Abstract
During an infection, inflammation mobilizes immune cells to eliminate the pathogen and protect the host. However, inflammation can be detrimental when exacerbated and/or chronic. The resolution phase of the inflammatory process is actively orchestrated by the specialized pro-resolving lipid mediators (SPMs), generated from omega-3 and -6 polyunsaturated fatty acids (PUFAs) that bind to different G-protein coupled receptors to exert their activity. As immunoresolvents, SPMs regulate the influx of leukocytes to the inflammatory site, reduce cytokine and chemokine levels, promote bacterial clearance, inhibit the export of viral transcripts, enhance efferocytosis, stimulate tissue healing, and lower antibiotic requirements. Metabolomic studies have evaluated SPM levels in patients and animals during infection, and temporal regulation of SPMs seems to be essential to properly coordinate a response against the microorganism. In this review, we summarize the current knowledge on SPM biosynthesis and classifications, endogenous production profiles and their effects in animal models of bacterial, viral and parasitic infections.
1. Introduction
Physiological response of the body to an infection consists in orchestrating a complex immunological defense, including triggering the inflammatory process. Acute inflammation is characterized by the production and release of molecules such as cytokines, chemokines, metalloproteinases, prostaglandins, and leukotrienes, which attract leukocytes, mainly neutrophils and macrophages, at the inflammation site [1,2]. Inflammatory mediators are essential to fight the pathogen, but may be detrimental to the host tissue, especially when inflammation becomes chronic. The “resolution” phase of the inflammation happens to prevent collateral damage and it is an active process: the so-called Specialized Pro-resolving lipid Mediators (SPMs) are key players produced by the metabolism of polyunsaturated fatty acids (PUFAs): arachidonic acid (AA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA). SPMs are currently classified into lipoxins (LX), resolvins (Rv), maresins (MaR), and protectins (PD) [3].
SPMs act as immunoresolvents by sending “stop signals” within the picogram to nanogram dose range [4] in a time- and context-dependent manner [5]. These compounds have also been described to act in a tissue- and disease-specific manner [6]. As a result of that, SPMs control the influx of granulocytes to the site of the inflammation, stimulate microbe killing and phagocytosis of cell debris and pathogens, limit pain, activate tissue-resident cells that promote repair [6,7,8,9], and lower antibiotic requirement [10].
Infectious diseases account for one in four deaths worldwide and represent one of the major causes of organ/tissue impairment due to both pathogen and uncontrolled inflammation-induced tissue damage [11,12]. In this sense, significant effort is constantly being made to find antimicrobial therapies that modulate the inflammatory process, avoid antibiotic resistance, stimulate innate and adaptive immune responses, and have low or no toxicity, reducing mortality.
Given that chronic inflammation is implicated in several diseases, there is a growing interest in discovering new pro-resolving mediators and elucidating how they act to promote resolution. In this review, we summarize and discuss the current knowledge on the biosynthetic pathways and classification regarding SPMs, including the ways in which production of these mediators occurs upon an infection, pointing out the main cell types, signaling molecules and pathways involved in this process. We also compile pre-clinical and clinical studies that have investigated the effect and/or levels of SPMs in infectious diseases.
2. SPM Biosynthesis
Resolution is an active and highly regulated process [13]. A class switch from pro-inflammatory mediators such as leukotrienes (LT) and prostaglandins (PG) to SPMs drives resolution of the inflammatory response. To promote their immunoresolvent actions, SPMs bind to specific G protein-coupled receptors (GPCR) expressed by several cells [14,15], which are summarized in Table 1. SPMs are synthesized endogenously from the metabolization of omega-3 (i.e., DHA, EPA, and DPA) PUFAs or omega-6 PUFAs (i.e., AA) (Figure 1) [16]. DHA can be converted into D-series resolvins (RvD; RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6) [17,18,19], protectins (PD1)/neuroprotectins (NPD1) [20,21], and maresins (MaRs; MaR1 and MaR2) [22]. EPA, on the other hand, is converted into the E-series resolvins (RvE; RvE1 and RvE2) [23]. There is an additional series of SPMs formed by the presence of aspirin, which have been coined aspirin-triggered SPMs [24]. Figure 1 presents a schematic representation of the SPM synthetic pathways and chemical structures.

Table 1.
Receptors for SPMs and their cellular expression.
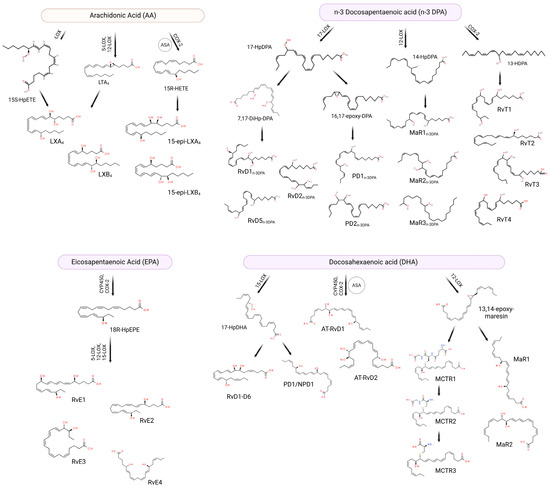
Figure 1.
SPMs can be biosynthesized from omega-3 and omega-6 fatty acids through different enzymes. Arachidonic acid (AA) originates lipoxins (LXs) and 15-epi lipoxins (15-epi-LXs), eicosapentaenoic acid (EPA) originates E-series resolvins (RvE), omega-3 docosapentaenoic acid (n-3 DPA) can be converted into D-series resolvinsn-3 DPA, protectins n-3 DPA, maresins n-3 DPA, and 13-series resolvins (RvTs), while docosahexaenoic acid (DHA) can be transformed into D-series resolvins (RvDs), protectins (PDs), aspirin-triggered D-series resolvins (AT-RvDs), maresins (MaRs) or maresin conjugate tissue regeneration (MCTRs). ASA: acetylsalicylic acid. Created using BioRender.com (accessed on 17th April 2023).
RvDs are formed from conversion of DHA through two lipoxygenations. First, DHA is converted to (17S,4Z,7Z,10Z,13Z,15E,19Z)-17-hydroperoxydocodahexaenoic acid (17S-HpDHA) through the action of 15-LOX in the carbon-17 (C-17) position. It then undergoes a second lipoxygenation by the same enzyme in C-7, generating an intermediate peroxide which can be reduced forming RvD5 or transformed into 7S, 8S-epoxide. 7S, 8S-epoxide can further undergo enzymatic hydrolysis generating RvD1 and RvD2. Alternatively, the second lipoxygenation may occur in the C-4 position forming another peroxide intermediate, which is similarly converted to RvD3, RvD4 and RvD6 [15,17]. There are also aspirin-triggered (AT) RvDs, such as AT-RvD1 and AT-RvD2—the difference in biosynthesis is in the initial lipoxygenation of C-17, which occurs in the presence of aspirin through acetylated COX-2 or via cytochrome P450 [25]. RvEs, in turn, are generated from the oxygenation of the EPA by acetylated COX-2 or via cytochrome P450. This oxygenation generates the intermediate acid 18R-hydroperoxy-eicosapentaenoic (18R-HpEPE), which is transformed into 18R-hydroxyEPA (18R-HEPE) by the action of a peroxidase [23]. Subsequently, 5-LOX promotes the lipoxygenation of 18R-HEPE into hydroperoxide, which can be transformed into epoxide and hydrolyzed into RvE1 [15] or may be reduced by means of a peroxidase in RvE2 [26]. Alternatively, the 18R-HEPE intermediate can undergo lipoxygenation through the action of 12-LOX or 15-LOX, becoming converted into 17,18-diHEPE, also called RvE3 [27]. Furthermore, EPA can be converted into 15S-HpEPE through lipoxygenation by 15-LOX, subsequently reduced to 15S-HEPE by peroxidase. The 15S-HEPE intermediate undergoes a second lipoxygenation by 5-LOX, becoming converted into 15S-hydroxy-5S-HpEPE (15S-H,5S-HpEPE), which is reduced to RvE4 through the action of a peroxidase [28,29].
Also synthesized from DHA, PD1 and NPD1 (when produced in neural tissues) are formed from the lipoxygenation of 17S-HpDHA through 15-LOX [20,30], generating the epoxide intermediate 16(17)-epoxydocosatriene. This intermediate is subsequently converted into PD1/NPD1 through the action of a hydrolase [31]. Additionally, maresins are synthesized from lipoxygenation that occurs in C-14 through 12-LOX, forming the 14S-hydroperoxiDHA (14S-HpDHA), which undergoes a second lipoxygenation by the same enzyme and forms the intermediate epoxide 13S,14S-epoxy-maresin [31]. This intermediate is subsequently converted into MaR1 and MaR2 by means of the action of a hydrolase or a soluble hydrolase, respectively [31]. Additionally, 13S,14S-epoxy-maresin can be converted into maresin conjugates in tissue regeneration (MCTRs). MCTR1 (13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid) is catalyzed by both leukotriene C4 synthase (LTC4S) and glutathione S-transferase Mu 4 (GSTM4). γ-glutamyl transferase (GGT) converts MCTR1 in MCTR2 (13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid) that, in turn, can be transformed into MCTR3 (13R-cysteinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid) by a dipeptidase enzyme [32].
In addition to DHA and EPA, arachidonic acid, derived from the enzymatic oxygenation of omega-6, is also involved in the biosynthesis of SPMs, more specifically in the synthesis of the lipoxin family (LX; LXA4 and LXB4) and aspirin-triggered lipoxins (ATLs) [33]. As previously mentioned, a lipid mediator class switch is necessary to begin resolution, and that happens by polymorphonuclear cells (PMNs) reducing production of LT and PG and that of increasing SPMs such as LX [14]. For LX synthesis to occur, cell–cell interaction is necessary. This occurs through a process known as transcellular biosynthesis, and can be achieved through two main pathways [34]. The first pathway occurs through lipoxygenation of AA in C-15 by 15-LOX, forming 15S-HpETE, which is secreted from cells (i.e., eosinophils, monocytes, and epithelial cells). 15S-HpETE is then converted into 5,6-epoxytetraene by the action of 5-LOX in PMNs and monocytes and subsequently hydrolyzed into LXA4 and LXB4 [34]. The second pathway occurs in an LTA4-dependent manner. Initially, the conversion of AA to LTA4 occurs through the action of 5-LOX, which is secreted and subsequently absorbed by adherent platelets. In platelets, LTA4 is transformed into LXA4 and LXB4 by 12-LOX [34,35]. In addition to the two main pathways, alternative pathway may occur where the presence of aspirin acts on COX-2, redirecting its catalytic activity and promoting the formation of LX. The initial acetylation of COX-2 by aspirin promotes the formation of 15R-hydroxyeicatetraenoic acid (15R-HETE), which is converted into 15-epimeric-LXs (15-epi-LXs) by the action of 5-LOX called aspirin-triggered lipoxins [24,34,36].
There are also reports in the literature of SPMs like RvD, PD and MaR being biosynthesized from docosapentaenoic acid (n-3 DPA), an intermediate metabolite in the conversion of EPA to DHA [53]. Among these SPMs are PD1n-3 DPA, PD2n-3 DPA, MaR1n-3 DPA, MaR2n-3 DPA, MaR3n-3 DPA, RvD1n-3 DPA, RvD2n-3 DPA, and RvD5n-3 DPA [53,54]. At the site of the inflammatory response, n-3 DPA is converted into the intermediate 17-hydroperoxy-8Z,10Z,13Z,15E,19Z-docosapentaenoic acid (17-HpDPA) through the action of 17-LOX, acting as a substrate for the formation of SPMs [53]. First, this intermediate can be transformed into the intermediate epoxide 7,17-dihydro(peroxy)-DPA, which is sequentially converted into RvD5n-3 DPA or into the 7,8-epoxy,17-hydroxy-DPA molecule; both actions are performed via 5-LOX. Finally, 7,8-epoxy,17-hydroxy-DPA can be converted into RvD1n-3 DPA and RvD2n-3 DPA, also by lipoxygenation by 5-LOX [53]. Furthermore, 17-HpDPA can be transformed into the epoxide intermediate 16-17-epoxy-DPA, which is enzymatically hydrolyzed into PD1n-3 DPA and PD2n-3 DPA [53]. Alternatively, n-3 DPA can be converted to the intermediate 14-hydroperoxy-7Z,10Z,12E,16Z,19Z-docosapentaenoic acid (14-HpDPA) through the action of 12-LOX. This intermediate can undergo a second lipoxygenation, becoming converted to MaR3n-3 DPA, or it can be converted to the intermediate epoxide 13,14-epoxy-DPA, which is enzymatically hydrolyzed to MaR1n-3 DPA and MaR2n-3 DPA [53]. In addition, n-3 DPA can generate 13-hydroxy-docosahexaenoic acid (13-HDPA) through COX-2, and neutrophils are able to convert 13-HDPA via lipoxygenation to the 13-series resolvins RvT1, RvT2, RvT3 and RvT4 [55]. To date, studies addressing n-3 DPA-derived SPMs are not as numerous as those for EPA- or DHA-derived SPMs. The lack of data makes it difficult to compare the potency of n-3 DPA-derived SPMs with that of EPA- or DHA-derived SPMs. The effects of n-3 DPA-derived SPMs, however, seem to be generally comparable to those of other SPMs [53,56].
Among the lipoxygenases involved in the biosynthesis of SPMs mentioned above, 15-LOX (ALOX15), mainly the 15-LOX-1 isoform, plays an important role in the pathways by catalyzing the initial transformation reactions of PUFAs [57]. LOX are found in organisms from two of the three domains, namely Bacteria and Eukarya [58]. In this context, it is important to understand the existence of orthologous enzymes between species (i.e., mice, rats, and humans). In humans, there are six LOX genes (ALOX5, ALOX15, ALOX15B, ALOX12, ALOX12B and ALOXE3) that encode six different isoforms of LOX. In comparison, mice have orthologs for all human ALOX isoforms [59]. However, these orthologs vary in their specificity, exhibiting differences in enzymatic oxygenation. For example, human ALOX15 acts enzymatically as 15-LOX, whereas murine ALOX15 acts enzymatically as 12-LOX [57,60,61]. Furthermore, human ALOX15 is expressed in epithelial cells, red blood cells and eosinophils, in addition to monocytes/macrophages and neutrophils through induction by interleukins (IL). In mice, on the other hand, it is found in resident macrophages [57,62]. Studies have shown that this difference in lipoxygenation from 12-LOX to 15-LOX improves the ability to synthesize LX, optimizing the process of inflammation resolution [61]. However, when it comes to DHA oxygenation, both ALOX15 (human and murine) produce similar amounts of 17-HDHA and 14-HDHA [60], showing similar actions.
SPMs actively induce the inflammation resolution process [63] by reestablishing tissue homeostasis, increasing host defense, and interfering with the maintenance of pain signals [15]. In addition, they promote the cessation of the influx of PMNs [23], reduce the production of pro-inflammatory mediators [24,64] and induce phagocytic activity of macrophages [65]. Failures in anti-inflammatory actions may contribute to the development of chronic inflammation [65,66]. Some factors, such as age, sex, and race, can influence the formation of SPMs and their pro-resolution abilities. In humans, aging contributes to the development of a heightened inflammatory state and decline in physiological functions, characterized by increased tumor necrosis factor (TNF)-α, nuclear factor kappa B (NF-κB), IL-1β and IL-6 [67]. In this context, studies demonstrate that elderly mice have reduced local levels of PMNs and increased levels of PMNs in inflammatory exudates, delaying the resolution process [65]. Regarding sex and race, one study with 53 participants found that after myocardial infarction (MI), plasma levels of metabolites from AA and DHA were higher in white individuals of both sexes (female and male) than those in Black male and female individuals. EPA levels were higher in white males than in white females and Black individuals of both sexes [68]. Regarding endogenous SPM levels, RvE1 was significantly lower in Black patients, while PD1 levels were lower in white, male patients [68]. Despite disparities in lifestyle (such as physical exercise and diet) that are suggested as the cause of higher incidence of MI among Black individuals [69], distinct SPM signatures may provide a better understanding and clinical guidance on personalized therapies in the future.
Cross-Linking Pro-Inflammatory and Pro-Resolving Mediator’s Biosynthesis
It is well described that some mediators of inflammation with contrasting activities share common precursors: one example is LX and PG that share arachidonic acid as the substrate during enzymatic conversions. It is also true that metabolization of one substrate can be performed by multiple enzymes, and the same enzyme can convert different substrates into multiple metabolites, sometimes with divergent actions. So, what regulates this process during inflammation?
While this question cannot be fully answered with the current available experimental data, some evidence suggests that there are substrate preferences for each enzyme. Conversion of omega-3 and omega-6 happens by oxidation by lipoxygenases, cyclooxygenases, or the cytochrome P450 oxidase/epoxygenases. Arachidonic acid is the preferred substrate for COX-2 [70,71], originating pro-inflammatory mediators. COX-2 oxygenates EPA at about 45% the rate of AA [71], despite AA and EPA displaying Km values similar to those of COX-2, individually [72]. When aspirin (ASA) acetylates COX-2, however, it prevents the formation of prostanoids, favoring the lipoxygenase-type reaction, generating 15R-HETE from AA, that can be metabolized into 15-epi-LXs [73].
Lipoxygenases, one of the main types of enzymes in SPM biosynthesis, accept AA, DHA and EPA as substrates, but with differences: 15-LOXs preferentially converts DHA > EPA > AA, while 12S-LOX’s preference is DHA > EPA > AA and that of 5-LOX’s is AA and 5S-HpETE [74]. In fact, human lipoxygenases have different kinetics with each metabolite serving as a substrate, which are deeply discussed by Kahnt et al. [75].
The third route of metabolization of PUFAs is through cytochrome P450, or CYP450. The first double bond on C3, present in omega-3 but not omega-6, is a preferred site of epoxidation catalyzed by CYP450 [76]. Most CYP isoforms have EPA as preferred substrate, while AA and DHA are converted at similar rates [76]. Examples of isoforms with higher rates of conversion of EPA over AA and DHA are CYP2J2 and CYP2C23 isoforms present in human heart and rat kidney, respectively [77,78].
Another factor that dictates SPM production over pro-inflammatory biosynthesis is that, depending on the activation status of macrophages on the inflammatory foci, these cells change the expression of lipoxygenases: macrophages that differentiate to the M2-like phenotype by IL-4 or IL-13 or, upon efferocytosis of apoptotic cells, upregulate the expression of 15-LOX-1 [79,80,81,82]. In addition, monocytes and macrophages minimally express 15-LOX-2 unless these cells undergo long-term stimulation by zymosan or lipopolysaccharide (LPS) via toll-like receptor activation [80,83]. In parallel with M2 polarization, PGE2 production decreases [84,85].
Additionally, SPM receptor expression likely influences regulation of resolution versus inflammation: a study published by Krishnamoorthy and colleagues [86] showed that less RvD1 is required to lower neutrophil migration in human ALX/FPR2-overexpressing transgenic mice. Proper or stimulated expression of receptors for SPMs in order to maximize beneficial effects during resolution seems to be decisive, but further evidence is needed.
As the same enzymes operate for both omega-3 and omega-6 conversion pathways, the bioavailability ratio of EPA, DHA and AA during inflammatory process may be critical, which further supports the intake of EPA- and DHA-rich diets to increase SPM production. There are several phospholipases A2 (PLA2s) responsible for remodeling the cell membrane that directly impacts PUFA bioavailability and metabolism. Evidence suggests that certain phospholipases also have preferences: human cytosolic cPLA2 prefers AA, calcium-independent iPLA2 prefers EPA, and secreted sPLA2 selectively prefers DHA as a substrate [87]. Secreted PLA2 group IID (PLA2G2D) appears to be linked to pro-resolution activity, and it is preferentially expressed by macrophages and dendritic cells [88]. Another study observed that DHA enrichment of mononuclear cell membranes was directly correlated with phospholipases D (PLD) activation by DHA [89].
It is important to highlight that each experimental condition is unique, and these results may not be fully extrapolated to the context of infections since not all results described above were observed upon pathogen stimuli. However, these data bring potential approaches to be investigated in order to potentiate SPM production and effectively induce resolution.
3. SPM Levels in Patients with Infectious Diseases
There are a limitingly small number of clinical studies on SPM levels in patients with infectious diseases. However, this also highlights the need of further studies in this field. Despite not being fully elucidated, a growing body of evidence correlates better clinical prognosis and better survival rates with increased levels of SPMs. Therefore, in this section, we discuss the current data on how SPMs levels can affect the evolution of the clinical picture of patients with infections.
3.1. Bacterial Infections
In a study led by Jesmond Dalli [90], the authors quantified the serum levels of LTB4, PGE2α and pro-resolving mediators RvD1, RvD2, and PD1 in patients with sepsis. It was found that non-survivors had lower levels of pro-resolution mediators, especially in the chronic phase of the disease, than survivors. It has also been demonstrated that the profile of lipid mediators can be directly related to the severity of the sepsis condition; patients with marked levels of pro-inflammatory lipid mediators have a marked and exaggerated response to the microorganism, resulting in worse prognoses. In addition, surviving patients needed smaller amounts of antibiotics since they had a more efficient immune response. The authors also point out that the severity of sepsis is directly associated with platelet aggregation, as this factor is directly linked to organ failure and death [90]. It is believed that high levels of AT-RvD1, AT-RvD3, and AT-PD1 may decrease the expression of COX-2 and LTB4. Hence, proper pro-resolving mediator production seems to regulate a balanced immune response and correlate with better survival rates, as the cytokine storm present during sepsis can be as detrimental as the infection.
In another study, plasma from 66 patients with sepsis in intensive care units and 20 healthy subjects (controls) were analyzed [91]. Sepsis patients were grouped into survivors or non-survivors, depending on their outcome on day 28 of the study. Pro-inflammatory and pro-resolutive mediators were detected: cytokines IL-6 and IL-8 were higher in concentration on non-survivors when compared to controls, while LXA4 and Annexin A1—pro-resolution mediators that inhibit leukocyte migration and eicosanoid production—levels were lower in survivors and non-survivors compared to the control patients [91]. In individuals with tuberculosis, AA-derived pro-inflammatory lipids were abundantly present, such as PGE2, LTB4 and PGF2α. Among the most prominent SPMs detected, there were LXB4 and 5S,15S-diHETE [92]. Indeed, infections trigger the generation of prominent inflammatory mediators, but the host capacity to correctly produce endogenous pro-resolving molecules that actively regulate the response also has a significant role in establishing or a chronic inflammatory process, or resolving it. In this sense, further investigation is needed to explore the correlation between higher circulating SPM levels and better outcomes.
As DHA and EPA, precursors of some SPMs, are present in plants and animal sources, consumption of omega-3 rich foods impact bioavailability of these precursors to form SPMs [93]. To investigate how omega-3 supplementation could affect the levels of PUFAs and oxylipins—metabolites of PUFAs that can be converted into SPMs—upon endotoxin (LPS) challenge in men, Walker and colleagues [94] designed a randomized crossover study. Blood samples were collected at baseline, 1, 2, 4 and 8 h after the challenge. The authors observed that EPA and DHA levels were increased in the omega-3-treated group by 432% and 142%, respectively, when compared to controls. Some oxylipins, such as HEPEs (from EPA), were strongly increased [94]. In a similar study, adults challenged with endotoxin and treated with omega-3 presented higher levels of circulating 18-HEPE, 17-HDHA, AT-LXA4, LXB4, RvE1 and RvD1 [95]. Despite the small number of participants in both studies, the results provide initial evidence of lipid profile modulation by the presence of LPS and how omega-3 bioavailability alters the synthesis of pro-resolution mediators during bacterial infections.
3.2. Viral Infections
The COVID-19 pandemic highlighted the importance and urgency of understanding the impact of this infectious disease on the immune system. To compare serum levels of pro-inflammatory molecules and SPMs between SARS-CoV-2 patients and healthy subjects, Turnbull and colleagues performed a lipidomic analysis where 44 bioactive lipids were quantified [96]. The SARS-CoV-2 infection was associated with a significant mobilization of both pro-resolutive and pro-inflammatory mediators’ production when compared to matching controls, as increased levels of LTB4, PGE2, 5-HETE, 13-HODE and 17-HDHA were detected [96]. In bronchoalveolar lavages, this strong sign of immune response was also observed—levels of LTB4, PGE2, DHA, n-3 DPA, RvD1, RvD2, RvD4, RvD5, PDX, 17-HDPAn-3, 14-HDHA and 17-HDHA were markedly boosted in COVID-19 subjects when compared to the non-infected group [97].
This increase, though, may differ in patients that are more affected by this infection. In a cohort study conducted in Beaumont Hospital, Ireland, 38 patients who tested positive for the SARS-CoV-2 virus had their plasma lipid mediators profile analyzed. Comparing two main groups, critically ill (patients that required invasive mechanical ventilation) and severe disease cohorts (the ones that required supplemental oxygen or non-invasive ventilation), a downregulation of the 5-lipoxygenase (ALOX5) pathway in critically ill patients who had lower levels of RvD1 and RvD3 was reported. Additionally, severe disease patients had higher concentrations of PD1n-3 DPA and MCTR1, and both cumulative SPM concentration and ratio of SPM concentration to pro-inflammatory mediators indicate that increased SPM production is linked to better outcomes [98]. Comparably, serum from severe patients showed a significant increase in RvE1 and MaR2 [99]. Moreover, COVID-19 alters the activation and function of circulating phagocytes, as PD1, RvT1, RvE3, and 10S,17S-diHDPA positively correlate with phagocytic ability of monocytes and neutrophils [100].
The possible mechanisms by which SPMs would improve COVID-19 outcomes are speculated elsewhere [101,102,103,104]. Recent evidence shows that the SARS-CoV-2 virion spike 1 glycoprotein (S1) can induce cytokine and chemokine release by macrophages and modulate microRNAs miR-103, miR-16 and miR-29a, known to control the inflammatory responses. Interestingly, in vitro treatment with RvD1 and RvD2 promoted resolution by lowering S1-induced production of IL-8, TNF-α, MCP-1, augmented macrophage phagocytic activity and regulated miRNAs expression to reduce IKK/NF-κB activation and downstream signaling cytokines [105].
This variation in SPM production among differently affected patients may not be restricted to COVID-19. Lipidomic profiling of nasal washes from patients with influenza, categorized in low, medium and high clinical scores, revealed that samples from individuals with a high clinical score and elevated levels of cytokines/chemokines also presented notable higher percentages of PGE2, LTE4, and mediators from the lipoxygenase, DHA, and EPA pathways [106].
Altogether, these data indicate that elucidating how each SPM is produced during different disease profiles may help clinicians to better understand a patient’s prognosis and plan effective therapies in the future. Moreover, using supervised machine-learning methodologies, a study highlighted that increased plasma levels of RvD4, 10S,17S-diHDPA, 15R-LXA4, and MaR1 are linked to therapy responsiveness in rheumatoid arthritis patients [107]. This might suggest a potential biomarker role for SPMs in the context of disease-modifying anti-rheumatic drug (DMARD) responsiveness [107]. These results might provide important disease monitoring information to clinicians, but also could be extrapolated to other diseases as well, including infectious ones.
4. SPM Regulation of Infection in Animal Models
During an infection, it is essential that the host immune system regulates the production and release of pro-inflammatory and pro-resolutive mediators to maintain the integrity of tissues and cells while it fights pathogens. This proper balance leads to homeostasis instead of chronic inflammation. Thus, SPMs have an important role regulating inflammatory infection in many animal models (Figure 2), as previously demonstrated. In general, SPMs limit the neutrophil-mediated tissue [4] in addition to increasing the repair capacity of macrophages [108]. Therefore, for a better understanding of their mechanisms, we divide the actions of SPMs into bacterial, viral, and parasitic animal models of infections, as summarized on Table 2 and Figure 2.
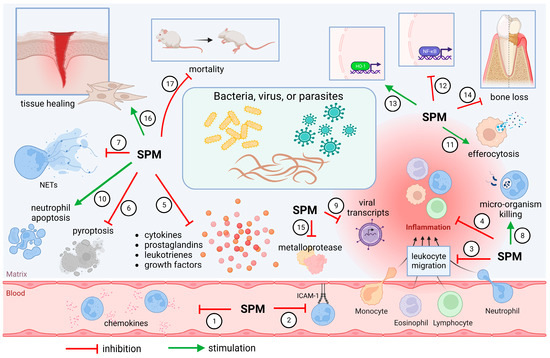
Figure 2.
SPMs control infection by different mechanisms. SPMs limit chemokine release (1) [120,125,133,136] and ICAM-1 expression (2) [115], reducing migration of leukocytes (3) [110,113,114,117,118,120,123,125,127,129,132,134,136,142] to the inflammatory site. Systemic inflammation (4) is also diminished [109,119], together with prominent reduction in pro-inflammatory molecules’ release (5) [10,120,121,124,125,128,130,133,134,136,137,139,141]. SPMs also decrease pyroptosis (6) [118] and NET formation (7) [132], without compromising microbicidal activities (8 and 9) [10,112,113,114,120,124,126,127,129,131,132,133,135,141]. Neutrophil accumulation is avoided also by stimulating neutrophil apoptosis (10) [110,123] and clearance of cell debris by macrophages (11) [123,126,130]. Inhibition of NF-κB (12) [129,134,137,138] and upregulation of HO-1 (13) [115] favor inflammation control. As a result, less bone loss is observed (14) [117,119,122], and tissue architecture is preserved by reducing the activity of metalloproteases (15) [121,125,136] and stimulating tissue healing (16) [119,122,128]. Improvement of survival was also observed (17) [109,110,112,116,118,120,124,129,131,133,135,137,141]. Created using BioRender.com (accessed on 6 April 2023).

Table 2.
Pharmacological activities of SPMs on animal models of infection.
4.1. Bacterial Infections
SPMs are known to control bacterial infections, and bacteria or their products can shape the production of SPMs in different steps of the inflammatory response.
Interestingly, if not by bactericidal effect per se, SPMs stimulate the phagocytosis and clearance of different pathogens. For instance, RvD1 synergizes with ciprofloxacin to promote the non-phlogistic phagocytosis of Pseudomonas aeruginosa during lung infection [140] and against Escherichia coli [10]. Similarly, other SPMs such as RvD2 [124], MaR1 [141], or PDX [133] decrease local and systemic bacterial burden, which leads to increased survival in a model of sepsis [82]. In this section, we review the literature on the possible outcomes of SPMs applied as treatments in disease models caused by bacteria.
Effects of SPMs in Animal Models of Bacterial Infections
One of the most extensively studied models of infectious disease is sepsis, a severe worldwide health concern and a leading cause of disability and mortality. Improper host immunological response to pathogens and excessive inflammation can lead to multi-organ disfunction and death [143]. Therefore, adequate innate and adaptive immune defense against the microorganism allied with a controlled inflammatory process is the key to reduce severity of sepsis. Due to SPMs ability to induce both features, bioactive lipid mediators have been studied in models of induced sepsis, mainly using cecal ligation and puncture (CLP), that results in a systemic polymicrobial infection that mimics sepsis in humans. LXA4 treatment significantly reduced mortality of CLP rats. Despite not affecting phagocytic activity, bacterial load was reduced. This was accompanied by reduced IL-6, monocyte chemotactic protein 1 (MCP-1) and IL-10 levels, in addition to inhibition of NF-κB activation in peritoneal macrophages [112]. Likewise, in a different study, LXA4 controlled neutrophil migration while increasing the phagocytic ability of the ones that were able to migrate to the inflammatory foci [113,114]. The authors observed that apoptotic, bacterial clearance and phagocytic activities were induced without uncontrolled free radical production [113]. Moreover, LXA4 can affect the virulence of Pseudomonas aeruginosa by acting as an antagonist and partial agonist of LasR, an important transcription factor that coordinates production and release of pathogenic factors of P. aeruginosa [114]. Treatment with LXB4 reduced inflammation and improved survival of mice after CLP by limiting neutrophil infiltration and protecting cells from pyroptosis [118]. In a model of E. coli-induced sepsis, 15-epi-LXA4 presented a synergic effect with antibiotics by regulating IL-6 and TNF-α production by macrophages, thus limiting bacterial replication, neutrophil migration, and resulting in better survival rates [109]. Administration of LXA4 during the late phase of Klebsiella pneumoniae-induced pneumosepsis reduced mortality significantly by ablating excessive inflammation and bacterial load [116].
Resolvins also demonstrated promising results in treating sepsis. Following CLP, mice that received RvD1 treatment had lower numbers of bacteria in blood and peritoneal fluid, and also inhibited uncontrolled neutrophil migration and NF-κB activation. RvD1 also diminished the rate of apoptosis of CD3+ T cells of the thymus [129], which is a relevant cause of immunosuppression that is highly detrimental during sepsis. Another member of the resolvins family, RvD2, exerted pro-resolutive effects upon CLP by lowering local and systemic bacterial burden, reducing neutrophil and increasing mononuclear peritoneal counts. This increase in macrophages was also correlated with improvement of phagocytosis of E. coli by macrophages as well as changes on IL-17, IL-10, PGE2, IL-6, IL-1β, IL-23, TNF-α, PGE2, and LTB4 levels [124].
Xia et al. [133] demonstrated that PDX also had an impact on sepsis outcomes after CLP. Following treatment with PDX, the authors observed improvement in survival rates, prevention of multiple-organ injury (as demonstrated by liver and kidney function markers), reduced bacterial colony formation unit (CFU) counts from both blood and peritoneal fluid, and suppressed neutrophil recruitment while increasing macrophage numbers with higher phagocytic abilities. Cytokine production and polarization of macrophages were also affected by PDX, as M2 macrophages (F4/80+CD206+) were increased and IL-6, TNF-α and MCP-1, markers of M1 macrophages, were decreased after treatment [133]. Similar promising results were attributed to MaR1 that effectively reduced lactate, acetate, and pyruvate levels in serum of CLP mice, downregulating proinflammatory cytokines and NF-κB, mitigating mitochondrial damage of lung tissues and resulting in better survival ratios [137,138].
Airway infections are caused by bacteria, fungus, or viruses, which can be spread through direct or indirect contact and eventually increase the risk of a secondary coinfection [144,145,146]. Respiratory tract infections gained particular attention in the last few years after the SARS-CoV-2 global pandemic. Commonly, prominent inflammation is one of the hallmarks of airborne infections, which leads to significant tissue injury. Hence, pre-clinical models are being extensively employed to study the role of SPMs on pathogen-induced airway diseases. Using a sepsis-induced acute lung injury (ALI) model, the authors determined that PDX ameliorated histopathological changes in lung tissue, reduced bacterial load, pulmonary edema, PMN migration, and production of IL-1β, IL-6, TNF-α, and MCP-1. Mechanistically, these effects were likely linked to suppression of NF-κB and upregulation of PPARγ, a natural receptor of PUFAs with regulatory role in the inflammatory process [134]. In mice, hydrochloric acid aspiration plus administration of E. coli mimics aspiration pneumonia in patients, one of the leading causes of ALI and acute respiratory distress. Treatment with 100 ng RvE1 promoted bacterial clearance, lowered PMN counts and several proinflammatory markers on lung homogenates. Inhibition of NF-κB and survival improvement were also observed [120].
In a slightly distinct model of pneumonia, induced by E. coli and P. aeruginosa, AT-RvD1 reduced CFU counts alone and potentialized ciprofloxacin activity when combined with it. Moreover, neutrophil numbers on bronchoalveolar fluid were reduced by AT-RvD1 but not by ciprofloxacin alone. Promotion of efferocytosis by macrophages also contributed to a better disease outcome [126]. Similar results were found by Isopi et al. [127]—RvD1 promoted resolution of P. aeruginosa infection and inflammation in cystic fibrosis. In addition, the Gram-negative bacterium Haemophilus influenzae is an opportunistic pathogen that can infect the upper respiratory tract and exacerbate inflammation in susceptible patients. AT-RvD1 alters the inflammatory cell profile and promotes efferocytosis of apoptotic neutrophils, dampening COX-2, IL-6, and TNF-α in a murine nontypeable H. influenzae infection model [130].
Pre-clinical models of peritonitis have been employed to study antimicrobial and/or anti-inflammatory therapies for several decades. It is an important and reproducible approach to investigate infections [147,148]. Using an E. coli-induced peritonitis model, the authors observed a protective effect of RvD2 by promoting PMN apoptosis, limiting neutrophil migration and enhancing macrophage phagocytic ability, effects that were absent in mice deficient in GPR18, an RvD2 receptor [123]. Similarly, RvD1 shortened the resolution interval of E. coli peritonitis, and when combined with antibiotic therapy, significantly raised bacterial phagocytosis and reduced IL-1β and IL-6 [10]. The culture of human neutrophils with RvE1 also enhances phagocytosis of opsonized E. coli [149]. When RvD1, RvD5 and PD1 (50 ng) were combined with a suboptimal dose of ciprofloxacin, bacterial titers were diminished and mice were protected from hypothermia. Additionally, levels of IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were reduced, and the production of the precursor of D-series resolvins, 17-HDHA, was increased [10]. These results highlight a promising ability to potentiate antibiotic efficacy. A combination of these three pro-resolving mediators as a treatment at endogenous levels induced phagocytosis of E. coli by human macrophages in vitro, and pro-inflammatory genes related to the expression of NF-κB and TNF-α were downregulated by RvD5 through activation of a GPR32 receptor [10]. In a different study, after E. coli intraperitoneal injection, treatment with LXA4 was able to induce neutrophil apoptosis through phosphorylation of the BCL-2-associated death promoter (BAD) and reduced expression of the myeloid leukemia sequence 1 (MCL1) anti-apoptotic protein [110]. Additionally, treatment with RvE1 can decrease MPO activity and production of IL-1β, and IL-6, and increase bacterial clearance [120].
Intestinal infection by the enteropathogenic Escherichia coli (EPEC) is a leading cause of mortality among infants. The Gram-negative bacteria Citrobacter rodentium shares many pathogenic mechanisms with EPEC in humans, and is therefore used as a model of intestinal infection in newborn rodents. Therapeutic effects of RvD1 and RvD5 were observed in C. rodentium infection, showing substantial decrease in bacterial load, lessening neutrophil influx and rescuing 33–100% of infected infants from death (depending on the number of CFU injected). In addition, the treated groups of neonates developed serum IgG responses comparable to those of infected adults, suggesting a remarkable impact of RvD1 and RvD5 on immunological memory establishment [131].
Periodontitis is an inflammatory disease primarily caused by a microbiota dysbiosis and raising of a pathobiont with excessive proliferation and invasion of oral cavity, destroying periodontal tissues; it is commonly associated with the onset of systemic illnesses if left untreated [150,151,152]. Experimental periodontitis shares pathogenic features with other inflammatory diseases such as septic arthritis and is employed to study inflammation and bone loss in oral cavity, which are easily observable. In rabbits, topical application of an LXA4 analog prevented loss of connective tissue and alveolar bone and significantly diminished inflammatory infiltrate [117]. Restoration of soft tissues and resolution of the intense inflammation were attributed to RvE1, in contrast to PGE2 and LTB4 administration which worsened the disease. In addition, RvE1 reduced bone resorption and serum IL-1β and C-reactive protein (CRP), which are markers of systemic inflammation [119]. Similar results were observed on a periapical periodontitis model in rats treated with RvD2, as well as healing of periapical lesions and lower bacterial burden [122].
Despite Staphylococcus aureus being a commensal microorganism of the body, it can become opportunistic and cause skin and articular infections. Commonly, murine skin pouch models are employed to assess S. aureus-induced infections. Coadministration of RvD2 and S. aureus via intra-pouch injection reduced bacterial titers and neutrophil numbers [123]. The combination of PD1, RvD5 and RvD1 reduced bacterial load by 10-fold; strikingly, a combination of PD1, RvD5 and RvD1 with suboptimal doses of vancomycin reduced bacterial load by 100-fold. This demonstrates that, in parallel with classic antibiotic strategies, SPMs may be suitable as a promising approach against pathogens.
Neutrophil extracellular traps (NETs) are scaffolds of chromatin released by neutrophils along with proteases and enzymes that help entrap invading microorganisms [153]. Excessive NET production is linked to collateral tissue damage [154]. During murine S. aureus infection, RvT1, RvT2, RvT3 and RvT4 reduced bacterial titers and NET formation, and in vitro, the treatment stimulated NET clearance by human monocyte-derived macrophages, promoting resolution [132]. This points to another mechanism by which SPMs could control tissue destruction caused by inflammatory products.
In addition, growing evidence highlights the importance of time-regulated production of SPMs. An interesting study published by Sordi and colleagues [116] demonstrated that LXA4 played a detrimental role when administered at an early phase of sepsis, worsening the infection, versus a protective effect when administered during the late phase of the disease. Additionally, distinct outcomes were observed when administering RvD2 at different timepoints in an E. coli peritonitis model. A higher number of apoptotic PMN were detected when treatment was provided during the peak of the inflammation, but no such effect was observed when RvD2 was administered during the onset of inflammation [123]. Therefore, these studies guide our attention to certain particularities of each context, timepoint of administration and disease profile that should be considered (and deeply explored) in future research to maximize SPMs’ beneficial potential.
Altogether, these effects stimulated by SPMs could be helpful because they might contribute to the reduction in new bacterial antibiotic resistance mechanisms given the pro-efferocytosis actions of SPMs. Moreover, some antibiotics, such as β-lactams, induce bacteriolysis releasing LPS and lipoteichoic acid (LTA) that can induce post-infection sequelae due to persistent activation of immune cells [155]. Therefore, the ability of SPMs to enhance bacterial clearance, lower antibiotic requirements, and shorten resolution time interval [10] can be essential to uncover new ways to treat infection. Ultimately, these effects can contribute to reduce antibiotic resistance and post-infectious sequelae.
4.2. Viral Infections
The span and magnitude of the immune response against viruses depends on how the virus interacts with host cells, the stages of replication, dissemination, and infection. Humoral immunity assumes that viruses or infected cells stimulate B lymphocytes to produce antibodies aimed at neutralizing and/or opsonizing the infected cell. The binding between antibodies with the virus/infected cell can block the interaction of the virus with the host cell, as well as facilitate recognition by cells of the immune system, especially by cytotoxic cells, and activate the lysis of the infected cell by the complement system [156]. Cell-mediated immunity, on the other hand, assumes that the infected cells will be recognized by cells of the immune system, through the recognition of MHC molecules, molecules related to DAMPs cell damage, or through the production of cytokines by the infected cells. Among the main cells involved in this mechanism are cytotoxic and helper T lymphocytes as well as NK cells [156]. Many viruses capable of causing chronic infections tend to activate dendritic cells and macrophages, stimulating them to produce TGFβ and IL-10, and these cytokines limit the inflammatory response against the virus [156,157,158,159,160].
Effects of SPMs in Animal Models of Viral Infections
Many studies involving viral infections and SPMs investigate the interaction of mediators with the influenza virus [102]. The current understanding is that there is a direct relationship between the virulence of the virus strain and the production of SPMs [102] since strains with high virulence (H5N1 and H1N1) can reduce lipoxin levels. It was previously demonstrated that this decrease caused by H5N1 occurs mainly due to the inhibition of ALOX5, the gene of the enzyme responsible for the synthesis of lipoxins, and that its inhibition results in the dissemination of the virus to other tissues, since the reparative tissue response was compromised [161]. PD1 was also previously demonstrated to be effective against influenza, improving mice survival rates, pulmonary functions, and infection by inhibiting nuclear export of viral transcripts [135]. In addition, SPMs may help humoral immunity against viruses. The production of IgG was stimulated by LXB4 in B cells collected from individuals vaccinated against influenza, and the authors concluded that the mechanism involved was related to the increase in COX2 induced by LXB4, which in turn led to an increase in BLIMP1 and XBP1 expression in B cells. Both are plasmatic cell differentiation factors that are directly linked to the activity of memory B cells [162].
Respiratory syncytial virus (RSV) is the most common cause of viral pneumonia among children. In mice, lung inflammation caused by RSV was mitigated by MaR1, with significant increase in viral clearance and amphiregulin production, an epithelial growth factor that aids in the resolution process [139]. These effects were, at least in part, attributed to MaR1 ability to modulate aberrant inflammatory regulatory T cells (Tregs) that express FoxP3 and signal through LGR6, as LGR6-deficient mice presented high viral load and exacerbated type-2 immune response [139]. Both macrophages and Tregs express LGR6 constitutively [139].
In a murine stromal keratitis model of ocular infection by herpes simplex virus (HSV)-1, untreated eye lesions do not regress even after viral clearance due to excessive local inflammatory process and Th1/Th17 cells influx. Corneal ulceration, edema and neovascularization arise from the local inflammatory response. Combined with excessive infiltration of neutrophils, macrophages, dendritic and natural killer cells [163], it may result in blindness [164]. Rajasagi and collaborators demonstrated that, in mice, the administration of RvE1 [121] or AT-RvD1 [125] minimized the disease severity by limiting leukocyte migration, neovascularization and production of pro-inflammatory cytokines, contributing to lesion healing. Also, the AT-RvD1 treatment upregulated IL-10 production, a major regulator of T-helper cell activation and secreted by resolution type macrophages in response to RvE1 [165,166] and suppressing STAT1, which influences in Th1 cell differentiation and IFN-γ expression [121,125]. Comparable results were described after topical application of NPD1, which mitigated the severity of the disease, dampening leukocyte infiltration, as well as cytokine and chemokine (IL-6, CXCL1, CXCL-10, CCL-20), metalloproteinases (MMP-2 and MMP-9) and vascular growth factor (VEGF-A) production, therefore controlling tissue destruction and abnormal vascularization [136]. Thus, SPMs may represent a safe and interesting addition to current therapies to mitigate viral infections.
4.3. Parasitic Infections
Diseases caused by protozoan parasites and helminths still account for an enormous social and health impact in tropical regions of the world, costing billions of dollars annually [167]. The pathogenesis of parasitic infections is complex and differ by pathogen. For example, eggs and larvae frequently induce granuloma formation and fibrosis, protozoans trigger Th1 response with high levels of IFN-γ and TNF-α, and helminths induce a strong Th2 response with significant eosinophilia [168]. Furthermore, parasitic infections often feature acute or chronic neuroinflammation and are linked to an assortment of clinical outcomes, as pro-inflammatory cytokines released by microglial cells and astrocytes are key players of the pathological process [169]. The presence of parasites can impair the activity of glial cells, and this is commonly related with pro-inflammatory mediators’ levels, cytotoxic action of nitric oxide, and reactive oxygen species [169]. When the infection becomes chronic, persistence of the pathogen leads to tissue damage and perpetuates inflammatory processes, markedly characterized by cellular infiltrate, composed mainly of T cytotoxic and T helper lymphocytes, macrophages, and B cells [170].
Effects of SPMs in Animal Models of Parasitic Infections
Although limited, some studies have addressed the role of SPMs as treatments for parasitic infections. Cerebral malaria, caused by the parasite Plasmodium berghei in mice, is mitigated by 15-epi-LXA4 [111]. The pathogenesis observed in this animal model resembles infection by P. falciparum in humans. The relevance of lipid mediators produced by 5-LOX, including LXA4, was demonstrated by the infection of mice deficient in this enzyme (Alox5-deficient mouse) that presented intense lymphocyte infiltration and high pro-inflammatory cytokine expression, and also accelerated mortality. These parameters were ameliorated by the LXA4 epimer, 15-epi-LXA4 [111]. In another study, Souza and colleagues [115] described protective effects of LXA4 on cerebral malaria by reducing vascular dysfunction and edema, inducing hemeoxigenase-1 (HO-1) expression and improving survival percentage. A distinct—but not mild—parasitic disease caused by Trypanosoma cruzi, Chagas disease, can lead to heart failure and death, if neglected [171]. A serious clinical manifestation of this disease is chronic cardiomyopathy, with significant focal leukocyte infiltration and fibrosis. Mice infected with T. cruzi received AT-RvD1, antiparasitic therapy or a combination of both; AT-RvD1 alone reduced serum levels of IFNγ and IL-1β, along with inflammatory infiltrate and molecular markers of cardiac hypertrophy and tissue fibrosis. Moreover, AT-RvD1 alone or in combination with an antiparasitic drug reduced parasitic load [128]. This indicates that, similarly to what happens in bacterial infections, SPMs could be used in combination to current clinically active drugs to improve their efficacy.
4.4. What Mechanisms Are Shared by SPMs upon Infection Caused by Different Pathogens?
When comparing the described effects of SPMs between different types of pathogens (e.g, bacteria vs. virus), some similarities can be noticed. As described above, these pro-resolution mediators are able to limit the production and release of chemokines [120,125,133,136], resulting in fewer cells recruited to the inflammatory site [110,113,114,117,118,120,123,125,127,129,132,134,136,142] and strongly downregulate pro-inflammatory cytokine release [10,120,121,124,125,128,130,133,134,136,137,139,141], without compromising the microbicidal capacity of phagocytes [10,112,113,114,120,124,126,127,129,131,132,133,135,141] that is an essential feature during an infection. As a consequence of controlled inflammation, tissue healing is improved [119,122,128] and survival rates are higher [109,110,112,116,118,120,124,129,131,133,135,137,141].
On the other hand, differences in SPM effects between different types of pathogens are harder to point out. This is due to two main reasons: first, the immunopathology mechanisms of infections can differ tremendously from each other, and naturally, different aspects are considered and measured in each study. Therefore, there are some knowledge gaps due to a lack of comparable data. And second, each SPM has different chemical structures and kinetics, and their activity can be similar or different depending on the context. Hopefully, novel studies will help fill this gap in the future.
5. Endogenous Production of SPMs in Animal Models of Infection
In addition to the studies evaluating the effect of exogenous (administrated) SPMs in different disease contexts, endogenous lipid mediator levels have been quantified upon infection in certain animal studies, as summarized in Table 3.

Table 3.
Studies that verified endogenous production of SPMs upon administration of bacteria, viruses, or parasites.
5.1. Production of SPMs in Animals upon Bacterial Infection
While SPMs have been shown to control E. coli infection, as discussed above, E. coli is also known to induce the production of SPMs by different cells. In a self-resolving model of the E. coli infection, endogenous RvD3 [172] and MaR1 [173] were detected in inflammatory peritoneal exudates (24 h and 4 h after infection, respectively), indicating that production of these mediators is induced upon infection. Similarly, after stimulus with E. coli, mouse and human vagus nerves produce SPMs to contribute to host homeostasis [174]. The human vagus nerve produces RvD4, RvD6, MaR1, 4S,14S-diHDHA, and 15-epi-LXA4 while the mouse vagus nerve produces only PDX [174], showing a species-specific production of SPM upon E. coli infection. This is in accordance with a study showing that vagotomy delays resolution of self-limiting E. coli infection by controlling the production of PD conjugate in tissue regeneration (PCTR1) and PD1 [175]. Therefore, in self-resolving E. coli infection, bacteria stimulate the vagus nerve to produce SPM that have a role in resolution of inflammation and infection. The role of the vagus nerve is biologically relevant since vagotomy delays resolution by impairing SPM production.
Acetylcholine derived from neurons acts on CD335+ILC3 to increase the production of the PCTR1 pathway marker 17-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid (17-HDHA). Additionally, adoptive transfer of ILC3 in E. coli-infected and vagotomized mice restore resolution index by reducing neutrophil recruitment and shortening resolution interval [175], demonstrating the relevance of vagus nerve–ILC3 communication. Expanding this cross-talk, in case of depletion of ILC3 or in vagotomized mice, peritoneal macrophages produce lower levels of PCTR1, indicating a role for ILC3–macrophage cross-talk in PCTR1 production. Reduction in peritoneal ILC3, furthermore, results in impaired E. coli phagocytosis, increased peritoneal bacterial load, and increased inflammation-initiating eicosanoids PGD2, PGE2, PGF2α, TXB2 and LTB4 [175]. In accordance, adoptive transfer of ILC3 treated with a lipoxygenase inhibitor abrogates the beneficial effect of these cells, indicating that ILC3 needs active lipoxygenase likely to produce SPMs, which then actively induce peritonitis resolution and host responses to infections [175]. Altogether, these data not only show the immunoresolvent properties of SPMs but also that cellular communications exist through the release of SPM. These cellular communications can be both from immune cell to immune cell and from neuron to immune cell. These types of cellular communications use SPMs to regulate infection outcome.
Pneumonia caused by E. coli in mice resulted in temporal synthesis and release of AT-RvD1, detected 24 h and peaking 72 h after infection, as determined by metabololipidomics performed on lung tissue [126]. In a similar model of pneumococcal pneumonia in mice, blockade of ALX/FPR2 (lipoxins and some D-series resolvins receptor) increased pulmonary edema, bacterial burden, and protein accumulation on air spaces [179]. This indicates that impairment of the resolution phase of the inflammation may contribute to disease worsening and progression to chronic disease. In fact, upregulation of the production of SPMs may be a protective mechanism exerted by beneficial microorganisms—the probiotic bacteria Clostridium butyricum MIYAIRI 588 helps protect the gut epithelial barrier from damage caused by antibiotic treatment by upregulating PD1, palmitoleic acid and 15d-prostaglandin J2 [176]. Furthermore, in animals lacking a nucleotide-binding domain, leucine-rich repeat-containing receptor, pyrin domain-containing-3 (NLRP3) inflammasome, CLP-induced sepsis resulted in decreased mortality due to reduced proinflammatory mediators and augmented LXB4 generation [118]; in addition, M. tuberculosis-infected mice produce LXA4 to enhance control of the infection [180].
Although not in a mouse model of infection induced by bacteria, α-Hemolysin (Hla) from S. aureus induces SPM formation. Intraperitoneal injection of Hla increases production of 15-lipoxygenase-1 (15-LOX-1) enzyme in murine M2-like macrophages. This effect is correlated to an increase in Hla phagocytosis and depletion of Hla impaired SPM formation, indicating that recognition of Hla by macrophages is important to promote bactericidal effects [181]. Interestingly, it was also noted that a particular threshold of bacterial challenge is required to activate different mediator pathways: 15-LOX-1-mediated SPM production required higher S. aureus multiplicity of infection (MOI) than that for 5-LOX or COX products. Also, SPM levels were detected later than COX/5-LOX products (starting at 90 min and 30 min, respectively) [181]. This suggests that different pathways may be activated during the response against S. aureus to promote distinct lipid mediator production.
5.2. Production of SPMs in Animals upon Viral Infection
Unfortunately, there is limited evidence on the quantification of SPM levels in animal models of viral infections. Animals that undergo severe influenza infection show increased levels of COX pathway products, such as PGE2, and decreased levels of DHA metabolites: 17-HDoHE—resolvin and protectin precursor—PD1, and LXA4 in lung tissue. Interestingly, these metabolites showed a large impact on the viral replication in vitro and on survival rates, when administered, in mice [135]. Similarly, 17-HDoHE was identified in murine samples following administration of sublethal doses of different influenza viruses, PR8/H1N1 (high pathogenicity) and X31/H3N2 (low pathogenicity). In the same study, different forms of hydroxylated DHA were detected in specific stages of the infection: 4-, 10-, 13-, and 20-HDoHE levels increased between days 3 and 5 after infection with lethal doses of PR8/H1N1, while 8-, 14-, and 16-HDoHE peaked at day 3 [106]. The importance of temporal identification of SPMs and their precursors relies on the fact that contrasting results have been reported on pro-resolving mediator administration in early versus late phase of the infection, highlighting the relevance of completely elucidating the way in which resolution is orchestrated as well as the beneficial SPM treatment administration time frame [116,123].
5.3. Production of SPMs in Animals upon Parasitic Infection
As previously mentioned, since SPMs are endogenous players of inflammation resolution, regulation of production of these mediators in a timely manner is essential. Parasitic infections are not different. Cytokines such as TNFα, IFN-γ and IL-12 are essential for host resistance against the protozoan Toxoplasma gondii. During the early phase (5–10 days after inoculation), IL-10 seems to be the main player regulating IL-12-dependent IFN-γ production, but after IL-10 levels decrease, there is a substantial production of LXA4, beginning at day 10 and reaching a plateau at day 15 after infection, limiting excessive cytokine production and mortality during a late phase of the infection. Therefore, LXA4 seems to work in synergy with IL-10, as the mouse strain deficient in the enzyme that produces LXA4, 5-LOX−/− succumb two weeks after inoculation [177]. In another article, the authors inoculated Toxocara canis and Toxocara cati eggs in mice to induce neurotoxocarosis, a common and underestimated parasitic infection that affects populations globally [178]. Temporal analysis of bioactive lipid metabolites during the infection showed a significant increase in LOX-derived metabolites when compared to noninfected group. In addition, significantly elevated levels of NPD1 were detected at days 14, 28 and 42 after infection (p.i.) in T. canis-infected and at days 14 and 42 p.i. in T. cati-infected mouse brain. In the cerebellum, increased levels of NPD1 were additionally detected on day 98 (T. canis) and day 28 (T. cati) [178]. Neuronal cell survival, control of leukocyte infiltration, oxidative stress protection, and inhibition of COX-2 expression and NF-κB activation are some of the effects of NPD1 in the brain [182,183]. These studies contribute to understanding the temporal production profile of these mediators and the ways in which they coordinate resolution, infection control and healing.
In this manner, these data suggest that impairment of the resolution phase of the inflammation, either by compromising cell signaling, pathogen recognition by the host or disruption of temporally regulated SPM production and release are directly related to worsening disease progression and outcomes.
6. Conclusions
Management of diseases caused by pathogens have been a challenge for centuries, and infections are one of the leading causes of morbidity and mortality globally. Factors that contribute to this problem can be pathogen-related, such as antibiotic resistance and virulence factors, or host-related, such as a lack of basic resources to prevent or treat infections and a debilitated immune response (due to comorbidities, stress, habits, etc.), and all directly impact morbidity and mortality of infections. In fact, perpetuation of the inflammatory response is commonly associated with high-prevalence neurodegenerative, cardiovascular, and rheumatic diseases [184]. A previous assumption was that resolution of inflammation would be a passive process; however, it is currently understood as an active process which is induced by SPMs.
As key players of resolution, SPMs are produced during self-resolving sterile and non-sterile inflammation. During infections, accumulating evidence shows that endogenous production of pro-resolving mediators correlates with lower cytokine levels and is linked to a better prognosis in patients [90,98], and disruption of SPM production or signaling negatively impacts microbicidal response in animals [175,179]. Pre-clinical studies point out the bacterial clearance capacity of SPMs [10,112,113,114,120,124,126,127,129,131,132,133,141], reduction in leukocyte influx [110,113,114,117,118,120,123,125,127,129,132,134,136,142] and enhanced phagocytosis/efferocytosis [113,114,118,123,124,126,130,133]. These activities are tissue- and disease-specific [6], and temporal regulation of SPM production seems to be essential to orchestrate a beneficial instead of a deleterious response [116,123]. We have summarized the SPM mechanisms in Figure 2.
Along with proper elucidation of the ways in which SPMs act during infections, future research is needed to address current limitations in this field. First, new families of SPMs are being discovered, and the receptors they may act on still need to be investigated. Second, orthologs from different species need to be explored and part of lipid mediator profiling in pre-clinical studies may not be transferable to humans [185]. There are a few interventional clinical trials involving SPMs or their precursors (ClinicalTrials.gov Identifier: NCT02719665; NCT01675570; NCT04088240; NCT04308889; NCT01865448); unfortunately, none of them involve infectious diseases and all lack results and conclusions thus far. This fact limits the discussion of the clinical pharmacological perspectives of SPMs in infections. Third, development of analogs with increased biostability may overcome challenges and help uncover physiologic roles of these mediators, so SPMs can become useful tools as biomarkers and effective treatments soon. Fourth, lipidomic studies are extremely useful; however, it seems that we also need information about the profile of SPM receptors and the cells that are expressing those receptors. Evidence has determined that enhancing SPM receptor increases the final biological activity without alteration of SPM concentration [186]. This indicates that in addition to the levels of SPMs, the profile of receptor expression is essential information to understand their contribution to disease outcomes. Therefore, knocking out specific receptors in selected cell types would be an essential approach to bring definitive evidence on the endogenous roles of SPMs in infections and other diseases. In any case, the pharmacological use of SPMs is also a valid approach evidencing the therapeutic application of exogenous SPMs administration.
Author Contributions
Conception and design of the article, F.S.R.-O., V.F., R.C. and W.A.V.J.; writing—original draft preparation, F.S.R.-O., M.D.V.d.S., G.M.-C. and V.F.; writing—critical review and editing for important intellectual content, V.F., R.C. and W.A.V.J.; supervision, V.F., R.C. and W.A.V.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; #309633/2021-4; #307852/2019-9; #405027/2021-4; #427946/2018-2; #203112/2020-2); PPSUS grant funded by Decit/SCTIE/MS intermediated by CNPq with support of Fundação Araucária and SESA-PR (agreement #041/2017); PRONEX grant supported by SETI/Fundação Araucária and MCTI/CNPq, and Governo do Estado do Paraná (agreement #014/2017); Fundação Araucária (PBA/PROPPG 13/2021 agreements #276/2022-PBA and #250/2022-PBA); Financiadora de Estudos e Projetos—FINEP; and CAPES (finance code #001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Images were created using BioRender.com. The authors would like to thank Benjamin Zhu for English language revisions.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Julliard, W.A.; Myo, Y.P.A.; Perelas, A.; Jackson, P.D.; Thatcher, T.H.; Sime, P.J. Specialized pro-resolving mediators as modulators of immune responses. Semin. Immunol. 2022, 59, 101605. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2015, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Li, Y.; Dalli, J.; Chiang, N.; Serhan, C.N. Self-Limited versus Delayed Resolution of Acute Inflammation: Temporal Regulation of Pro-Resolving Mediators and MicroRNA. Sci. Rep. 2012, 2, 639. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Fattori, V.; Ferraz, C.R.; Rasquel-Oliveira, F.S.; Verri, W.A. Neuroimmune communication in infection and pain: Friends or foes? Immunol. Lett. 2020, 229, 32–43. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A. Specialized pro-resolving lipid mediators: A new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol. Res. 2019, 151, 104549. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; E Kaufmann, S.H.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Abbasoglu Ozgoren, A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, A.J.; Perretti, M.; Rossi, A.G.; John, L. Resolution of Inflammation: State of the Art, Definitions and Terms. FASEB J. 2011, 21, 325–332. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr. Opin. Pharmacol. 2006, 6, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment That Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and Its Aspirin-triggered 17R Epimer: Stereochemical Assignments, Anti-Inflammatory Properties, and Enzymatic Inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Hellmann, J.; Sansbury, B.E.; Wong, B.; Li, X.; Singh, M.; Nuutila, K.; Chiang, N.; Eriksson, E.; Serhan, C.N.; Spite, M. Biosynthesis of D-Series Resolvins in Skin Provides Insights into Their Role in Tissue Repair. J Invest Dermatol 2018, 138, 2051–2060. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Lu, Y.; Siegelman, J.; Baer, T.; Yang, R.; Colgan, S.P.; Petasis, N.A. Anti-Inflammatory Actions of Neuroprotectin D1/Protectin D1 and Its Natural Stereoisomers: Assignments of Dihydroxy-Containing Docosatrienes. J. Immunol. 2006, 176, 1848–1859. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2008, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-Inflammatory and Pro-Resolving Lipid Mediators. Annu. Rev. Pathol. 2009, 3, 279–312. [Google Scholar] [CrossRef]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef]
- Tjonahen, E.; Oh, S.F.; Siegelman, J.; Elangovan, S.; Percarpio, K.B.; Hong, S.; Arita, M.; Serhan, C.N. Resolvin E2: Identification and Anti-Inflammatory Actions: Pivotal Role of Human 5-Lipoxygenase in Resolvin E Series Biosynthesis. Chem Biol 2006, 13, 1193–1202. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and Structure Determination of Novel Anti-inflammatory Mediator Resolvin E3, 17,18-Dihydroxyeicosapentaenoic Acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2021, 11, 631319. [Google Scholar] [CrossRef]
- Reinertsen, A.F.; Primdahl, K.G.; Shay, A.E.; Serhan, C.N.; Hansen, T.V.; Aursnes, M. Stereoselective Synthesis and Structural Confirmation of the Specialized Pro-Resolving Mediator Resolvin E4. J. Org. Chem. 2021, 86, 3535–3545. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2015, 785, 144–155. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Vlasakov, I.; Riley, I.R.; Rodriguez, A.R.; Spur, B.W.; Petasis, N.A.; Chiang, N.; Serhan, C.N. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 12232–12237. [Google Scholar] [CrossRef]
- Serhan, C.N. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1994, 1212, 1–25. [Google Scholar] [CrossRef]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Serhan, C.N. Lipoxin generation by permeabilized human platelets. Biochemistry 1992, 31, 8269–8277. [Google Scholar] [CrossRef]
- Romano, M. Lipoxin and Aspirin-Triggered Lipoxins. ScientificWorldJournal 2010, 10, 1048–1064. [Google Scholar] [CrossRef]
- Maderna, P.; Cottell, D.C.; Toivonen, T.; Dufton, N.; Dalli, J.; Perretti, M.; Godson, C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010, 24, 4240–4249. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Abdulnour, R.-E.E.; Walker, K.H.; Engstrom, B.D.; Levy, B.D. Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases. Physiol. Rev. 2018, 98, 1335–1370. [Google Scholar] [CrossRef]
- Chiang, N.; Arita, M.; Serhan, C.N. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 163–177. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.-Y.C.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Potent Immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 Formation and Impact in Inflammation Resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef]
- Toda, A.; Yokomizo, T.; Shimizu, T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 575–585. [Google Scholar] [CrossRef]
- Flak, M.B.; Koenis, D.S.; Sobrino, A.; Smith, J.; Pistorius, K.; Palmas, F.; Dalli, J. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J. Clin. Investig. 2019, 130, 359–373. [Google Scholar] [CrossRef]
- Liu, G.-J.; Tao, T.; Wang, H.; Zhou, Y.; Gao, X.; Gao, Y.-Y.; Hang, C.-H.; Li, W. Functions of resolvin D1-ALX/FPR2 receptor interaction in the hemoglobin-induced microglial inflammatory response and neuronal injury. J. Neuroinflamm. 2020, 17, 239. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 Binds Human Phagocytes with Evidence for Proresolving Receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Dalli, J.; Norling, L.V.; Colas, R.A.; Perretti, M.; Serhan, C.N. Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J. Immunol. 2016, 197, 2362–2368. [Google Scholar] [CrossRef]
- Qu, L.; Caterina, M.J. Accelerating the reversal of inflammatory pain with NPD1 and its receptor GPR37. J. Clin. Investig. 2018, 128, 3246–3249. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Zhao, M.; Li, C.; Zhang, J.; Yin, Z.; Zheng, Z.; Wan, J.; Wang, M. Maresin-1 and its receptors RORα/LGR6 as potential therapeutic target for respiratory diseases. Pharmacol. Res. 2022, 182, 106337. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Serhan, C.N. Novel n-3 Immunoresolvents: Structures and Actions. Sci. Rep. 2013, 3, srep01940. [Google Scholar] [CrossRef]
- Vik, A.; Dalli, J.; Hansen, T.V. Recent advances in the chemistry and biology of anti-inflammatory and specialized pro-resolving mediators biosynthesized from n-3 docosapentaenoic acid. Bioorganic. Med. Chem. Lett. 2017, 27, 2259–2266. [Google Scholar] [CrossRef]
- Dalli, J.; Chiang, N.; Serhan, C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 2015, 21, 1071–1075. [Google Scholar] [CrossRef]
- Weylandt, K.H. Docosapentaenoic Acid Derived Metabolites and Mediators – The New World of Lipid Mediator Medicine in a Nutshell. Eur J Pharmacol 2016, 785, 108–115. [Google Scholar] [CrossRef]
- Jordan, P.M.; Werz, O. Specialized pro-resolving mediators: Biosynthesis and biological role in bacterial infections. FEBS J. 2021, 289, 4212–4227. [Google Scholar] [CrossRef]
- Horn, T.; Adel, S.; Schumann, R.; Sur, S.; Kakularam, K.R.; Polamarasetty, A.; Redanna, P.; Kuhn, H.; Heydeck, D. Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signaling. Prog. Lipid Res. 2014, 57, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Chen, X.-S.; Johnson, E.N.; Zhao, L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002, 68–69, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Rao, G.N. Emerging Role of 12/15-Lipoxygenase (ALOX15) in Human Pathologies. Prog Lipid Res 2019, 73, 28. [Google Scholar] [CrossRef] [PubMed]
- Adel, S.; Karst, F.; González-Lafont, À.; Pekárová, M.; Saura, P.; Masgrau, L.; Lluch, J.M.; Stehling, S.; Horn, T.; Kuhn, H.; et al. Evolutionary alteration of ALOX15 specificity optimizes the biosynthesis of antiinflammatory and proresolving lipoxins. Proc. Natl. Acad. Sci. USA 2016, 113, E4266–E4275. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, P.; Yang, J.; Wu, Y. The Regulatory Effect of Specialized Pro-Resolving Mediators on Immune Cells. Biomed Pharmacother 2022, 156. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Proudman, S.; Cleland, L.; James, M.; Proudman, S.; Cleland, L. Dietary n-3 fats as adjunctive therapy in a prototypic inflammatory disease: Issues and obstacles for use in rheumatoid arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nano-Proresolving Medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Dillion, C.; Beasley, M.; Dudenbostel, T.; Oparil, S.; Limdi, N.A. Race-based and sex-based differences in bioactive lipid mediators after myocardial infarction. ESC Hear. Fail. 2020, 7, 1700–1710. [Google Scholar] [CrossRef]
- Howard, G.; Cushman, M.; Moy, C.S.; Oparil, S.; Muntner, P.; Lackland, D.T.; Manly, J.J.; Flaherty, M.L.; Judd, S.E.; Wadley, V.G.; et al. Association of Clinical and Social Factors with Excess Hypertension Risk in Black Compared with White US Adults. JAMA 2018, 320, 1338–1348. [Google Scholar] [CrossRef]
- Laneuville, O.; Breuer, D.K.; Xu, N.; Huang, Z.; Gage, D.A.; Watson, J.T.; Lagarde, M.; DeWitt, D.L.; Smith, W.L. Fatty Acid Substrate Specificities of Human Prostaglandin-endoperoxide H Synthase-1 and −2. J. Biol. Chem. 1995, 270, 19330–19336. [Google Scholar] [CrossRef]
- Sharma, N.P.; Dong, L.; Yuan, C.; Noon, K.R.; Smith, W.L. Asymmetric Acetylation of the Cyclooxygenase-2 Homodimer by Aspirin and Its Effects on the Oxygenation of Arachidonic, Eicosapentaenoic, and Docosahexaenoic Acids. Mol. Pharmacol. 2010, 77, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; DeLong, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and Receptors of Prostaglandin Pathways with Arachidonic Acid-derived Versus Eicosapentaenoic Acid-derived Substrates and Products. J. Biol. Chem. 2007, 282, 22254–22266. [Google Scholar] [CrossRef] [PubMed]
- Fierro, I.M.; Kutok, J.L.; Serhan, C.N. Novel Lipid Mediator Regulators of Endothelial Cell Proliferation and Migration: Aspirin-Triggered-15R-Lipoxin A(4) and Lipoxin A(4). J Pharmacol Exp Ther 2002, 300, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, L.; Goloshchapova, K.; Heydeck, D.; Stehling, S.; Kuhn, H.; Schebb, N.H. Mammalian ALOX15 orthologs exhibit pronounced dual positional specificity with docosahexaenoic acid. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 666–675. [Google Scholar] [CrossRef]
- Kahnt, A.S.; Schebb, N.H.; Steinhilber, D. Formation of lipoxins and resolvins in human leukocytes. Prostaglandins Other Lipid Mediat. 2023, 166, 106726. [Google Scholar] [CrossRef]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.-H. Cytochrome P450–dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef]
- Fer, M.; Dréano, Y.; Lucas, D.; Corcos, L.; Salaün, J.-P.; Berthou, F.; Amet, Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch. Biochem. Biophys. 2008, 471, 116–125. [Google Scholar] [CrossRef]
- Barbosa-Sicard, E.; Markovic, M.; Honeck, H.; Christ, B.; Muller, D.N.; Schunck, W.-H. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem. Biophys. Res. Commun. 2005, 329, 1275–1281. [Google Scholar] [CrossRef]
- Wuest, S.J.; Crucet, M.; Gemperle, C.; Loretz, C.; Hersberger, M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis 2012, 225, 121–127. [Google Scholar] [CrossRef]
- Ebert, R.; Cumbana, R.; Lehmann, C.; Kutzner, L.; Toewe, A.; Ferreirós, N.; Parnham, M.J.; Schebb, N.H.; Steinhilber, D.; Kahnt, A.S. Long-term stimulation of toll-like receptor-2 and -4 upregulates 5-LO and 15-LO-2 expression thereby inducing a lipid mediator shift in human monocyte-derived macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158702. [Google Scholar] [CrossRef]
- Conrad, D.J.; Kuhn, H.; Mulkins, M.; Highland, E.; Sigal, E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 1992, 89, 217–221. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Benatzy, Y.; Schmid, T.; Namgaladze, D.; Mainka, M.; Schebb, N.H.; Lütjohann, D.; Brüne, B. Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation. Cell Death Differ. 2020, 28, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- von Hegedus, J.H.; Kahnt, A.S.; Ebert, R.; Heijink, M.; Toes, R.E.; Giera, M.; Ioan-Facsinay, A. Toll-like receptor signaling induces a temporal switch towards a resolving lipid profile in monocyte-derived macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158740. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Jordan, P.M.; Romp, E.; Czapka, A.; Rao, Z.; Kretzer, C.; Koeberle, A.; Garscha, U.; Pace, S.; Claesson, H.E.; et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019, 33, 6140–6153. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 Receptor Stereoselectivity and Regulation of Inflammation and Proresolving MicroRNAs. Am. J. Pathol. 2012, 180, 2018–2027. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Omega-3 versus Omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A2s. J. Lipid Res. 2021, 62, 100113. [Google Scholar] [CrossRef]
- Miki, Y.; Yamamoto, K.; Taketomi, Y.; Sato, H.; Shimo, K.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; Nakanishi, H.; Ikeda, K.; et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013, 210, 1217–1234. [Google Scholar] [CrossRef]
- Diaz, O.; Berquand, A.; Dubois, M.; Di Agostino, S.; Sette, C.; Bourgoin, S.; Lagarde, M.; Némoz, G.; Prigent, A.-F. The Mechanism of Docosahexaenoic Acid-induced Phospholipase D Activation in Human Lymphocytes Involves Exclusion of the Enzyme from Lipid Rafts. J. Biol. Chem. 2002, 277, 39368–39378. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Quintana, C.; Barragan-Bradford, D.; Hurwitz, S.; Levy, B.D.; Choi, A.M.; Serhan, C.N.P.; Baron, R.M. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations with Survival and Clinical Outcomes. Crit. Care Med. 2017, 45, 58–68. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Shih, C.-H.; Yu, Y.-B.; Hsu, H.-C. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein-3a, and neutrophil gelatinase-associated lipocalin. J. Chin. Med. Assoc. 2013, 76, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Dalli, J.; Kadam, D.; Gaikwad, S.; Barthwal, M.; Colas, R.A.; Mazzacuva, F.; Lokhande, R.; Dharmshale, S.; Bharadwaj, R.; et al. Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes. Prostaglandins Other Lipid Mediat. 2020, 147, 106398. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.E.; Richter, C.K.; Skulas-Ray, A.C.; Flock, M.R.; Harsch, B.A.; Annevelink, C.E.; Kris-Etherton, P.M.; Jensen, G.L.; Shearer, G.C. Effect of omega-3 ethyl esters on the triglyceride-rich lipoprotein response to endotoxin challenge in healthy young men. J. Lipid Res. 2023, 64, 100353. [Google Scholar] [CrossRef]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050. [Google Scholar] [CrossRef]
- Turnbull, J.; Jha, R.R.; A Ortori, C.; Lunt, E.; Tighe, P.J.; Irving, W.L.; A Gohir, S.; Kim, D.-H.; Valdes, A.M.; Tarr, A.W.; et al. Serum Levels of Proinflammatory Lipid Mediators and Specialized Proresolving Molecules Are Increased in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 and Correlate With Markers of the Adaptive Immune Response. J. Infect. Dis. 2022, 225, 2142–2154. [Google Scholar] [CrossRef]
- Archambault, A.-S.; Zaid, Y.; Rakotoarivelo, V.; Turcotte, C.; Doré, É.; Dubuc, I.; Martin, C.; Flamand, O.; Amar, Y.; Cheikh, A.; et al. High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB J. 2021, 35, e21666. [Google Scholar] [CrossRef]
- Palmas, F.; Clarke, J.; Colas, R.A.; Gomez, E.A.; Keogh, A.; Boylan, M.; McEvoy, N.; McElvaney, O.J.; McElvaney, O.; Alalqam, R.; et al. Dysregulated plasma lipid mediator profiles in critically ill COVID-19 patients. PLoS ONE 2021, 16, e0256226. [Google Scholar] [CrossRef]
- Irún, P.; Gracia, R.; Piazuelo, E.; Pardo, J.; Morte, E.; Paño, J.R.; Boza, J.; Carrera-Lasfuentes, P.; Higuera, G.A.; Lanas, A. Serum lipid mediator profiles in COVID-19 patients and lung disease severity: A pilot study. Sci. Rep. 2023, 13, 6497. [Google Scholar] [CrossRef]
- Koenis, D.S.; Beegun, I.; Jouvene, C.C.; Aguirre, G.A.; Souza, P.R.; Gonzalez-Nunez, M.; Ly, L.; Pistorius, K.; Kocher, H.M.; Ricketts, W.; et al. Disrupted Resolution Mechanisms Favor Altered Phagocyte Responses in COVID-19. Circ. Res. 2021, 129, e54–e71. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: A promising therapeutic strategy for SARS-CoV-2 cytokine storm. Arch. Pharmacal Res. 2021, 44, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2021, 52, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Al-Gareeb, A.I.; Elekhnawy, E.; Al-Kuraishy, H.M. Potential role of lipoxin in the management of COVID-19: A narrative review. Inflammopharmacology 2022, 30, 1993–2001. [Google Scholar] [CrossRef]
- Recchiuti, A.; Patruno, S.; Mattoscio, D.; Isopi, E.; Pomilio, A.; Lamolinara, A.; Iezzi, M.; Pecce, R.; Romano, M. Resolvin D1 and D2 reduce SARS-CoV-2-induced inflammatory responses in cystic fibrosis macrophages. FASEB J. 2021, 35, e21441. [Google Scholar] [CrossRef]
- Tam, V.C.; Quehenberger, O.; Oshansky, C.M.; Suen, R.; Armando, A.M.; Treuting, P.M.; Thomas, P.G.; Dennis, E.A.; Aderem, A. Lipidomic Profiling of Influenza Infection Identifies Mediators that Induce and Resolve Inflammation. Cell 2013, 154, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.A.; Colas, R.A.; Souza, P.R.; Hands, R.; Lewis, M.J.; Bessant, C.; Pitzalis, C.; Dalli, J. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat. Commun. 2020, 11, 5420. [Google Scholar] [CrossRef]
- Prieto, P.; Rosales-Mendoza, C.E.; Terron, V.; Toledano, V.; Cuadrado, A.; López-Collazo, E.; Bannenberg, G.; Martín-Sanz, P.; Fernández-Velasco, M.; Boscá, L. Activation of Autophagy in Macrophages by Pro-Resolving Lipid Mediators. Autophagy 2015, 11, 1729–1744. [Google Scholar] [CrossRef]
- Ueda, T.; Fukunaga, K.M.; Seki, H.M.; Miyata, J.M.; Arita, M.; Miyasho, T.; Obata, T.; Asano, K.M.; Betsuyaku, T.M.; Takeda, J.M. Combination Therapy of 15-Epi-Lipoxin A4 With Antibiotics Protects Mice from Escherichia coli–Induced Sepsis. Crit. Care Med. 2014, 42, e288–e295. [Google Scholar] [CrossRef]
- El Kebir, D.; József, L.; Pan, W.; Wang, L.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. 15-Epi-lipoxin A4 Inhibits Myeloperoxidase Signaling and Enhances Resolution of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2009, 180, 311–319. [Google Scholar] [CrossRef]
- Shryock, N.; McBerry, C.; Gonzalez, R.M.S.; Janes, S.; Costa, F.T.M.; Aliberti, J. Lipoxin A4 and 15-Epi-Lipoxin A4 Protect against Experimental Cerebral Malaria by Inhibiting IL-12/IFN-γ in the Brain. PLoS ONE 2013, 8, e61882. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Dichter, E.; Lacorte, G.; Kerner, D.; Spur, B.; Rodriguez, A.; Yin, K. Lipoxin A4 Increases Survival by Decreasing Systemic Inflammation and Bacterial Load in Sepsis. Shock 2011, 36, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Walker, J.; Spur, B.; Rodriguez, A.; Yin, K. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot. Essent. Fat. Acids 2014, 94, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Capilato, J.; Pham, M.P.; Walker, J.; Spur, B.; Rodriguez, A.; Perez, L.J.; Yin, K. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J. 2016, 30, 2400–2410. [Google Scholar] [CrossRef]
- Souza, M.C.; Pádua, T.A.; Torres, N.D.; Costa, M.F.S.; Candéa, A.P.; Maramaldo, T.; Seito, L.N.; Penido, C.; Estato, V.; Antunes, B.; et al. Lipoxin A 4 attenuates endothelial dysfunction during experimental cerebral malaria. Int. Immunopharmacol. 2015, 24, 400–407. [Google Scholar] [CrossRef]
- Sordi, R.; Menezes-De-Lima, O., Jr.; Horewicz, V.; Scheschowitsch, K.; Santos, L.F.; Assreuy, J. Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int. Immunopharmacol. 2013, 17, 283–292. [Google Scholar] [CrossRef]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced Inflammation and Tissue Damage in Transgenic Rabbits Overexpressing 15-Lipoxygenase and Endogenous Anti-inflammatory Lipid Mediators. J. Immunol. 2003, 171, 6856–6865. [Google Scholar] [CrossRef]
- Lee, S.; Nakahira, K.; Dalli, J.; Siempos, I.I.; Norris, P.C.; Colas, R.A.; Moon, J.-S.; Shinohara, M.; Hisata, S.; Howrylak, J.A.; et al. NLRP3 Inflammasome Deficiency Protects against Microbial Sepsis via Increased Lipoxin B4 Synthesis. Am. J. Respir. Crit. Care Med. 2017, 196, 713–726. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A.; Goguet-Surmenian, E.; Blackwood, A.; Andry, C.; Serhan, C.N.; Van Dyke, T.E. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. J. Immunol. 2007, 179, 7021–7029. [Google Scholar] [CrossRef]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The Anti-Inflammatory and Proresolving Mediator Resolvin E1 Protects Mice from Bacterial Pneumonia and Acute Lung Injury. J. Immunol. 2009, 184, 836–843. [Google Scholar] [CrossRef]
- Rajasagi, N.K.; Reddy, P.B.J.; Suryawanshi, A.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Controlling Herpes Simplex Virus-Induced Ocular Inflammatory Lesions with the Lipid-Derived Mediator Resolvin E1. J. Immunol. 2011, 186, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.D.; Omori, K.; Ito, T.; Yamashiro, K.; Nakamura, S.; Okamoto, K.; Ono, M.; Yamamoto, T.; Dyke, T.E.V.; Takashiba, S. Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodotitis. Front. Immunol. 2019, 10, 307. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef]
- Rajasagi, N.K.; Bhela, S.; Varanasi, S.K.; Rouse, B.T. Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J. Leukoc. Biol. 2017, 102, 1159–1171. [Google Scholar] [CrossRef]
- Abdulnour, R.; Sham, H.; Douda, D.; Colas, R.; Dalli, J.; Bai, Y.; Ai, X.; Serhan, C.; Levy, B. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2015, 9, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Isopi, E.; Mattoscio, D.; Codagnone, M.; Mari, V.C.; Lamolinara, A.; Patruno, S.; D’aurora, M.; Cianci, E.; Nespoli, A.; Franchi, S.; et al. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front. Immunol. 2020, 11, 581. [Google Scholar] [CrossRef]
- Carrillo, I.; Rabelo, R.A.N.; Barbosa, C.; Rates, M.; Fuentes-Retamal, S.; González-Herrera, F.; Guzmán-Rivera, D.; Quintero, H.; Kemmerling, U.; Castillo, C.; et al. Aspirin-triggered resolvin D1 reduces parasitic cardiac load by decreasing inflammation in a murine model of early chronic Chagas disease. PLOS Negl. Trop. Dis. 2021, 15, e0009978. [Google Scholar] [CrossRef]
- Chen, F.; Fan, X.H.; Wu, Y.P.; Zhu, J.L.; Wang, F.; Bo, L.L.; Li, J.B.; Bao, R.; Deng, X.M. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 33, 457–464. [Google Scholar] [CrossRef]
- Croasdell, A.; Lacy, S.H.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P. Resolvin D1 Dampens Pulmonary Inflammation and Promotes Clearance of Nontypeable Haemophilus influenzae. J. Immunol. 2016, 196, 2742–2752. [Google Scholar] [CrossRef]
- Diaz, L.A.; Altman, N.H.; Khan, W.; Serhan, C.N.; Adkins, B. Specialized Proresolving Mediators Rescue Infant Mice from Lethal Citrobacter Rodentium Infection and Promote Immunity against Reinfection. Infect. Immun. 2017, 85, e00464-17. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Sakuma, M.; Rodriguez, A.R.; Spur, B.W.; Irimia, D.; Serhan, C.N. Resolvin T-series reduce neutrophil extracellular traps. Blood 2022, 139, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, L.; Liu, H.; Sun, Z.; Yang, W.; Yang, Y.; Cui, S.; Li, S.; Wang, Y.; Song, L.; et al. Protectin DX increases survival in a mouse model of sepsis by ameliorating inflammation and modulating macrophage phenotype. Sci. Rep. 2017, 7, 99. [Google Scholar] [CrossRef]
- Xia, H.; Ge, Y.; Wang, F.; Ming, Y.; Wu, Z.; Wang, J.; Sun, S.; Huang, S.; Chen, M.; Xiao, W.; et al. Protectin DX Ameliorates Inflammation in Sepsis-Induced Acute Lung Injury through Mediating PPARγ/NF-ΚB Pathway. Immunol. Res. 2020, 68, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, N.K.; Reddy, P.B.J.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Neuroprotectin D1 Reduces the Severity of Herpes Simplex Virus–Induced Corneal Immunopathology. Investig. Opthalmology Vis. Sci. 2013, 54, 6269–6279. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ma, Z.; Ma, M.; Wang, D.; Xie, G.; Yin, Y.; Zhang, P.; Tao, K. Maresin 1 Mitigates Inflammatory Response and Protects Mice from Sepsis. Mediat. Inflamm. 2016, 2016, 3798465. [Google Scholar] [CrossRef]
- Sun, S.J.; Wang, J.M.; Wang, J.X.; Wang, F.Q.; Yao, S.L.; Xia, H.F. Maresin 1 Mitigates Sepsis-Associated Acute Kidney Injury in Mice via Inhibition of the NF-ΚB/STAT3/MAPK Pathways. Front Pharmacol 2019, 10. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Walker, K.H.; Brüggemann, T.R.; Tavares, L.P.; Smith, E.W.; Nijmeh, J.; Bai, Y.; Ai, X.; Cagnina, R.E.; Duvall, M.G.; et al. The Maresin 1–LGR6 Axis Decreases Respiratory Syncytial Virus-Induced Lung Inflammation. Proc. Natl. Acad. Sci. USA 2023, 120, e2206480120. [Google Scholar] [CrossRef]
- Codagnone, M.; Cianci, E.; Lamolinara, A.; Mari, V.C.; Nespoli, A.; Isopi, E.; Mattoscio, D.; Arita, M.; Bragonzi, A.; Iezzi, M.; et al. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol. 2018, 11, 35–49. [Google Scholar] [CrossRef]
- Hao, Y.; Zheng, H.; Wang, R.-H.; Li, H.; Yang, L.-L.; Bhandari, S.; Liu, Y.-J.; Han, J.; Smith, F.G.; Gao, H.-C.; et al. Maresin1 Alleviates Metabolic Dysfunction in Septic Mice: A 1H NMR-Based Metabolomics Analysis. Mediat. Inflamm. 2019, 2019, 2309175. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-W.; Zhang, L.; Lian, Q.-Q.; Liu, D.; Wu, P.; Yao, S.-L.; Ye, D.-Y. Posttreatment with Aspirin-Triggered Lipoxin A4 Analog Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice: The Role of Heme Oxygenase-1. Obstet. Anesth. Dig. 2007, 104, 369–377. [Google Scholar] [CrossRef]
- Cutuli, S.L.; Cascarano, L.; Lazzaro, P.; Tanzarella, E.S.; Pintaudi, G.; Grieco, D.L.; De Pascale, G.; Antonelli, M. Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review. Microorganisms 2023, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Sandhaus, S.; Swick, A.G. Specialized proresolving mediators in infection and lung injury. Biofactors 2020, 47, 6–18. [Google Scholar] [CrossRef]
- Stetzenbach, L.D. Airborne Infectious Microorganisms. In Encyclopedia of Microbiology, 3rd ed.; Academic Press: San Diego, CA, USA, 2009; pp. 175–182. [Google Scholar] [CrossRef]
- Iqbal, H.; Rhee, D.-K. Ginseng alleviates microbial infections of the respiratory tract: A review. J. Ginseng Res. 2019, 44, 194–204. [Google Scholar] [CrossRef]
- Frimodt-Møller, N.; Knudsen, J.D.; Espersen, F. Chapter 14 - The Mouse Peritonitis/Sepsis Model. In Handbook of Animal Models of Infection; Zak, O., Sande, M.A., Eds.; Academic Press: Cambridge, MA, USA, 1999; pp. 127–136. [Google Scholar] [CrossRef]
- Frimodt-Møller, N. The mouse peritonitis model: Present and future use. J. Antimicrob. Chemother. 1993, 31, 55–60. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Gjorstrup, P.; Filep, J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 14983–14988. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Baehni, P.C. Periodontal health and global public health. Periodontol. 2000 2012, 60, 7–14. [Google Scholar] [CrossRef]
- Vitkov, L.; Muñoz, L.E.; Knopf, J.; Schauer, C.; Oberthaler, H.; Minnich, B.; Hannig, M.; Herrmann, M. Connection between Periodontitis-Induced Low-Grade Endotoxemia and Systemic Diseases: Neutrophils as Protagonists and Targets. Int. J. Mol. Sci. 2021, 22, 4647. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, 4227. [Google Scholar] [CrossRef] [PubMed]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I. The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. Apmis 2002, 110, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Elrefaei, M.; Burke, C.M.; Baker, C.A.R.; Jones, N.G.; Bousheri, S.; Bangsberg, D.R.; Cao, H. TGF-β and IL-10 Production by HIV-Specific CD8+ T Cells Is Regulated by CTLA-4 Signaling on CD4+ T Cells. PLoS ONE 2009, 4, e8194. [Google Scholar] [CrossRef]
- Hyodo, N.; Nakamura, I.; Imawari, M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2004, 135, 462–466. [Google Scholar] [CrossRef]
- Brockman, M.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009, 114, 346–356. [Google Scholar] [CrossRef]
- Schultz-Cherry, S.; Hinshaw, V.S. Influenza virus neuraminidase activates latent transforming growth factor beta. J. Virol. 1996, 70, 8624–8629. [Google Scholar] [CrossRef]
- Cilloniz, C.; Pantin-Jackwood, M.J.; Ni, C.; Goodman, A.G.; Peng, X.; Proll, S.C.; Carter, V.S.; Rosenzweig, E.R.; Szretter, K.J.; Katz, J.M.; et al. Lethal Dissemination of H5N1 Influenza Virus Is Associated with Dysregulation of Inflammation and Lipoxin Signaling in a Mouse Model of Infection. J. Virol. 2010, 84, 7613–7624. [Google Scholar] [CrossRef]
- Kim, N.; Lannan, K.L.; Thatcher, T.H.; Pollock, S.J.; Woeller, C.F.; Phipps, R.P. Lipoxin B4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression. J. Immunol. 2018, 201, 3343–3351. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Xu, C.; Zhou, H. Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators. Front. Immunol. 2020, 11, 766. [Google Scholar] [CrossRef]
- Deshpande, S.; Banerjee, K.; Biswas, P.S.; Rouse, B.T. Herpetic eye disease: Immunopathogenesis and therapeutic measures. Expert Rev. Mol. Med. 2004, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Xavier, R.A.; Newson, J.; Silveira, V.L.F.; Farrow, S.N.; Gilroy, D.W.; Bystrom, J. A New Strategy for the Identification of Novel Molecules with Targeted Proresolution of Inflammation Properties. J. Immunol. 2009, 184, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Neglected Tropical Diseases—Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Rogerio, A.P.; Anibal, F.F. Role of Leukotrienes on Protozoan and Helminth Infections. Mediat. Inflamm. 2012, 2012, 595694. [Google Scholar] [CrossRef] [PubMed]
- Alloo, J.; Leleu, I.; Grangette, C.; Pied, S. Parasite infections, neuroinflammation, and potential contributions of gut microbiota. Front. Immunol. 2022, 13, 1024998. [Google Scholar] [CrossRef]
- Argüello, R.J.; Vigliano, C.; Cabeza-Meckert, P.; Viotti, R.; Garelli, F.; Favaloro, L.E.; Favaloro, R.R.; Laguens, R.; Laucella, S.A. Presence of Antigen-Experienced T Cells with Low Grade of Differentiation and Proliferative Potential in Chronic Chagas Disease Myocarditis. PLOS Negl. Trop. Dis. 2014, 8, e2989. [Google Scholar] [CrossRef]
- Echeverria, L.E.; Morillo, C.A. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef]
- Norris, P.C.; Arnardottir, H.; Sanger, J.M.; Fichtner, D.; Keyes, G.S.; Serhan, C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fat. Acids 2016, 138, 81–89. [Google Scholar] [CrossRef]
- Colas, R.A.; Dalli, J.; Chiang, N.; Vlasakov, I.; Sanger, J.M.; Riley, I.R.; Serhan, C.N. Identification and Actions of the Maresin 1 Metabolome in Infectious Inflammation. J. Immunol. 2016, 197, 4444–4452. [Google Scholar] [CrossRef]
- Serhan, C.N.; de la Rosa, X.; Jouvene, C.C. Cutting Edge: Human Vagus Produces Specialized Proresolving Mediators of Inflammation with Electrical Stimulation Reducing Proinflammatory Eicosanoids. J. Immunol. 2018, 201, 3161–3165. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Colas, R.A.; Arnardottir, H.; Serhan, C.N. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity 2017, 46, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N.; et al. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience 2019, 23, 100772. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, J.; Serhan, C.; Sher, A. Parasite-induced Lipoxin A4 Is an Endogenous Regulator of IL-12 Production and Immunopathology in Toxoplasma gondii Infection. J. Exp. Med. 2002, 196, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Waindok, P.; Janecek-Erfurth, E.; Lindenwald, D.; Wilk, E.; Schughart, K.; Geffers, R.; Balas, L.; Durand, T.; Rund, K.M.; Schebb, N.H.; et al. Multiplex profiling of inflammation-related bioactive lipid mediators in Toxocara canis- and Toxocara cati-induced neurotoxocarosis. PLoS Negl. Trop. Dis. 2019, 13, e0007706. [Google Scholar] [CrossRef] [PubMed]
- Siegel, E.R.; Croze, R.H.; Fang, X.; Matthay, M.A.; Gotts, J.E. Inhibition of the lipoxin A4 and resolvin D1 receptor impairs host response to acute lung injury caused by pneumococcal pneumonia in mice. Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L1085–L1092. [Google Scholar] [CrossRef]
- Bafica, A.; Scanga, C.A.; Serhan, C.; Machado, F.; White, S.; Sher, A.; Aliberti, J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase–dependent lipoxin production. J. Clin. Investig. 2005, 115, 1601–1606. [Google Scholar] [CrossRef]
- Jordan, P.M.; Gerstmeier, J.; Pace, S.; Bilancia, R.; Rao, Z.; Börner, F.; Miek, L.; Gutiérrez-Gutiérrez, Ó.; Arakandy, V.; Rossi, A.; et al. Staphylococcus aureus-Derived α-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution. Cell Rep. 2020, 33, 108247. [Google Scholar] [CrossRef]
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-Derived Mediator that Protects Brain and Retina Against Cell Injury-Induced Oxidative Stress. Brain Pathol. 2006, 15, 159–166. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2016, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Humeniuk, L.; Kozlov, N.; Roigas, S.; Adel, S.; Heydeck, D. The evolutionary hypothesis of reaction specificity of mammalian ALOX15 orthologs. Prog. Lipid Res. 2018, 72, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Devchand, P.R.; Arita, M.; Hong, S.; Bannenberg, G.; Moussignac, R.; Gronert, K.; Serhan, C.N.; Southgate, R.J.; Bruce, C.R.; Carey, A.L.; et al. Human ALX receptor regulates neutrophil recruitment in transgenic mice: Roles in inflammation and host defense. FASEB J. 2003, 17, 652–659. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).