Synthesis of Organic–Inorganic Hybrid Perovskite/MOF Composites from Pb–MOF Using a Mechanochemical Method
Abstract
1. Introduction
2. Results and Discussion
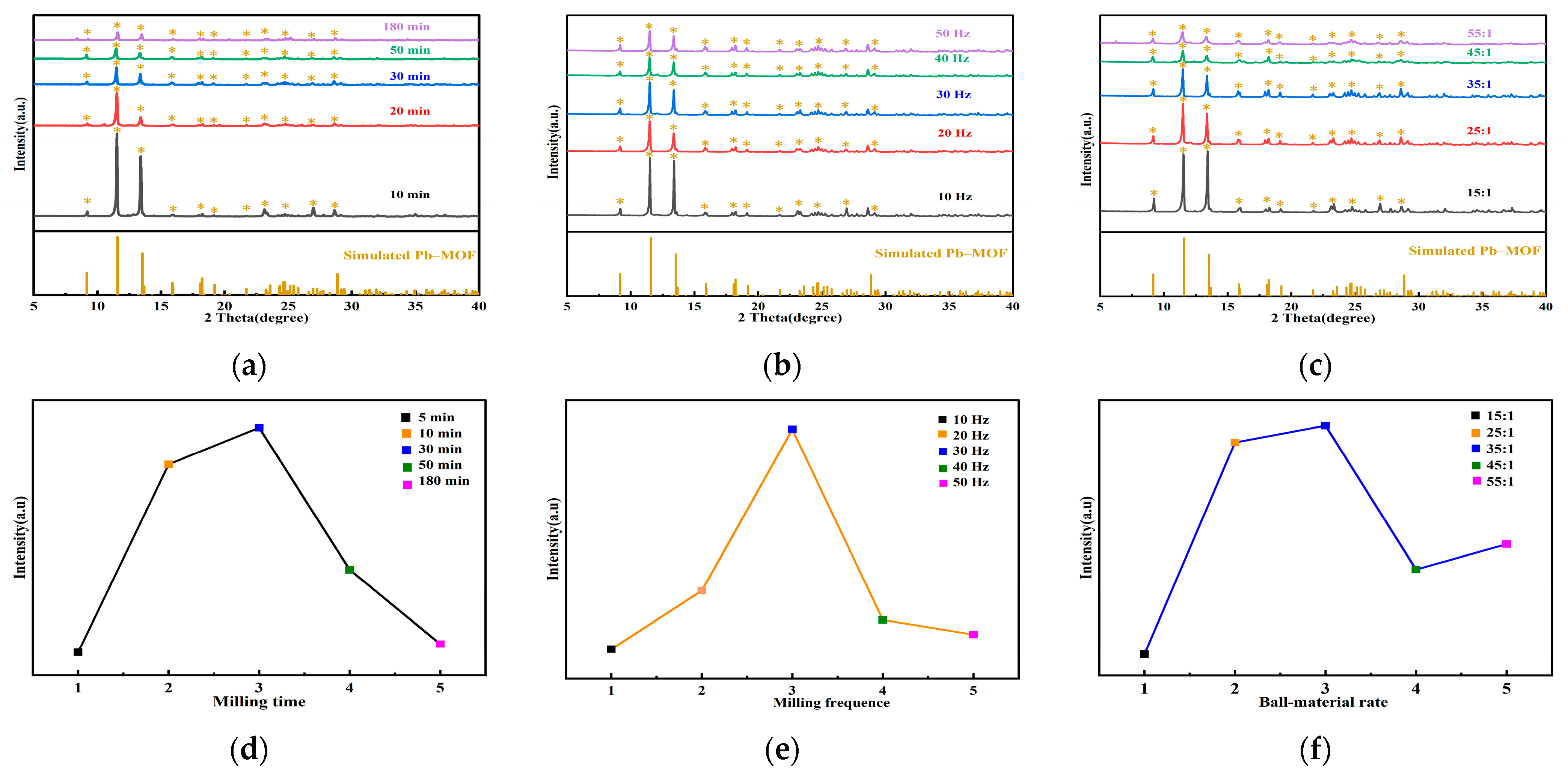
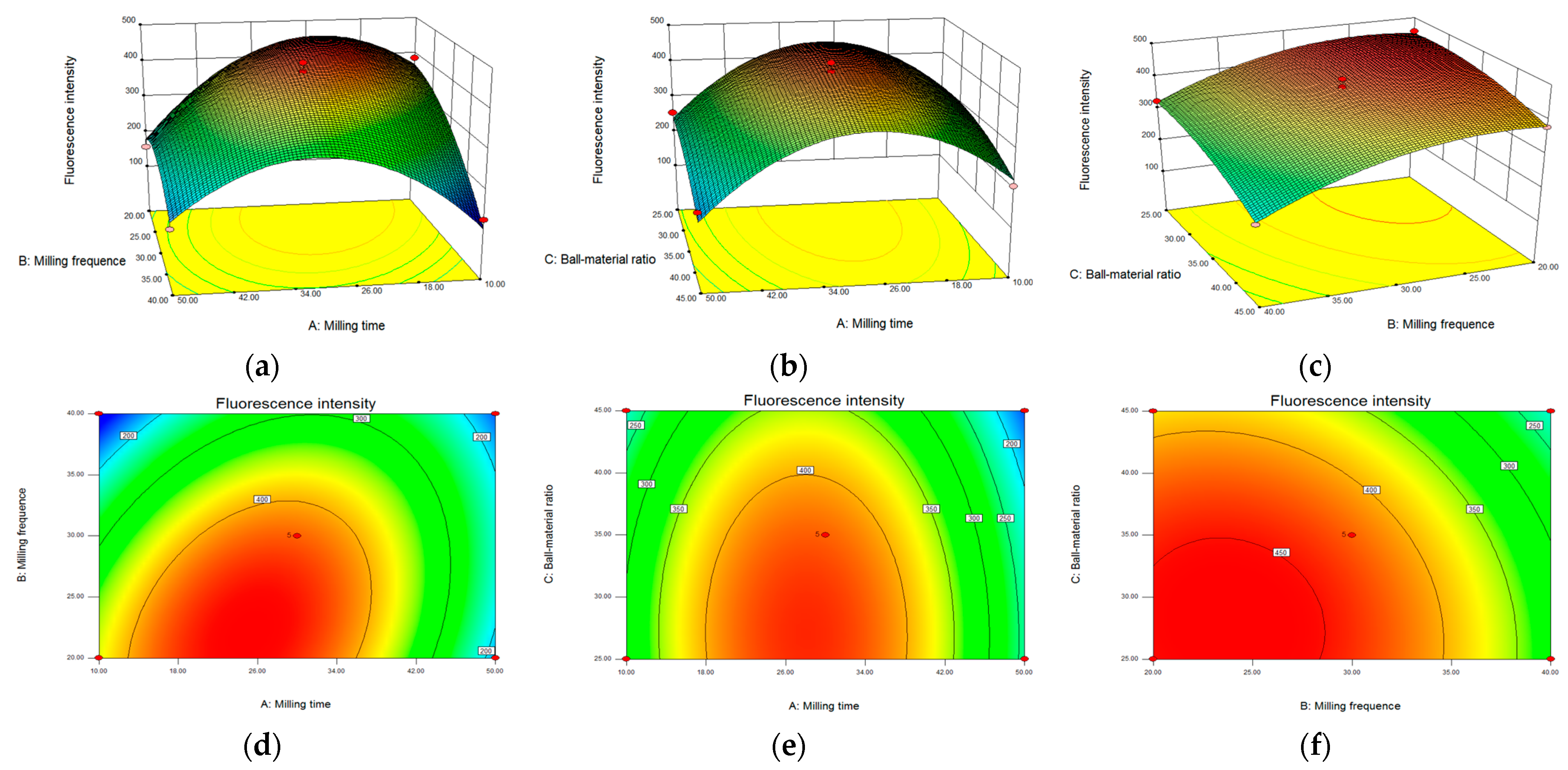
2.1. Preparation of Pb–MOF
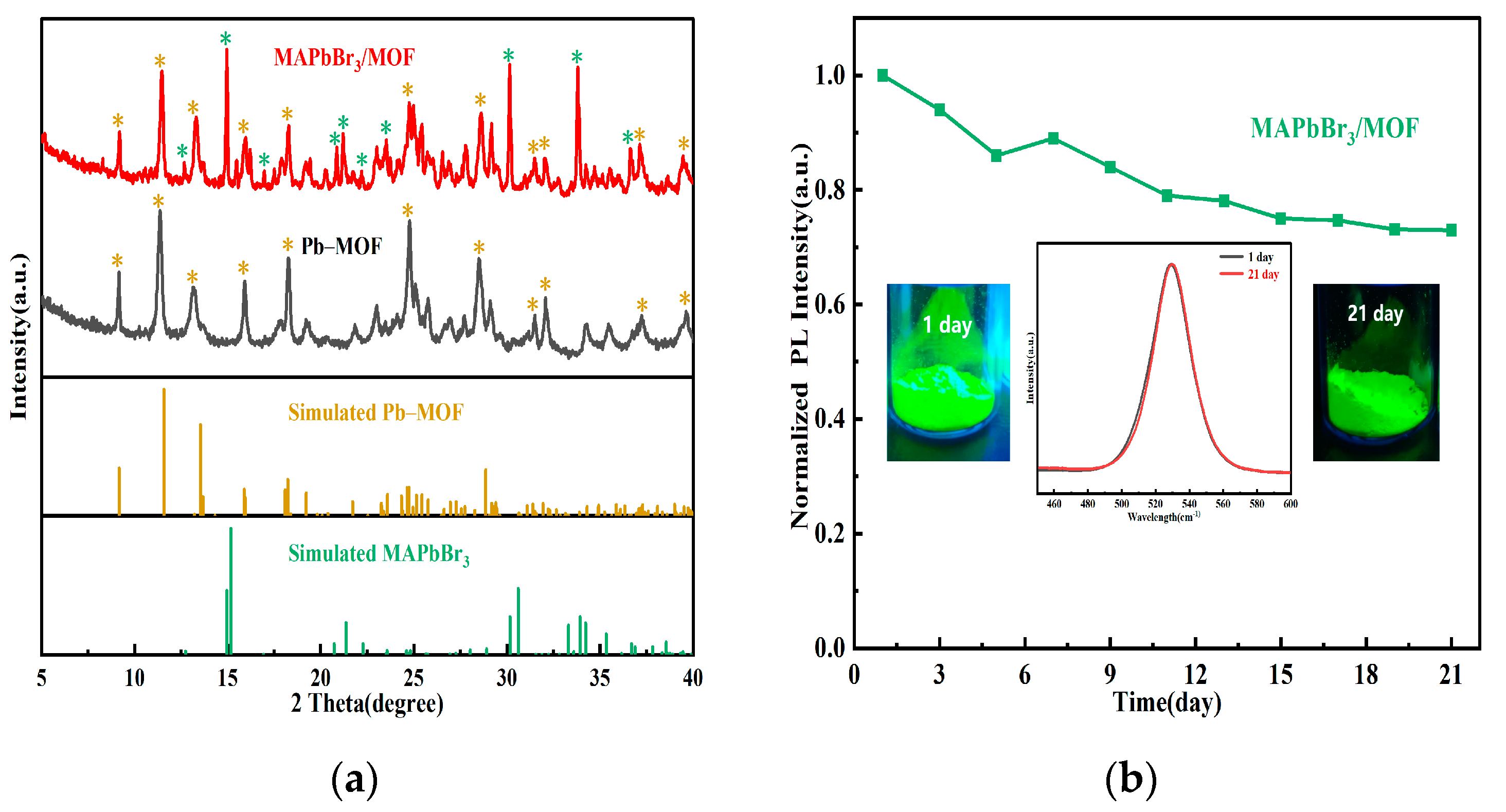
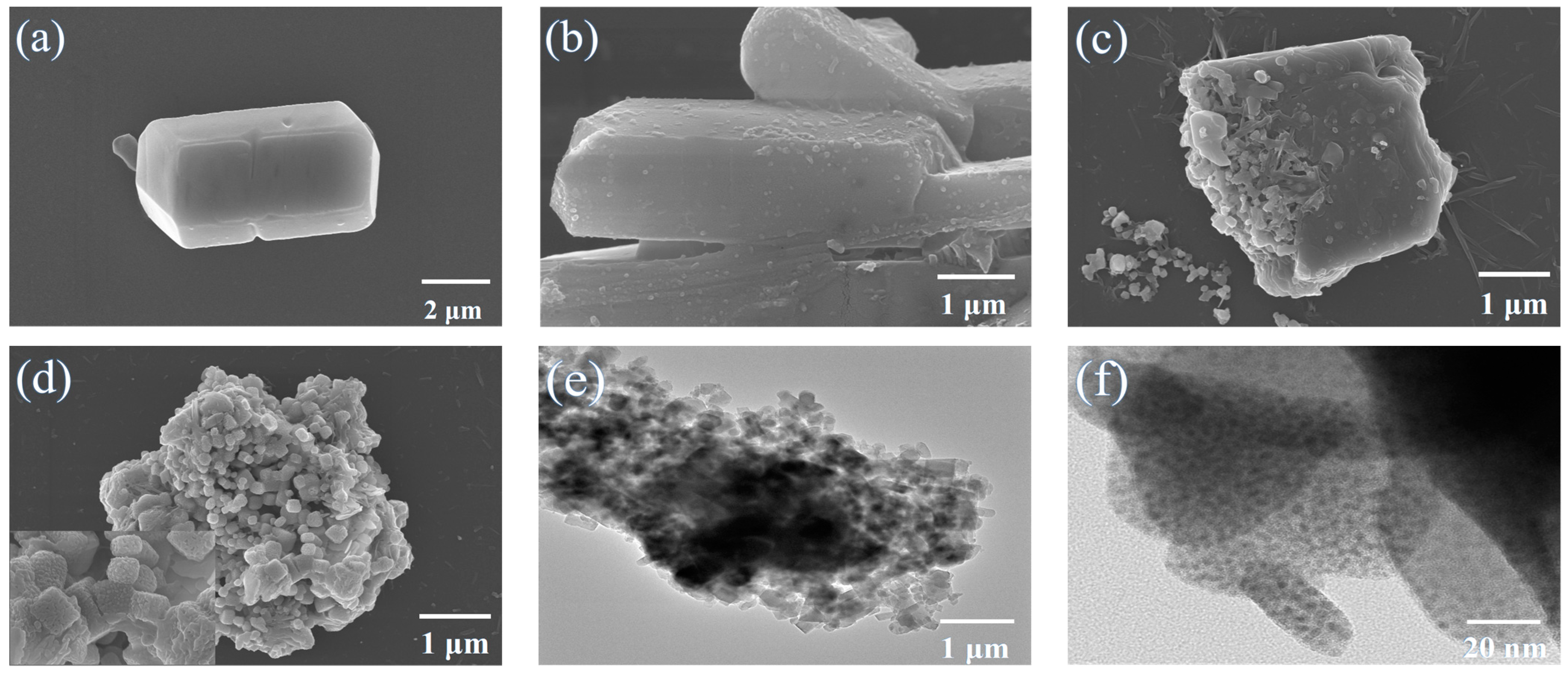
2.2. The Structural and Elemental Characterization of Pb–MOF and MAPbBr3/MOF Composites
2.3. Stabilities of Pb–MOF and MAPbBr3/MOF Composite
2.4. The Transformation Process of MAPbBr3/MOF Composite during Mechanochemical Synthesis
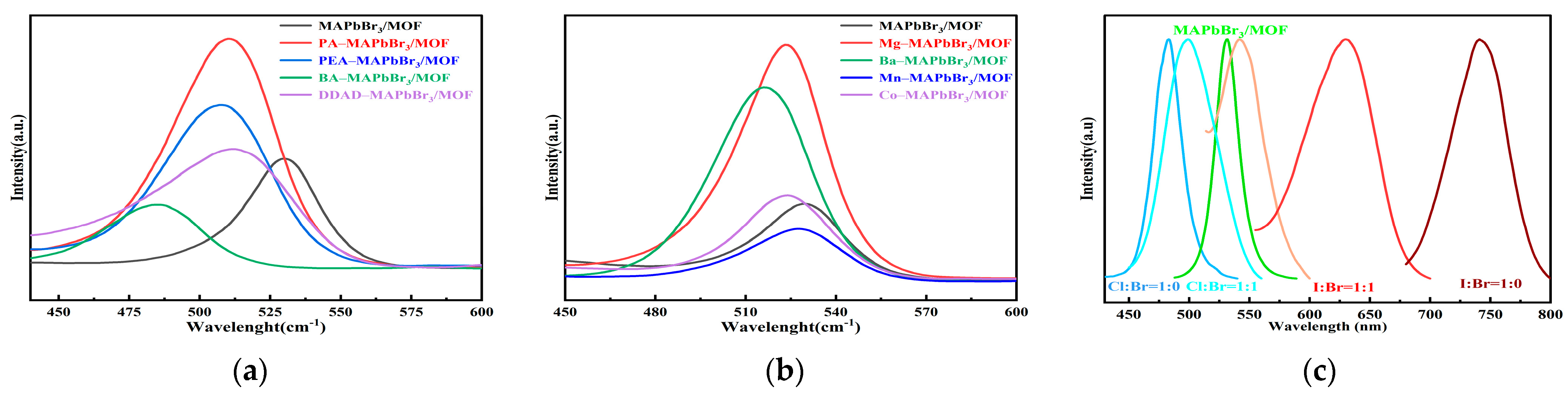
2.5. The Universality of the Mechanochemical Method in the Preparation of Organic–Inorganic Hybrid Perovskite/MOF Composites
3. Materials and Methods
3.1. Preparation of Pb–MOF
3.2. Perovskite/MOF Composites
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chouhan, L.; Ghimire, S.; Subrahmanyam, C.; Miyasaka, T.; Biju, V. Synthesis, Optoelectronic Properties and Applications of Halide Perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photon. 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Xiong, J.; Yuan, C.; Semin, S.; Rasing, T.; Bu, X.H. Halide Perovskites for Nonlinear Optics. Adv. Mater. 2020, 32, 1806736. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Li, H.; Liu, S.H.; Yue, F.Y.; Jing, C.B.; Cheng, Y.; Chu, J. A “Turn-on” Fluorescence Perovskite Sensor Based on MAPbBr3/Mesoporous TiO2 for NH3 and Amine Vapor Detections. Sens. Actuators B Chem. 2021, 327, 128918. [Google Scholar] [CrossRef]
- Walsh, A. Principles of Chemical Bonding and Band Gap Engineering in Hybrid Organic-Inorganic Halide Perovskites. J. Phys. Chem. C 2015, 119, 5755–5760. [Google Scholar] [CrossRef]
- El-Mellouhi, F.; Marzouk, A.; Bentria, E.T.; Rashkeev, S.N.; Kais, S.; Alharbi, F.H. Hydrogen Bonding and Stability of Hybrid Organic-Inorganic Perovskites. ChemSusChem 2016, 9, 2648–2655. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, X.; Xiang, H.; Gong, X.; Walsh, A.; Wei, S. Intrinsic Instability of the Hybrid Halide Perovskite Semiconductor CH3NH3PbI3. Chin. Phys. Lett. 2018, 35, 036104. [Google Scholar] [CrossRef]
- Akbulatov, A.; Luchkin, S.; Frolova, L.; Dremova, N.; Zhidkov, I.; Anokhin, D.; Gerasimov, K.; Kurmaev, E.; Stevenson, K.; Troshin, P. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Wu, W.; Jiang, S.; Zhang, H.; Li, B. Water-Mediated Promotion of Direct Oxidation of Benzene over the Metal–Organic Framework HKUST-1. RSC Adv. 2015, 5, 56020–56027. [Google Scholar] [CrossRef]
- Yin, Z.; Ma, W.M.; Wang, C.; Luo, X.P.; Hu, X.T.; Cao, L.H.; Li, X.Y.; Yu, X.Y.; Ma, Y.M.; Zeng, M.H. Color-Tuning and Near-Sunlight White Emission in Highly Stable Rod-Spacer MOFs with Defective Dicubane Based Lead(II)-Carboxyl Chains. Inorg. Chem. 2019, 58, 16171–16179. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Li, W.; Huang, S.; Kong, L.; Li, Z.; Li, L. Conversion of Invisible Metal-Organic Frameworks to Luminescent Perovskite Nanocrystals for Confidential Information Encryption and Decryption. Nat. Commun. 2017, 8, 1138. [Google Scholar] [CrossRef]
- Tsai, H.; Shrestha, S.; Vilá, R.A.; Huang, W.; Liu, C.; Hou, C.; Huang, H.; Wen, X.; Li, M.; Gary, W.; et al. Bright and Stable Light-Emitting Diodes Made with Perovskite Nanocrystals Stabilized in Metal–Organic Frameworks. Nat. Photon. 2021, 15, 843–849. [Google Scholar] [CrossRef]
- Głowniak, S.; Barbara, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward Green Synthesis of Metal–Organic Frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Tao, C.; Wang, J. Synthesis of Metal Organic Frameworks by Ball-Milling. Crystals 2021, 11, 15. [Google Scholar] [CrossRef]
- Julien, P.A.; Uzarevic, K.; Katsenis, A.D.; Kimber, S.A.; Wang, T.; Farha, O.K.; Zhang, Y.; Casaban, J.; Germann, L.S.; Etter, M.; et al. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef]
- Prochowicz, D.; Franckevičius, M.; Cieślak, A.M.; Zakeeruddin, S.M.; Grätzel, M.; Lewiński, J. Mechanosynthesis of the Hybrid Perovskite CH3NH3PbI3: Characterization and the Corresponding Solar Cell Efficiency. J. Mater. Chem. A 2015, 3, 20772–20777. [Google Scholar] [CrossRef]
- Rambabu, D.; Bhattacharyya, S.; Singh, T.; Chakravarthy, M.L.; Maji, T.K. Stabilization of MAPbBr3 Perovskite Quantum Dots on Perovskite MOFs by a One-Step Mechanochemical Synthesis. Inorg. Chem. 2020, 59, 1436–1443. [Google Scholar] [CrossRef]
- Burlakov, V.M.; Hassan, Y.; Danaie, M.; Snaith, H.J.; Goriely, A. Competitive Nucleation Mechanism for CsPbBr3 Perovskite Nanoplatelet Growth. J. Phys. Chem. Lett. 2020, 11, 6535–6543. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhou, L.; Shen, W.; Li, M.; He, R. Highly Luminescent and Stable Quasi-2D Perovskite Quantum Dots by Introducing Large Organic Cations. Nanoscale Adv. 2021, 3, 5393–5398. [Google Scholar] [CrossRef]
- Kim, G.M.; Sato, H.; Ohkura, Y.; Ishii, A.; Miyasaka, T. Phenethylamine-Based Interfacial Dipole Engineering for High Voc Triple-Cation Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2102856. [Google Scholar] [CrossRef]
- Solari, S.F.; Poon, L.N.; Wörle, M.; Krumeich, F.; Li, Y.; Chiu, Y.; Shih, C. Stabilization of Lead-Reduced Metal Halide Perovskite Nanocrystals by High-Entropy Alloying. J. Am. Chem. Soc. 2022, 144, 5864–5870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yu, F.; et al. Two-Inch-Sized Perovskite CH3NH3PbX3 (X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Roztocki, K.; Jedrzejowski, D.; Hodorowicz, M.; Senkovska, I.; Kaskel, S.; Matoga, D. Isophthalate-Hydrazone 2D Zinc-Organic Framework: Crystal Structure, Selective Adsorption, and Tuning of Mechanochemical Synthetic Conditions. Inorg. Chem. 2016, 55, 9663–9670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wei, S.; Kang, X.; Tegus, O.; Pan, D. Short-Chain Ligand Assisted Synthesis of CH3NH3PbX3 (X = Cl, Br, I) Perovskite Quantum Dots and Improved Morphology of CH3NH3PbBr3 Thin Films. J. Lumin. 2016, 211, 26–31. [Google Scholar] [CrossRef]


| Sample | Elements | Peak | Height | Atomic % | MAPbBr3 % |
|---|---|---|---|---|---|
| Br 3d | 67.83 | 18,687.72 | 4.52 | ||
| Pb 4f | 138.1 | 169,444.58 | 3.39 | ||
| MAPbBr3/MOF | C 1s | 284.21 | 90,371.65 | 63.07 | 44.4 |
| N 1s | 401.22 | 8541.33 | 3.69 | ||
| O 1s | 531.03 | 96,104.52 | 25.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, J.; Tao, S.; Dou, G.; Mansur, S.; Zhang, X. Synthesis of Organic–Inorganic Hybrid Perovskite/MOF Composites from Pb–MOF Using a Mechanochemical Method. Molecules 2023, 28, 5021. https://doi.org/10.3390/molecules28135021
Han X, Li J, Tao S, Dou G, Mansur S, Zhang X. Synthesis of Organic–Inorganic Hybrid Perovskite/MOF Composites from Pb–MOF Using a Mechanochemical Method. Molecules. 2023; 28(13):5021. https://doi.org/10.3390/molecules28135021
Chicago/Turabian StyleHan, Xinlan, Jinhua Li, Siyu Tao, Guowei Dou, Sanawar Mansur, and Xinqian Zhang. 2023. "Synthesis of Organic–Inorganic Hybrid Perovskite/MOF Composites from Pb–MOF Using a Mechanochemical Method" Molecules 28, no. 13: 5021. https://doi.org/10.3390/molecules28135021
APA StyleHan, X., Li, J., Tao, S., Dou, G., Mansur, S., & Zhang, X. (2023). Synthesis of Organic–Inorganic Hybrid Perovskite/MOF Composites from Pb–MOF Using a Mechanochemical Method. Molecules, 28(13), 5021. https://doi.org/10.3390/molecules28135021





