Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study
Abstract
1. Introduction
2. Results and Discussion
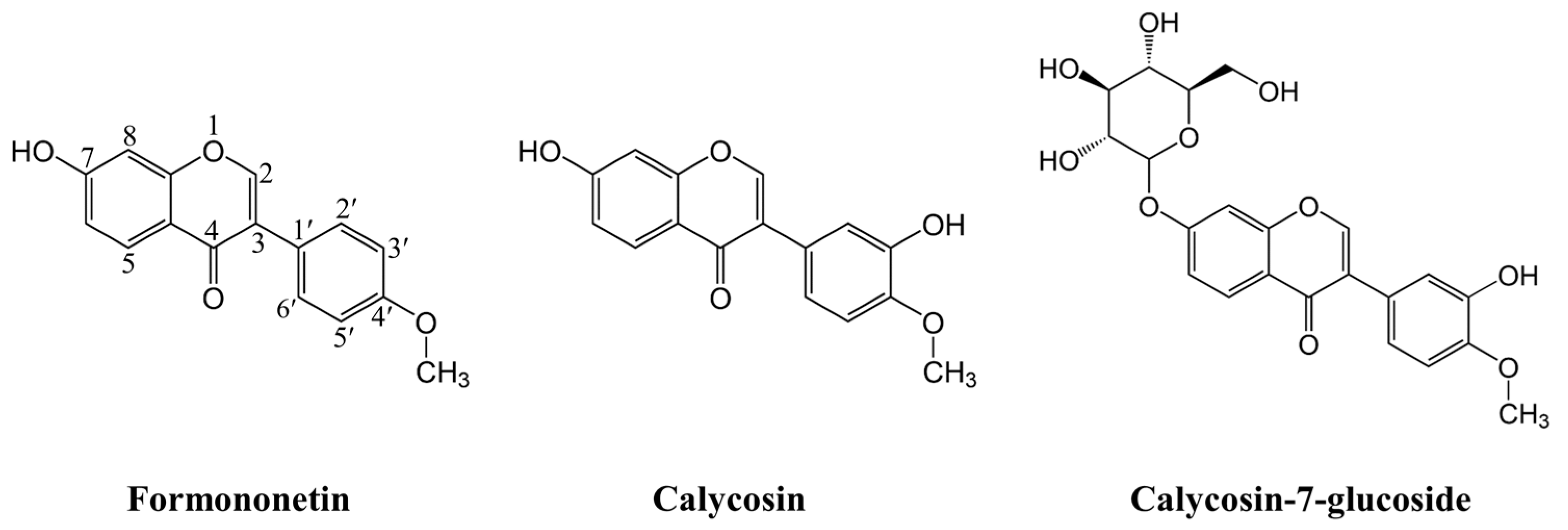
2.1. Conformational Analysis
2.2. Analysis of Free Radical Scavenging Reaction Paths
2.2.1. HAT Mechanism
2.2.2. SET-PT Mechanism
2.2.3. SPLET Mechanism
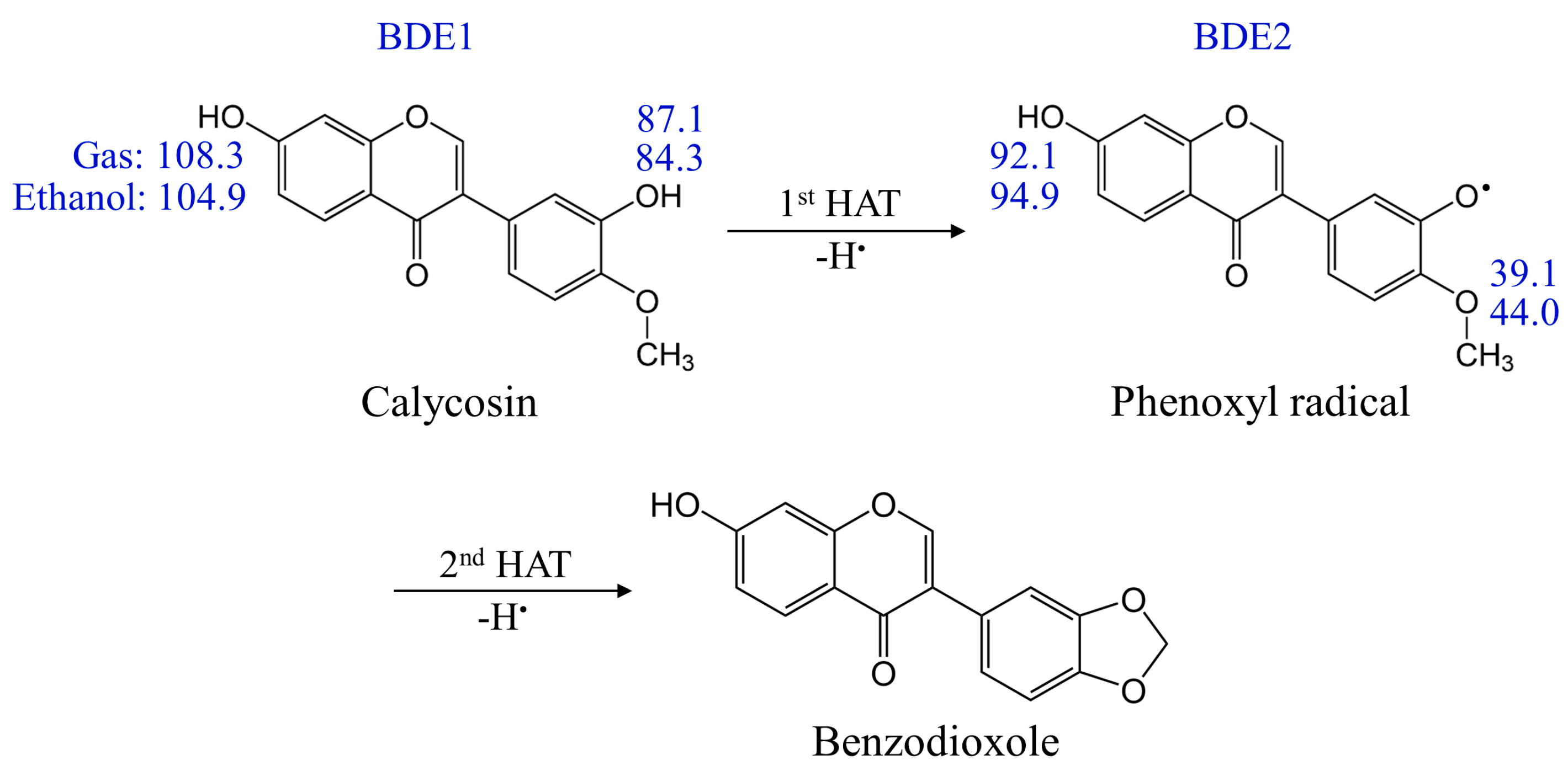
2.2.4. Double HAT Mechanism
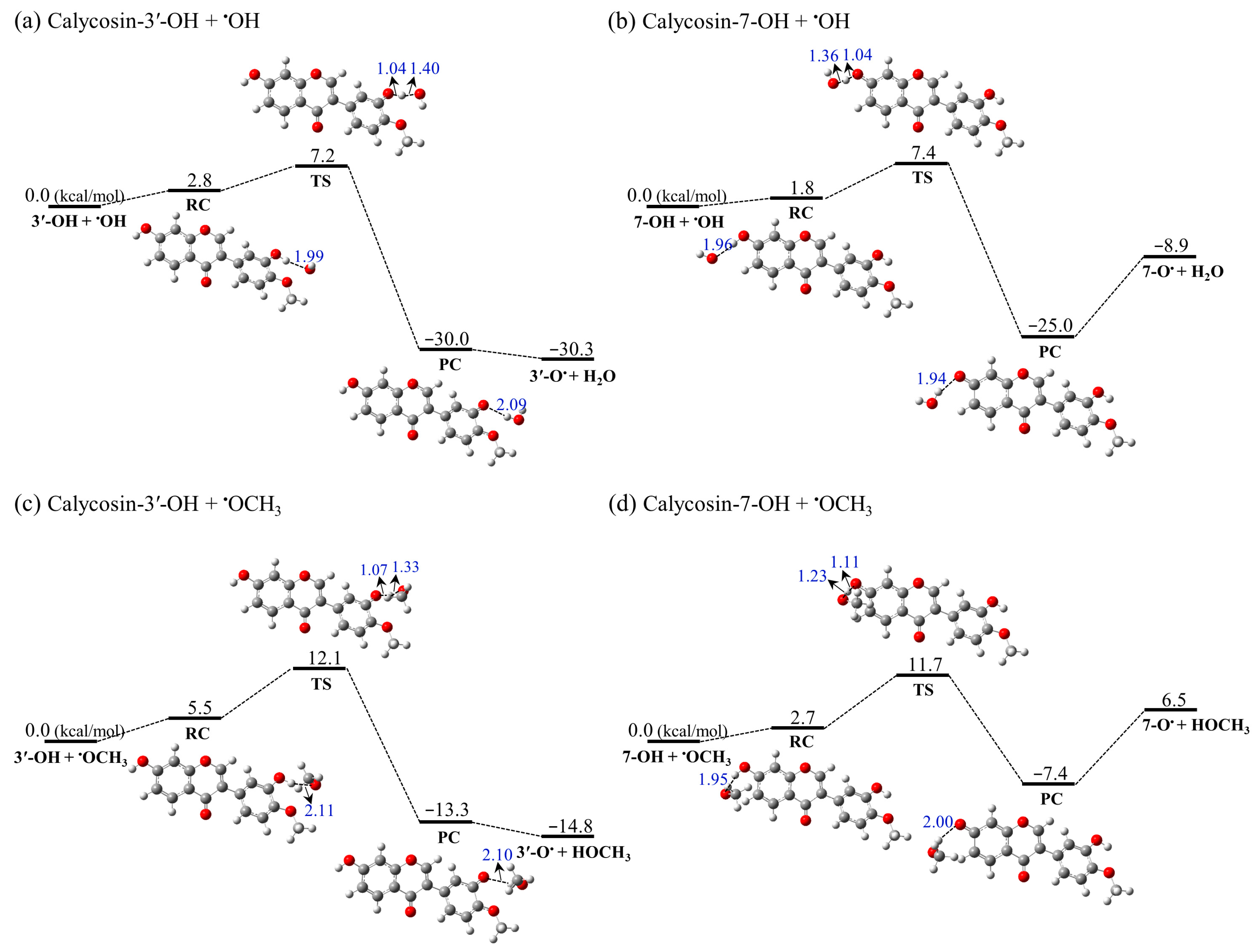
2.3. Kinetic Study
2.3.1. Reaction with •OH
2.3.2. Reaction with •OCH3
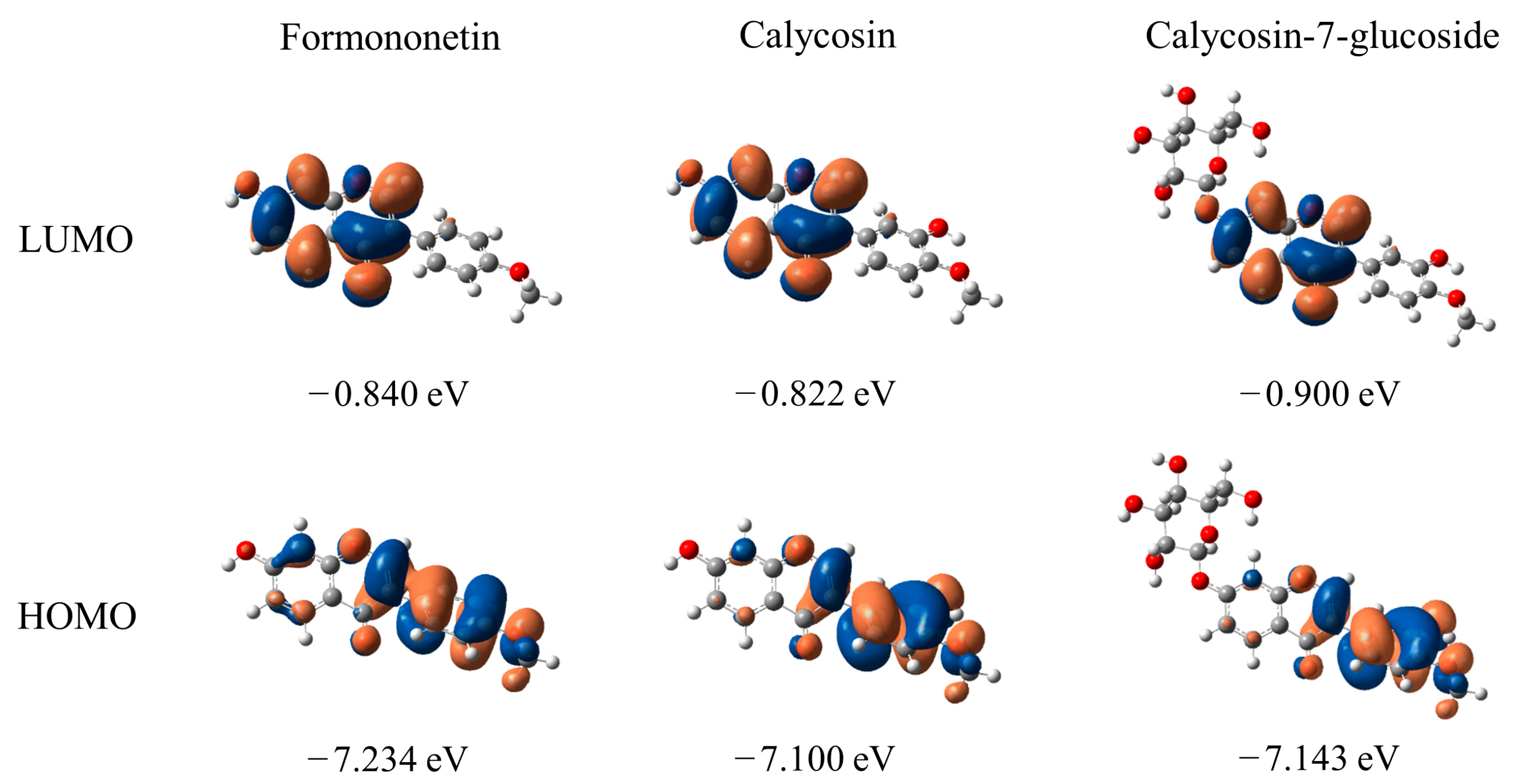
2.4. Molecular Orbital Analysis
3. Computational Details
3.1. Antioxidant Mechanisms and Thermochemical Parameters
- (a)
- HAT is a one-step mechanism in which hydrogen atoms are transferred from flavonoid hydroxyl groups to the free radicals through homolytic cleavage of the O-H bond (Equation (1)). The activity of the antioxidants can be characterized by the BDE for this mechanism (Equation (2)):
- (b)
- The SET-PT mechanism consists of two-steps. In the SET-PT mechanism, electron transfer from ArOH is followed by proton transfer (Equation (3)). The first and second step of the SET-PT mechanism are governed by IP and PDE, respectively (Equations (4) and (5)):
- (c)
- For the SPLET mechanism, it also consists of two-steps. Proton transfer from ArOH is followed by electron transfer (Equation (6)). The PA and ETE were used to drive the first and second steps, respectively (Equations (7) and (8)):
3.2. Kinetic Parameters
4. Conclusions
- (1)
- The hydroxyl group on the O3′ position has a higher H-atom donation ability than that on the O7 position for the investigated compounds. A comparison of the intrinsic thermodynamic properties including BDEs, IPs, and PAs demonstrated that the HAT action is thermodynamically preferred in the gas phase and SPLET is more preferred in the solvent phase in the first H+/e− reaction. The sequence of free radical scavenging capability for the three isoflavonoid compounds is calycosin > calycosin-7-glucoside > formononetin.
- (2)
- The calycosin preferentially undergoes the first H+/e− reaction on the 3′-OH site, followed by the second H+/e− reaction from the ortho-OCH3 group to form stable benzodioxazole with considerably reduced energy via the double HAT mechanism.
- (3)
- The potential energy profiles and kinetic calculations show that the reaction of •OH into the 3′-OH site of calycosin has a lower energy barrier (7.2 kcal/mol) and higher rate constant (4.55 × 109 M−1 s−1) compared with other reactions. It is worth noting that the reaction between the 7-OH of calycosin and •OCH3 is endothermic.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yao, X.-S. Strengthen the research on the medicinal and edible substances to advance the development of the comprehensive healthcare industry of TCMs. Chin. J. Nat. Med. 2019, 17, 1–2. [Google Scholar] [CrossRef]
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1. [Google Scholar]
- Li, S.S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.N.; Fang, Y.X.; Liu, Y.Y.; Wang, S.J.; Guo, Y.; Wang, H.; et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef]
- Chan, J.Y.; Koon, J.C.; Leung, P.-C.; Che, C.-T.; Fung, K.-P. Suppression of lowdensity lipoprotein oxidation, vascular smooth muscle cell proliferation and migration by a herbal extract of Radix Astragali, Radix Codonopsis and Cortex Lycii. BMC Complement. Altern. Med. 2011, 11, 32. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Yang, S.; Hua, Y.; Su, J.; Shang, Y.; Wang, Z.; Feng, K.; Zhang, J.; Yang, X.; et al. Astragalus flavone ameliorates atherosclerosis and hepatic steatosis Via inhibiting lipid-disorder and inflammation in apoE−/− mice. Front. Pharm. 2020, 11, 610550. [Google Scholar] [CrossRef]
- Jalsrai, A.; Grecksch, G.; Becker, A. Evaluation of the effects of Astragalus mongholicus Bunge saponin extract on central nervous system functions. J. Ethnopharmacol. 2010, 131, 544–549. [Google Scholar] [CrossRef]
- Liu, L.J.; Li, H.F.; Xu, F.; Wang, H.Y.; Zhang, Y.F.; Liu, G.X.; Shang, M.Y.; Wang, X.; Cai, S.Q. Exploring the in vivo existence forms (23 original constituents and 147 metabolites) of astragali radix total flavonoids and their distributions in rats using HPLC-DAD-ESI-IT-TOF-MSn. Molecules 2020, 25, 22. [Google Scholar] [CrossRef]
- Sheng, Z.; Jiang, Y.; Liu, J.; Yang, B. UHPLC–MS/MS analysis on flavonoids composition in astragalus membranaceus and their antioxidant activity. Antioxidants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Bao, X.-F.; Cao, P.-H.; Zeng, J.; Xiao, L.-M.; Luo, Z.-H.; Zou, J.; Wang, C.-X.; Zhao, Z.-X.; Zhou, Z.-Q.; Zhi, H.; et al. Bioactive pterocarpans from the root of Astragalus membranaceus var. mongholicus. Phytochemistry 2022, 200, 113249. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the genus Astragalus: Phytochemistry and biological activity. Pharm. Rev. 2016, 10, 11. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ho, C.-T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D.; Ma, L. The bio-inspired heterogeneous single-cluster catalyst Ni100–Fe4S4 for enhanced electrochemical CO2 reduction to CH4. Nanoscale 2023, 15, 2756–2766. [Google Scholar] [CrossRef]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.-H.; Xu, H. The dual-site adsorption and high redox activity enabled by hybrid organic-inorganic vanadyl ethylene glycolate for high-rate and long-durability lithium–sulfur batteries. Small 2023, 19, 2206750. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xu, H.; Xu, W.; Peng, B.; Zhao, C.; Xie, M.; Lv, X.; Gao, Y.; Hu, K.; Fang, Y.; et al. Quasi-topological intercalation mechanism of Bi0.67NbS2 enabling 100 C fast-charging for sodium-ion batteries. Adv. Energy Mater. 2023, 2300790. [Google Scholar] [CrossRef]
- Lu, X.-Q.; Chen, Q.; Tian, X.-X.; Mu, Y.-W.; Lu, H.-G.; Li, S.-D. Predicting lanthanide boride inverse sandwich tubular molecular rotors with the smallest core–shell structure. Nanoscale 2019, 11, 21311. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem. 2020, 304, 125446. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Teng, Y.; Chen, M.; Li, Z.; Wang, G. Antioxidant activity and mechanism of avenanthramides: Double H+/e− processes and role of the catechol, guaiacyl, and carboxyl groups. J. Agric. Food Chem. 2021, 69, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Teena Rose, K.S.; Jacob, J.M.; Varatharaj, R.; Shashikala, K.; Janardanan, D. Curcumin analogues with improved antioxidant properties: A theoretical exploration. Food Chem. 2022, 373, 131499. [Google Scholar] [CrossRef] [PubMed]
- Amic, A.; Markovic, Z.; Markovic, J.M.D.; Milenkovic, D.; Stepanic, V. Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Structure-antioxidant activity relationship of ferulic acid derivatives: Effect of ester groups at the end of the carbon side chain. LWT-Food Sci. Technol. 2020, 120, 108932. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Li, Z.; Zhou, J.; Qu, L.; Chen, K. Theoretical study on the radical scavenging activity and mechanism of four kinds of Gnetin molecule. Food Chem. 2022, 378, 131975. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Xia, D.; Wang, S. Antioxidant properties of camphene-based thiosemicarbazones: Experimental and theoretical evaluation. Molecules 2020, 25, 1192. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Stepanić, V.; Trošelj, K.G.; Lučić, B.; Marković, Z.; Amić, D. Bond dissociation free energy as a general parameter for flavonoid radical scavenging activity. Food Chem. 2013, 141, 1562–1570. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Amića, A.; Markovićb, Z.; Kleinc, E.; Dimitrić Markovićd, J.M.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro- and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Lu, T. Molclus Program. Beijing Kein Research Center for Natural Science. Version 1.9.9.9. 2022. Available online: https://www.keinsci.com/research/molclus.html (accessed on 5 June 2023).
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Glendening, P.E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0. Available online: https://nbo6.chem.wisc.edu/ (accessed on 5 June 2023).
- Zheng, Y.-Z.; Fu, Z.-M.; Guo, R.; Chen, D.-F.; Zhang, Y.-C. The important role of benzylic C–H bond in the antioxidant behaviours of the xanthones. J. Food Compos. Anal. 2021, 103, 104082. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Luo, Q.; Wang, H.; Chen, R.; Liu, Y.; Li, Y. Antiradical activity and mechanism of coumarin−chalcone hybrids: Theoretical insights. J. Phys. Chem. A 2018, 122, 8520–8529. [Google Scholar] [CrossRef]
- Canneaux, S.; Bohr, F.; Henon, E. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem. 2014, 35, 82–93. [Google Scholar] [CrossRef]
- Vo, V.Q.; Bay, M.V.; Nam, P.C.; Mechler, A. Is indolinonic hydroxylamine a promising artificial antioxidant? J. Phys. Chem. B 2019, 123, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Dimić, D.; Milenković, D.; Marković, J.D.; Marković, Z. Antiradical activity of catecholamines and metabolites of dopamine: Theoretical and experimental study. Phys. Chem. Chem. Phys. 2017, 19, 12970. [Google Scholar] [CrossRef] [PubMed]
- Garzón, A.; Bravo, I.; Barbero, A.J.; Albaladejo, J. Mechanistic and kinetic study on the reactions of coumaric acids with reactive oxygen species: A DFT approach. J. Agric. Food Chem. 2014, 62, 9705–9710. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. T. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363. [Google Scholar] [CrossRef]
- Bartmess, J.E. Thermodynamics of the electron and the proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Rimarcík, J.; Lukes, V.; Klein, E.; Ilcin, M. Study of the solvent effect on the enthalpies of homolytic and heterolytic N–H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J. Mol. Struc. Theor. Chem. 2010, 952, 25–30. [Google Scholar] [CrossRef]
- Markovic, Z.; Tosovic, J.; Milenkovic, D.; Markovic, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Wigner, E. On the quantum correction for thermodynamic equilibrium. Phys. Rev. 1932, 40, 749–759. [Google Scholar] [CrossRef]
| Mechanism | HAT | SET-PT | SPLET | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | |||||||||||
| Compounds | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol | Gas | Water | Ethanol |
| Formononetin | 175.2 | 132.9 | 127.2 | ||||||||||||
| 7-OH | 108.3 | 95.6 | 94.4 | 246.5 | 16.7 | 14.3 | 328.9 | 30.7 | 31.7 | 92.8 | 118.9 | 109.8 | |||
| Calycosin | 172.9 | 129.3 | 123.7 | ||||||||||||
| 3′-OH | 87.1 | 85.1 | 84.3 | 227.6 | 9.7 | 7.6 | 348.4 | 36.4 | 38.7 | 52.1 | 102.6 | 92.7 | |||
| 7-OH | 108.3 | 104.2 | 104.9 | 248.7 | 28.8 | 28.2 | 329.2 | 30.7 | 31.7 | 92.5 | 127.4 | 120.3 | |||
| Calycosin-7-glucoside | 173.0 | 129.7 | 124.1 | ||||||||||||
| 3′-OH | 87.3 | 85.1 | 84.5 | 227.7 | 9.4 | 7.4 | 347.3 | 36.4 | 38.7 | 53.3 | 102.7 | 92.8 | |||
| Reactions | ΔG (kcal/mol) | ΔG≠ (kcal/mol) | k (M−1 s−1) |
|---|---|---|---|
| Calycosin-3′-OH + •OH | −30.3 | 7.2 | 4.55 × 109 |
| Calycosin-7-OH + •OH | −8.9 | 7.4 | 2.03 × 109 |
| Calycosin-3′-OH + •OCH3 | −14.8 | 12.1 | 6.72 × 105 |
| Calycosin-7-OH + •OCH3 | 6.5 | 11.7 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.-Q.; Qin, S.; Li, J. Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study. Molecules 2023, 28, 5039. https://doi.org/10.3390/molecules28135039
Lu X-Q, Qin S, Li J. Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study. Molecules. 2023; 28(13):5039. https://doi.org/10.3390/molecules28135039
Chicago/Turabian StyleLu, Xiao-Qin, Shu Qin, and Jindong Li. 2023. "Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study" Molecules 28, no. 13: 5039. https://doi.org/10.3390/molecules28135039
APA StyleLu, X.-Q., Qin, S., & Li, J. (2023). Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study. Molecules, 28(13), 5039. https://doi.org/10.3390/molecules28135039









