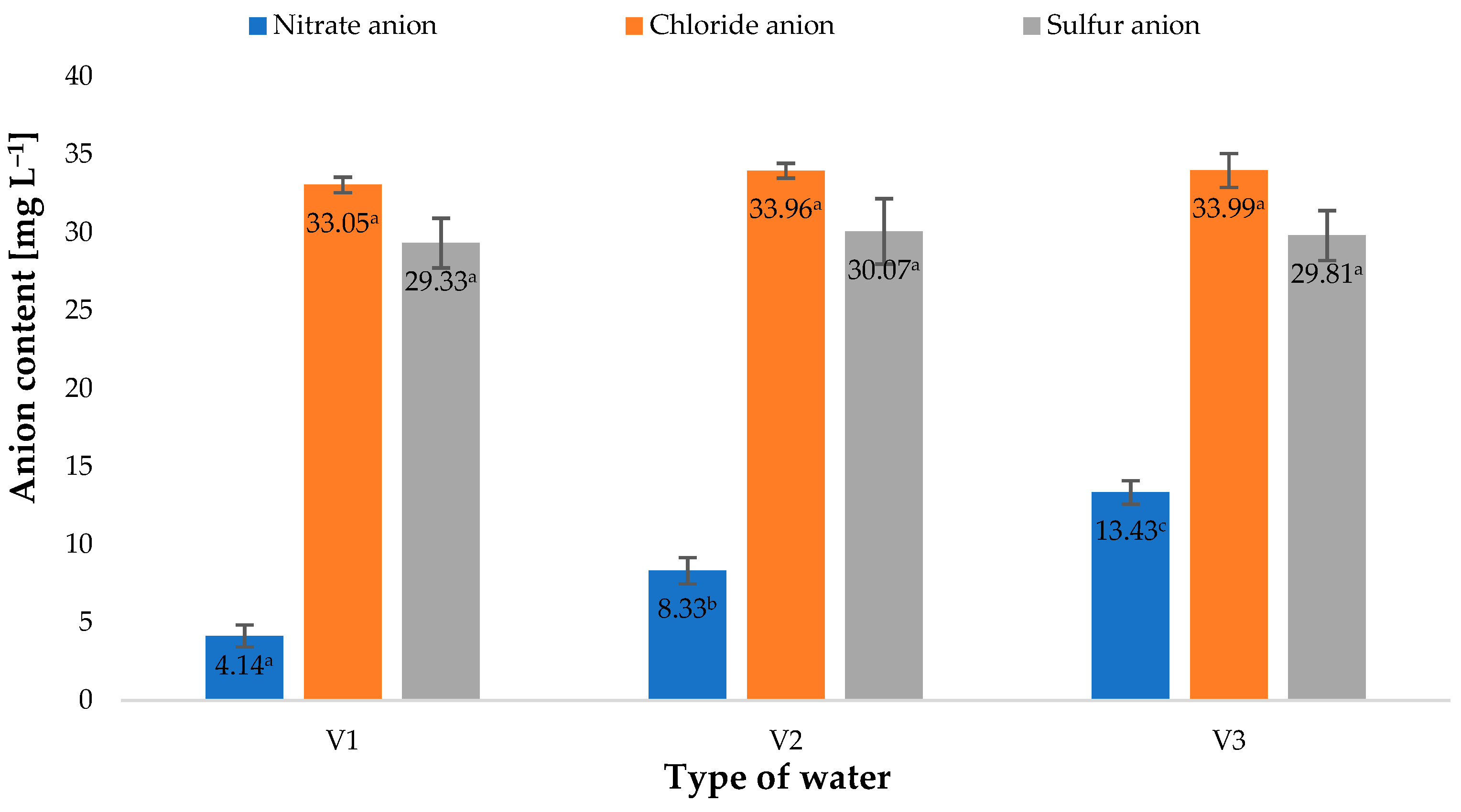

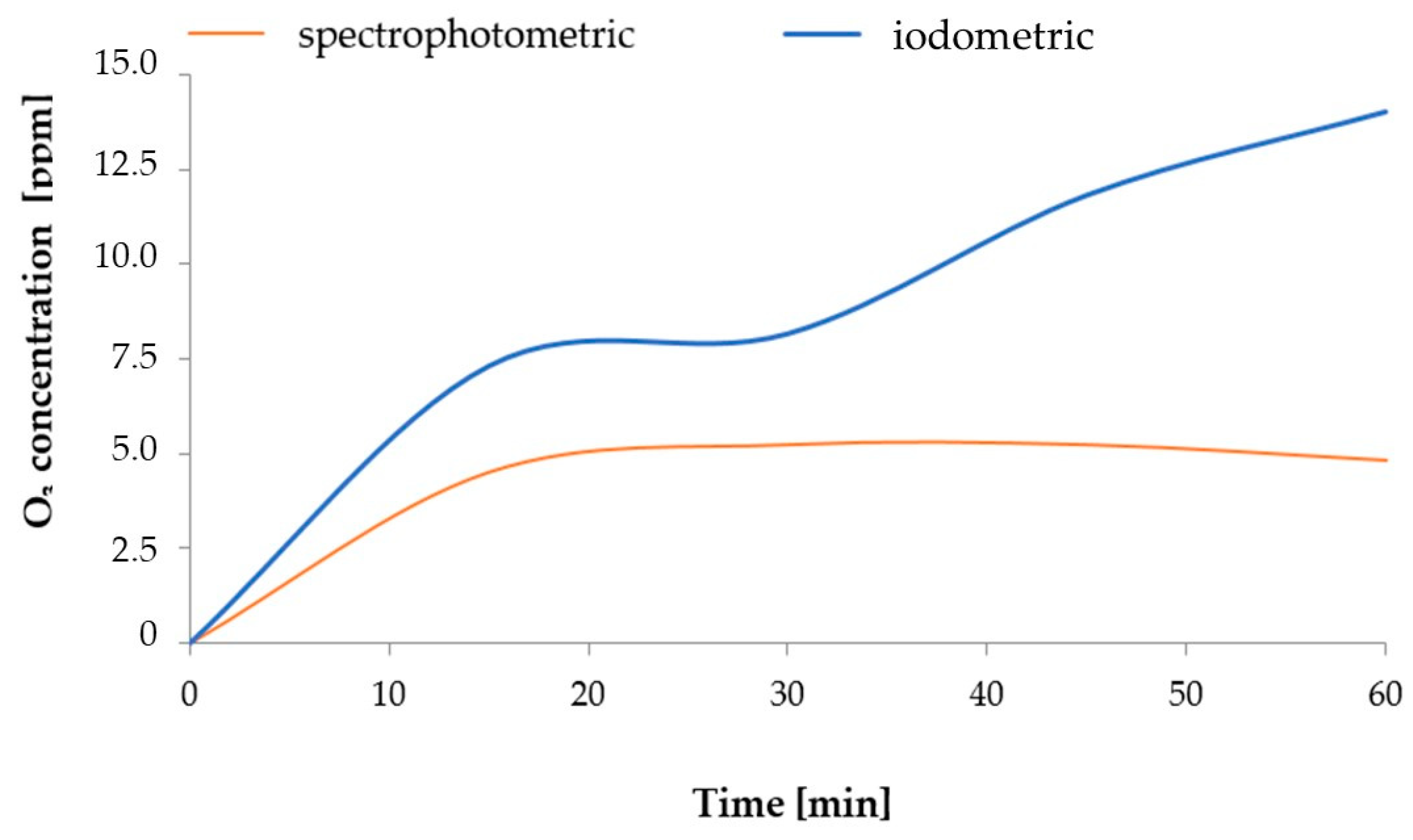
| Type of Water | pH | Iodometric Concentration of O3 [ppm] | Spectrophotometric Concentration of O3 [ppm] | Oxidizability [mmol S2O4−] |
|---|---|---|---|---|
| V1 | 8.0 | 0 | 0 | 0 |
| V2 | 8.4 | 7.68 | 3.0 | 0.008 |
| V3 | 8.5 | 4.80 | 0.5 | 0.005 |
| Compound | Rt | λmax | [M − H]− m/z | Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| min | nm | MS | MS/MS | V1 | V2 | V3 | ||
| 1 | p-coumaric acid | 2.54 | 309 | 163 | 119 | 0.13 a | 1.20 b | 0.16 a |
| 2 | Caftaric acid | 2.66 | 288sh. 327 | 311 | 179 | 0.23 a | 0.91 c | 0.32 b |
| 3 | Feruloyl-caffeic acid | 3.05 | 288sh. 322 | 367 | 193 | 0.88 b | 0.42 a | 0.83 b |
| 4 | Unspecified caffeic derivative | 3.13 | 288sh. 324 | 425 | 367 | 0.44 a | 0.41 a | 0.42 a |
| 5 | Luteolin 6-C-arabinoside-8-C-glucoside | 3.22 | 274. 341 | 579 | 447. 285 | 0.36 a | 0.64 b | 0.35 a |
| 6 | Isoscoparin 7-O-glucoside | 3.32 | 276. 333 | 623 | 461. 299 | 0.65 b | 0.26 a | 0.58 b |
| 7 | Isoorientin 7-O-glucoside | 3.48 | 269. 347 | 609 | 447. 285 | 0.27 a | 0.33 a | 0.32 a |
| 8 | Isovitexin 7-O-glucoside | 3.60 | 269. 331 | 593 | 431 | 86.63 a | 83.93 a | 86.71 a |
| 9 | Isovitexin 7-O-(6′-p-coumaroyl)-glucoside | 3.67 | 271. 324 | 739 | 593 | 2.10 a | 3.71 b | 2.46 a |
| 10 | Isovitexin | 3.84 | 270. 338 | 431 | 269 | 0.64 a | 0.62 a | 0.63 a |
| 11 | Isoorientin 7-O-(6″-sinapoyl)-glucoside | 3.99 | 271. 338 | 815 | 447 | 0.74 b | 0.63 a | 0.61 a |
| 12 | Isoorientin 7-O-(6″-feruloyl)-glucoside | 4.26 | 271. 338 | 785 | 447 | 0.70 a | 0.75 a | 0.72 a |
| 13 | Isovitexin 7-O-(6″-sinapoyl)-glucoside | 4.57 | 271. 334 | 799 | 431 | 1.66 a | 1.88 b | 1.39 a |
| 14 | Isovitexin 7-O-(6″-sinapoyl)-glucoside-4′-O-glucoside | 4.67 | 270. 338 | 961 | 799. 593 | 0.43 b | 0.39 a | 0.34 a |
| 15 | Isovitexin 7-O-(6″-feruloyl)-glucoside | 4.77 | 271. 331 | 769 | 431 | 3.56 a | 3.41 a | 3.64 a |
| 16 | Apigenin 6-C-arabinoside-8-C-glucoside | 4.89 | 269. 329 | 563 | 443 | 0.57 a | 0.51 a | 0.54 a |
| Total polyphenolic content [mg 100 g−1 f.m.] | 15.61 a | 14.82 a | 18.07 b | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Piechowiak, T.; Kapusta, I.; Józefczyk, R.; Balawejder, M. Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water. Molecules 2023, 28, 5038. https://doi.org/10.3390/molecules28135038
Matłok N, Piechowiak T, Kapusta I, Józefczyk R, Balawejder M. Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water. Molecules. 2023; 28(13):5038. https://doi.org/10.3390/molecules28135038
Chicago/Turabian StyleMatłok, Natalia, Tomasz Piechowiak, Ireneusz Kapusta, Radosław Józefczyk, and Maciej Balawejder. 2023. "Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water" Molecules 28, no. 13: 5038. https://doi.org/10.3390/molecules28135038
APA StyleMatłok, N., Piechowiak, T., Kapusta, I., Józefczyk, R., & Balawejder, M. (2023). Variability of Properties Modulating the Biosynthesis of Biologically Active Compounds in Young Barley Treated with Ozonated Water. Molecules, 28(13), 5038. https://doi.org/10.3390/molecules28135038









