Abstract
Dandelion (Taraxacum genus) is a perennial herb belonging to the Asteraceae family. As a well-known and extensively studied genus, dandelion comprises numerous species. Some species have been widely used in both complementary and alternative medicine to clear heat, detoxify, activate blood circulation, dispel stasis, and discharge urine. Multiple pharmacological studies have highlighted its therapeutic potential, including anti-bacterial, anti-oxidant, anti-cancer, and anti-rheumatic activities. Furthermore, bioactive compounds associated with these effects include sesquiterpenoids, phenolic compounds, essential oils, saccharides, flavonoids, sphingolipids, triterpenoids, sterols, coumarins, etc. Based on recent studies about the Taraxacum genus, the present review critically evaluates the current state of dandelion utilization and summarizes the significant roles of dandelion and its constituents in different diseases. We also focus on the reported phytology, chemical composition, pharmacology, and toxicity of dandelion, along with the main possible action mechanisms behind their therapeutic activities. Meanwhile, the challenges and future directions of the Taraxacum genus are also prospected in this review, thus highlighting its pharmaceutical research and practical clinical applications.
1. Introduction
Dandelion (Taraxacum genus), named “Pugongying” in China, is a perennial plant belonging to the Asteraceae family. It has a complex classification, comprising over three hundred species [1]. In Asia, the Taraxacum genus is widely cultivated and also found wild in most parts of China, North Korea, Mongolia, and Russia [2]. It grows in temperate regions globally, including on lawns, on roadsides, on disturbed banks and shores of waterways, and in other areas with moist soils.
As an edible medicinal herb and vegetable, dandelion (Taraxacum genus) has long been utilized in traditional medicine, folk remedies, and substitution therapies in many countries to treat diverse diseases (Figure 1) [3]. Taraxacum genus as a drug was first used to treat liver and spleen diseases in Arabian medicine. In the 16th century, the German botanist Fuchs discovered that Taraxacum can be used to treat gout, diarrhea, blisters, and spleen and liver diseases. It has been used as a common drug for detoxification, swelling, and lactation since the 16th century in China. Since the 19th century, several authors have relied on the existing traditional knowledge to provide scientific explanations about how Taraxacum works on diseases and their symptoms [4]. Taraxacum can be used as diuretics, antioxidants, bile agents, anti-inflammatory, analgesic, and anti-cancer agents. Corresponding studies in the 20th century revealed that Taraxacum can be used medicinally, while its inflorescences, leaves, and roots can be processed into different foods. For example, the leaves of cultivated or wild Taraxacum species can be eaten in salads, while roots are baked and used as a coffee substitute [5]. Additionally, the Taraxacum leaf extract can be used as a flavoring agent for various foods, including alcoholic and soft drinks, frozen dairy desserts, candies, baked goods, pudding, and cheese [6,7].

Figure 1.
The morphology of Taraxacum officinale.
The Chinese Pharmacopoeia (2020 edition) records over forty Chinese patent medicines containing Taraxacum genus, which can be clinically utilized for treating over fifty types of diseases. Since Taraxacum genus could clear away heat, remove toxicity, disperse swelling, dissipate binds, and induce diuresis, dandelion in clinic is commonly used to cure inflammation, stomach trouble, tumors, gynecological diseases, male urinary system diseases, etc. For example, clinical studies demonstrated that the Kangfuxiaoyan suppository containing dandelion could attenuate symptoms and improve immunity in pelvic inflammatory patients; Rupixiao granule containing dandelion possessed the beneficial activity to induce diuresis so as to mitigate edema and resolve hard lumps, which had a satisfactory effective rate for treating breast hyperplasia. Additionally, according to the ClinicalTrials.gov database resource (https://www.clinicaltrials.gov, accessed on 14 June 2023) supported by the U.S. National Library of Medicine, for the interventional clinical trials of Taraxacum genus, one clinical study (NCT00442091) has also been conducted by Odense University Hospital to explore the beneficial effect of dandelion juice on dyshidrotic hand eczema.
Therefore, a comprehensive review of the Taraxacum genus studies is necessary considering its numerous benefits. In this work, we reviewed the recent studies of the Taraxacum genus. Firstly, we introduced the dandelion herbs and described them both botanically and ethnopharmacologically. We then discussed the main chemical composition of Taraxacum genus and outlined its pharmacological effects and toxicity, as these previously have not been fully reviewed to date. Finally, we focused on the challenges and future directions of Taraxacum genus, which could elucidate their pharmaceutical research and practical clinical applications. Thus, this review will provide a better understanding of the Taraxacum genus and its biological activities by comprehensively summarizing the existing literature.
2. Materials and Methods
The literature review was performed using several resources, including Web of Science, ScienceDirect, PubMed, Wiley Online Library, Google Scholar, Europe PMC, Baidu Scholar, American Chemical Society (ACS), and SpringerLink as well as books on Chinese Pharmacopoeia and China Flora, by using different relevant keywords. Additionally, we used Ph.D. and M.S. dissertations, local magazines, and books on toxicology, such as Ben Cao Gang Mu and the Handbook for the Toxicity of Traditional Chinese Medicine.
The keywords used included Taraxacum, dandelion, phytology, genus, ethnopharmacology, sesquiterpenoids, phenolics, other main active components, toxicology, and pharmacological and clinical study. We verified the Latin names of all plants mentioned in this paper from http://www.theplantlist.org/, accessed on 1 January 2023 or http://mpns.kew.org/mpns-portal/, accessed on 1 January 2023 and also provided their validated species names.
3. Phytology and Ethnopharmacology
3.1. Phytology
Dandelion is widely distributed in the northern hemisphere and belongs to the Asteraceae family. The whole grass possesses medicinal values, such as clearing away heat, detoxification, reduction of swelling, and phlegm dispersion. Generally, the leaves of various dandelions are ≥5–25 cm long, simple, lobed, and form a basal rosette above the central taproot. Their flower heads open during the daytime and close at night, and have colors ranging from yellow to orange. The heads are borne singly on a hollow stem (scape), which is usually leafless and rises 1–10 cm or more above the leaves. Stems and leaves exude white and milky latex when broken. A rosette may simultaneously produce several flowering stems. The flower heads are 2–5 cm in diameter and entirely comprise ray florets. The flower heads mature into spherical seed heads, sometimes called blowballs or clocks, which contain many single-seeded fruits called achenes. Each achene is attached to a pappus with fine hair-like material, which facilitates wind-aided dispersal over long distances (https://en.wikipedia.org/wiki/Taraxacum/, accessed on 1 January 2023 ).
3.2. Ethnopharmacology
In traditional Chinese medicine, dandelion was first recorded in Tang Bencao—the oldest classical medical book written in the Tang Dynasty (657–659 AD). In this book, dandelion was regarded as a powerful medicine to treat breast swelling and pain. Later, the Yunnan Materia Medica written by Ren Lanmao (in the Ming Dynasty) described that dandelion displayed a great detoxification effect and could treat various sores. Additionally, dandelion has also been included in the Ming Dynasty masterpiece Compendium of Materia Medica edited by Chinese pharmacologist Li Shizhen. This book describes the detailed functions of dandelion and its wide use in many Chinese medicinal prescriptions.
Currently, dandelion has been recorded in the 2020 edition of the Pharmacopoeia of the People’s Republic of China. Thirty-nine prescriptions included dandelion as the principal active component, and these are listed in the Chinese Pharmacopeia and approved by the state administration of traditional Chinese medicine (TCM) of the People’s Republic of China (Table 1). Furthermore, many countries also recognize the medical applications of dandelion ever since the advent of ancient medicine, especially in Latin America, Europe, and Asia. Ancient medicine records showed that every part of the dandelion could be used. Based on the symptoms, the usage forms of dandelion may be countless, including infusion, decoction, tincture, plaster, and powder. Furthermore, the administration route may either be oral or topical. The main therapeutic indications include gastrointestinal, skin, and respiratory diseases [1]. Table 2 lists the different uses of the various species of Taraxacum.

Table 1.
Preparations in which Taraxacum mongolicum Hand.-Mazz. is the main component listed in Chinese Pharmacopoeia and approved by the government.

Table 2.
Use of Taraxacum spp. plants in traditional medicine.
4. Chemical Compounds
Traditional medicinal plants have a corresponding therapeutic effect depending on their constituent compounds [32]. Dandelion is highly regarded for its unique biological characteristics and good biological activity. Considering its excellent pharmacological properties, researchers have isolated their active ingredients over the past few decades. Its biological activity is determined by complex chemical components, mainly sesquiterpenoids, phenolic compounds, essential oils, saccharides, flavonoids, sphingolipids, triterpenoids, sterols, coumarins, etc. In vivo and in vitro studies have displayed outstanding bioactivities of dandelion, such as anti-bacterial, anti-oxidant, anti-cancer, anti-rheumatic, etc. Undoubtedly, it is the diverse phytoconstituents that provide dandelion with remarkable pharmacological properties. For example, phenolic acids such as caffeic acid, coumaric acid, dihydrosyingin, chicoric acid, vanillin, etc., in dandelion possess anti-oxidative and immunostimulant properties. The main sesquiterpene compounds in dandelion are sesquiterpene lactones, usually in the form of glycosides, such as sonchuside, cichorioside C, ixerin D, taraxafolide, and so on, which have anti-inflammatory and anti-bacterial activities. Triterpenoids and sterols in dandelion such as lupenyl acetate, α-amyin acetate, β-amyin acetate, β-sitosterol, daucosterol, etc., can alleviate cardiovascular diseases. Flavonoids in dandelion such as quercetin, chrysoeriol diosmetin, luteolin, etc., usually have anti-oxidative activity; While coumarins in dandelion such as aesculin, cichoriin, esculetin, scopoletin, etc., possess anti-inflammatory, bacteriostatic, anti-coagulant, and anti-cancer effects.
4.1. Sesquiterpenoids and Phenolic Compounds
Dandelion contains different sesquiterpenoid and phenolic compounds. The bitter taste of dandelion is mainly imparted by sesquiterpenoids (Figure 2). The only known sesquiterpene lactone components in this plant are two germacranolides, namely, taraxinic acid and the β-glucopyranosyl ester and its 11, 13-dihydroderivative and two eudesmanolides (4a(15),11β(13)-tetrahydroridentin B, and taraxacolide-1-O-β-glucopyranoside), which were isolated from T. officinale [33]. From the ethanolic root extracts of two different species of the Taraxacum genus (T. laevigatum and T. disseminatum), eight types of germacrane and eudesmane sesquiterpenoids, including 1β,3β,6α-trihydroxy-4α(15)-dihydrocosticacid methyl ester and its 1-O-β-glucopyranoside, were obtained [34]. A sesquiterpenoid ketolactone and a new guaianolide were isolated from an ethyl acetate-soluble part of a methanolic extract of T. wallichii [35]. Furthermore, eight sesquiterpenes, including 1β,3β-dihydroxy-eudesman-11(13)-en-6α,12-olide, 1β,3β-dihydroxyeudesman-6α,12-olide, loliolide, 1β,3β-dihydroxyeudesman-11(13)-en-6α,12-olide, ainslioside 1β,3β-dihydroxyeudesman-6α,12-olide, and 11β,13-dihydrotaraxinic acid were successfully obtained from T. mongolicum [36,37,38]. Five germacrane- and guaiane-type sesquiterpene lactones together with benzyl glucoside, dihydroconiferin, syringing, and dihydrosyringin were isolated from the roots of T. officinale [39]. Eleven sesquiterpene lactones, including the new guaianolide 11β-hydroxydeacetylmatricarin-8-O-β-glucopyranoside and four other known phenolic glucosides, were also isolated from the roots of T. hondoense [40]. T. erythrospermum, T. serotinum, T. obovatum, T. alpinum, and T. udum are species of the Taraxacum genus. Additionally, sixteen sesquiterpenoids were also isolated from the aforementioned varieties [41,42,43,44,45]. Two eudesmane-type sesquiterpene lactones, (2β-hydroxysantamarine-1β-D-glucopyranoside and 3β-hydroxy-4αH-3-dihydrosantamarine-β-d-glucopyranoside) were successfully isolated from the methanolic extract of T. linearisquameum [46]. New sesquiterpenoids were also obtained from the roots of T. platycarpum [47]. In 1998, a guaianolide sesquiterpene, desacetylmatricarin, was isolated from T. platycarpum and reported as an active ingredient with the anti-allergic property [48]. 14-O-β-d-Glucosyl-l1,13-dihydro-taraxinic acid and 14-O-β-d-glucosyl-taraxinic acid were extracted from the roots of T. officinale [49].
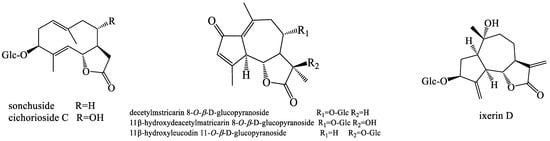

Figure 2.
The chemical structures of representative sesquiterpenoids from Taraxacum genus.
Polyphenolic compounds are widely present in plants. Many potentially active phenolic compounds are isolated from different species of the Taraxacum genus [50,51,52] (Figure 3).
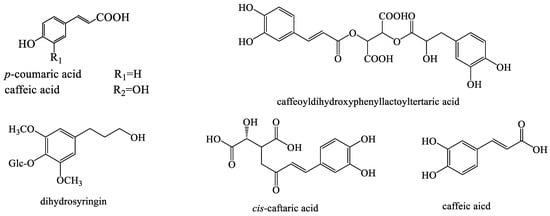

Figure 3.
The chemical structures of representative phenolic compounds from Taraxacum genus.
4.2. Essential Oils
Bylka et al. analyzed T. officinale L. by gas chromatography–mass spectrometry (GC–MS) and obtained 25 volatile compounds, with 1,3-dimethylbenzene, 1,2-dimethylbenzene, 1-ethyl-3-methylbenzene, heneicosane, and tricosane as the main components [53]. The separated compounds also included straight-chain aliphatic hydrocarbons (nonadecane, hexadecane, heneicosane, pentadecane, tricosane, eicosane, and 1-tridecyne), branched aliphatic hydrocarbons (2,5,5-trimethylheptane and 6-ethyl-2-methyloctane), esters (benzyl benzoate), alkylated benzenes (1,3-dimethylbenzene, 1,2-dimethylbenzene, 1-ethyl-3-methylbenzene, and 1-hydroxymethyl-4-methylbenzene), alcohols (2-nonen-1-ol, 1,9-nonanediol, and 1-tridecanol), aldehydes (octanal, phenylacetaldehyde, 2-methylbenzaldehyde, nonanal, pentadecanal, and 10-undecenal), and ketones (5-methyl-2-hexanone and hexadecanoic acid). Upon analyzing the volatile components of T. officinale using GC–MS, the essential oil components obtained were butyl acetate, 2-methyl-propanol, n-butanol, 4-phenyl-1-butanol, 4-hydroxyl 4-methyl-2-pentanone, acetic acid, 4-terpineol, fluoro-terpineol, and alpha-terpineol [54].
4.3. Saccharides
A recent study reported the successful extraction of a water-soluble heteropolysaccharide from T. mongolicum Hand.-Mazz comprising three monosaccharides, namely pika, arabinose, and galactose in a molar ratio of 1.0:10.7:11.9 [55]. Schütz et al. isolated fructooligosaccharides and fructopolysaccharides from the root of T. officinale WEB. ex WIGG [56].
4.4. Flavonoids
Flavonoids are a class of natural compounds with a 2-phenylchromanthone structure and a ketone carbonyl group. The oxygen atom in the first position is basic and can form a salt with a strong acid. The hydroxy derivative has a yellow color and is called a xanthophyll or a flavonoid. Dandelion contains diverse flavonoids, which are important in plant growth, development, flowering, fruiting, and anti-bacterial defense (Figure 4). Six flavonoids, including apigenin, luteolin, quercetin, luteolin-7-β-d-glucopyranoside, quercetin-7-β-d-glucopyranoside, and quercetin-37-O-β-d-diglucopyranoside, were obtained and identified from T. mongolicum [57]. Two new flavone glycosides, namely, isoetin-7-O-β-d-glucopyranosyl-2′-O-α-L-arabinopyranoside and isoetin-7-O-β-d-glucopyranosyl-2′-O-α-d-glucopyranoside, were isolated from the aerial part of T. mongolicum. The structures of these compounds were elucidated mainly by spectral analyses [58]. Shi et al. established an online rapid screening method, namely, high-performance liquid chromatography (HPLC) diode array for detection and electrospray mass spectrometry system for separation and identification of free radical scavengers in T. mongolicum methanol extract. Additionally, the detected anti-oxidant was directly separated by preparative HPLC (PHPLC) and Sephadex LH-20. The purified compound was sampled using an off-line nuclear magnetic resonance (NMR) spectrometer to obtain the corresponding spectrum. Thirty-two kinds of free radical scavenging compounds were screened, isolated, and identified, including 16 flavonoids, 10 phenylpropyl compounds, and 6 benzoic acid compounds. Among them, 17 compounds were isolated for the first time from T. mongolicum, including three new compounds [59]. Five flavonoid glycosides were isolated and purified from the gas phase of T. mongolicum (a traditional Chinese medicinal herb) using high-speed counter-current chromatography (HSCCC) [60,61,62]. Moreover, two polymethoxylated flavones were isolated from T. mongolicum in 2009 [63]. In 1996, three flavonoid glycosides, including luteolin 7-glycoside and two luteolin 7-diglucosides, were isolated from the flowers and leaves, while free luteolin was isolated from the flower tissues of T. officinale [63]. Eight flavones and eight flavonol glycosides were isolated from T. officinale WEB. ex WIGG. and identified using HPLC/electrospray ionization mass spectrometry [50]. Ten flavonoids were identified from T. formosanum and quantified with concentrations of 9.9–325.8 μg g−1 [51].
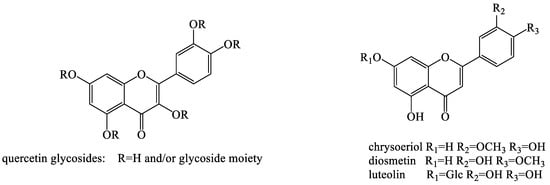

Figure 4.
The chemical structures of representative flavonoids from Taraxacum genus.
4.5. Sphingolipids
A sphingolipid comprises a long-chain fatty acid, a sphingosine molecule or its derivative, and a polar head alcohol. The polar head group of the sphingolipid binds to the hydroxyl group of the sphingosine, while the fatty acid moiety forms an amide bond with its amino group. Two sphingolipids, namely, gynuramide II and phytolacca cerebroside, were obtained and identified from the root of T. mongolicum [57] (Figure 5).

Figure 5.
The chemical structures of representative sphingolipids from Taraxacum genus.
4.6. Triterpenoids and Sterols
Triterpenoids are substances formed by the end-to-end joining of several isoprenes with their hydroxyl groups being removed. Most triterpenoids comprise a chain of 30 carbon atoms, while few contain 27 carbon atoms. In dandelion, pentacyclic triterpenoids are the main type. A sterol, which is a general term for a group of compounds with a fluorene nucleus, has a cyclopentane polyhydrophenanthrene skeleton. There are different kinds of sterols in different parts of the dandelion. In dandelions, triterpenoids and sterols exhibit remarkable anti-oxidative and anti-inflammatory activities (Figure 6). Six triterpenoids and sterols, such as gigantursenol A, taraxasterol, β-sitosterol, β-sitosterol-3-O-β-d-glucoside, stigmasterol, and β-sigmasterol-3-O-β-d-glucoside were successfully obtained from the root of T. mongolicum [57]. Warashina et al. extracted eight new triterpenes from dandelion roots [47]. Later, three novel triterpenoids, including the lupane-, bauerane-, and euphane-type triterpenoids were isolated from the roots of T. officinale [64].

Figure 6.
The chemical structures of representative triterpenoids and sterols from Taraxacum genus.
4.7. Coumarins
Coumarin-based compounds are relatively less distributed in dandelions (Figure 7); 6,7-dihydroxycoumarin (escin) and scutellarin were isolated from the stems and leaves of T. officinale in 1981 [65]. Nine coumarin compounds (including umbelliferone, coumestrol, lactucin, hachibate, east azlactone, resveratrol, lactucin, chicory, and esculin) were isolated from T. officinale and T. mongolicum Hand.-Mazz [30,63,66].

Figure 7.
The chemical structures of representative coumarins from Taraxacum genus.
4.8. Others
Some studies have reported that T. mongolicum also contains glycerin, inositol, polysaccharides, and other compounds that are indispensable for plant growth [67,68] (Figure 8). With the advancement of identification techniques, different classes of compounds can be assigned by comparing them with standards. Ma et al. employed ultra-performance liquid chromatography (UPLC) and identified three compounds, namely, chlorogenic acid, caffeic acid, and taraxasterol [69]. Oh et al. used HPLC to identify quercetin from the ethanolic extract of T. mongolicum [70]. Lignans, including mongolicumin A and rufescidride, were obtained from T. mongolicum [38,60]. The leaf extract of T. officinale contained houttuyin and aescin. This study is the first to report the discovery of free pterin (luteolin 3′-methyl ether). The contents of cichoric acid, chlorogenic acid, houttuyin, and aescin in chicory plants were initially identified. Chicoric acid and its related monocaffeine tartaric acid are major phenolics, which are also used in pharmaceutical preparations and found in alfalfa [63]. Jia et al. isolated two compounds, namely, caffeic acid and luteolin 7-O-β-d-glucopyranoside, from T. mongolicum [71]. HPLC was used to identify an organic acid from T. mongolicum Hand.-Mazz; the acid plays an important role in the treatment of acute tracheobronchitis and has good anti-inflammatory activity [72,73]. Kao utilized HPLC–MS spectrometry and carotenoid column chromatography and successfully separated 25 carotenoids from T. formosanum. Furthermore, all-trans-canthaxanthin was found to be an appropriate internal standard for quantitation; all-trans-carotene and its cis isomers had the largest amount (413.6 μg·g−1), followed by all-trans-violoxanthin and its cis isomers (209.5 μg·g−1), all-trans-lutein and its cis isomers (212.4 μg·g−1), all-trans-neoxanthin and its cis isomers (134.6 μg·g−1), antheraxanthin (16.5 μg·g−1), all-trans-cryptoxanthin and its cis isomers (5.8 μg·g−1), all-trans-zeaxanthin (3.6 μg·g−1), and neochrome (0.1 μg·g−1). Two inositol derivatives, namely, (1S,2S,4R,5S)-2,3,4,6-tetrahydroxy-5-[2-(4-hydroxyphenyl)acetyl] oxycyclohexyl-2-(4-hydroxyphenyl) acetate and (2S,3R,5R,6S)-2,3,5,6-tetrahydroxy-4-[2-(4-hydroxyphenyl) acetyl]oxycyclohexyl-2-(4-hydroxy- phenyl) acetate, were successfully isolated from the methanolic extract of T. linearisquameum [74]. Additionally, the known compound taraxinic acid β-D-glucopyranosyl ester was isolated [46]. Kenny et al. used liquid chromatography–mass spectrometry to separate and identify different 4-hydroxyphenylacetic acid derivatives, including, 9-hydroxyoctadecatrienoic acid and 9-hydroxyoctadecadienoic acid, from the root fraction of T. officinale and characterized these compounds using spectroscopy [75,76]. Lutein epoxide was successfully isolated from the petals of T. officinale F. Weber ex Wiggers. Moreover, all-E-lutein epoxide was the major carotenoid and had high amounts of (9Z)- and (9′Z)-isomers [77]. Phytochemical investigation of the roots of T. coreanum led to the isolation of two new inositol derivatives [52].
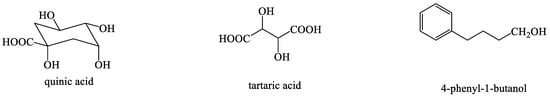

Figure 8.
The chemical structures of other types of compounds from Taraxacum genus.
5. Pharmacological Effects
Dandelion has been reported to have multiple pharmacological effects, including anti-bacterial, anti-oxidant, anti-cancer, anti-rheumatic, etc. This section reviews recent findings on the pharmacological effects of Taraxacum (Figure 9) (Table 3).

Figure 9.
The pharmacological mechanisms of the key chemical constituents of the dandelion.

Table 3.
The bioactivity of extracts from different species of Taraxacum.
5.1. Anti-Bacterial and Anti-Oxidant Effects
Dandelion reportedly possesses excellent anti-bacterial activity. Díaz and his colleagues isolated diverse chemical compounds from T. officinale leaves, mainly triterpenoids and other unknown compounds. Subsequently, the leaves’ extract could markedly inhibit Gram-positive bacteria with a minimum inhibitory concentration (MIC) of 200 g mL−1), thus suggesting dandelion had promising anti-bacterial potential [127]. In another experiment, the content, anti-oxidant activity, and cytotoxicity of phenols and flavonoids in three different types of dandelion methanolic extracts were investigated. The total phenolic content was 1000 mg·kg−1, with the aboveground content being higher than the root. T. mongolicum had the highest phenolic content in the stems (76.8 mg·kg−1) and roots (40.0 mg·kg−1), followed by T. coreanum and T. officinale (p < 0.05). Furthermore, the total flavonoid content also showed a consistent trend with the total phenolic content. The anti-oxidant activity of each methanolic extract increased in a dose-dependent manner. The maximum 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activities of T. mongolicum shoot and root extracts (89.6% and 83.4%) were obtained at a concentration of 1000 mg·kg−1. The overall experimental results proved that the total phenolic and flavonoid levels were highly correlated with anti-oxidant activity, but their content and activity varied across species [87,89]. In another study of the anti-bacterial activity of the extracts of T. mongolicum in different solvents, Gao reported that only the ethanolic extract had varying degrees of anti-bacterial activity (inhibition zone >7 mm in diameter), while the aqueous extract did not [78].
Flavonoids and coumaric acid derivatives were extracted from dandelion flowers. In the study of anti-oxidant properties, the extracts had scavenged effects on superoxide and hydroxyl radical-induced damage; meanwhile, the inhibition of hydroxyl radicals was nonspecific. The reduction in the phenolic content of the extract reduced the DPPH capacity and showed a synergistic effect with α-tocopherol. After the addition of the corresponding extract, the bacterial lipopolysaccharide (LPS) stimulated macrophage RAW264.7 cells in mice, thereby significantly reducing the NO concentration in a concentration-dependent manner. Additionally, adding a certain extract concentration significantly inhibited the peroxide radical-induced intracellular oxidation of RAW264.7 cells. The extract demonstrated a significant anti-oxidant activity in biological and chemical models. In addition, the inhibitory effect of the extract on reactive oxygen species (ROS) and NO was related to its phenolic content [93].
T. mongolicum extract inhibited four Gram-negative bacteria and two Gram-positive bacteria, especially Pseudomonas aeruginosa and Bacillus subtilis, with MIC values of 125 and 62.5 μg·mL−1, respectively. The ethyl acetate soluble component extracted from dandelion had high anti-bacterial activity and can be used as a natural preservative in the pharmaceutical industry [79]. The relationship between osteoporosis and oxidative stress induced by ROS was also studied and food and plants with anti-oxidant effects are being increasingly focused upon to reduce the ROS-induced damage caused during bone metabolism. The anti-oxidative effect of T. mongolicum on the proliferation and the differentiation of MC3T3-E1 cells induced by hydrogen peroxide was investigated, and the total contents of polyphenols and flavonoids were 33.65 and 4.45 mg·g−1, respectively. Under hydrogen peroxide-induced oxidative stress, dandelion extract promoted the proliferation of MC3T3-E1 cells and differentiation of osteoblasts. Hence, dandelion extract can inhibit oxidative stress-induced damage to osteoblasts and serve as a potential anti-oxidant material for preventing bone diseases [94].
With the increasing resistance of cow mastitis to bacteria and considering the safety of dairy products, anti-bacterial extracts should be used instead of antibiotics for the treatment of mastitis in dairy cows. The anti-bacterial effects of purslane and T. mongolicum aqueous and ethanolic extracts on the main pathogens of cow mastitis (Escherichia coli, Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus agalactiae) were studied using disk diffusion method. The aqueous and ethanolic extracts of the two traditional Chinese medicines had different inhibitory effects on the four pathogens of cow mastitis. The anti-bacterial activity of the two Chinese herbal extracts against E. coli was higher than that against other bacteria. The ethanolic extract had higher anti-bacterial activity against E. coli than purslane. However, the anti-bacterial activity of the T. mongolicum ethanol extract was lower than that of the aqueous extract. Hence, purslane and the T. mongolicum extract may be used for the treatment of mastitis in dairy cows [66].
5.2. Anti-Cancer Effects
A previous report investigating the use of T. mongolicum extracts for the prevention and treatment of bovine mastitis discovered that different concentrations of the extract had no noticeable cytotoxic effect on MAC-T cells, thus being the first to report that the T. mongolicum extract significantly inhibited the production of NO and pro-inflammatory cytokines in MAC-T cells. This finding has a certain clinical application value for the prevention and treatment of bovine mastitis [108]. Breast cancer is an aggressive and fatal breast disease with limited treatment options. Although T. mongolicum (a Chinese herbal medicine with anti-cancer activity) has been used for the treatment of breast abscess and breast hyperplasia since ancient times, its mechanism of action needs further scientific studies [128]. T. mongolicum extract significantly inhibited the activity of MDA-MB-231 cells by causing the G2/M phase arrest and apoptosis. The extract also significantly increased the levels of cleaved caspase-3 and PARP proteins, with the caspase inhibitor Z-VAD-FMK inhibiting T. mongolicum extract-induced apoptosis. Three ER stress-related signals were strongly induced by the T. mongolicum treatment, including increased expression of ATF4, ATF6, XBP1s, GRP78, and cleavage-related genes along with elevated phosphorylation levels of proteins, eIF-2αIRE1, and downstream molecular GRP78 impermanence. MDA-MB-231 cells transfected with CHOP siRNA significantly inhibited the T. mongolicum extract-induced apoptosis. The underlying mechanism is partially attributed to the strong activation of the active/p-eIF2α/ATF4/cut axis. In conclusion, apoptosis induced by endoplasmic reticulum stress generates the anti-cancer effect of the T. mongolicum extract, thereby suggesting that the T. mongolicum extract may be a potential treatment for triple-negative breast cancer (TNBC) [57]. However, the use of dandelion for breast cancer treatment is mainly based on anecdotal evidence and has no sufficient scientific evidence. Therefore, Oh et al. hypothesized that T. mongolicum can act as a selective estrogenic receptor modulator and hormone replacement therapy for postmenopausal women. T. mongolicum ethanol extract significantly increased the cell proliferation and estrogenic response element-driven luciferase activity. Hence, T. mongolicum ethanol extract can induce estrogenic activity mediated by the classical estrogenic receptor pathway, thereby providing a scientific basis for its anti-cancer application in traditional medicine [70].
5.3. Anti-Inflammatory Effects
Inflammation plays an important role in the pathogenesis of acute tracheobronchitis. The main component of T. mongolicum Hand.-Mazz—an organic acid, has good anti-inflammatory activity. Furthermore, organic acids can improve the regulation of the TLR4/NF-κB (TLR4/IKK/NF-κB) signaling pathway in LPS-mediated histopathological damage, which may provide a basis for the treatment of acute tracheobronchitis [72,73].
T. mongolicum is widely used in the Eastern Hemisphere. Since T. mongolicum has a high mineral content, it causes potential problems with the absorption of quinolones. Since a previous study reported the occurrence of multifactorial drug interactions between T. mongolicum and ciprofloxacin, the effects of their simultaneous use should not be ignored. Ciprofloxacin is a fluoroquinolone antibiotic that has good anti-bacterial activity against Gram-positive, Gram-negative, and mycobacteria. However, its oral absorption greatly diminished the effect of simultaneous administration of metal-containing cations. This phenomenon has been extensively studied for antacids, mineral supplements, and dairy products. However, information about this interaction is not yet available in mineral-rich herbal and health foods. Drug–drug interactions may occur between ciprofloxacin and a mineral-rich anti-inflammatory/anti-bacterial herb, T. mongolicum Hand-Mazz. Traditionally, T. mongolian dried plants are used for the treatment of lice, ulcers, mastitis, lymphadenitis, inflamed eyes, sore throat, lung and breast abscesses, acute appendicitis, jaundice, and urinary tract infections. Furthermore, this herb exerts a bactericidal effect on multiple pathogens, and its water extract has MIC values ranging from 1:10 to 1:640. In addition, the in vitro antifungal, anti-leptospiral, and antiviral effects of the herbs have been proven. Chemical testing of T. mongolicum indicates the presence of triterpenoids (such as tartaric alcohol and tartaric acid), inulin, pectin, asparagine, and phenolic compounds. A comprehensive pharmacokinetic assessment of the rat was performed and demonstrated the potential of drug–drug interactions between T. mongolicum and ciprofloxacin [80].
Different solvent extracts of T. officinale were successfully prepared by Jeon et al. in a carrageenan-induced balloon model [104]. The ethanolic extract inhibited the production of exudates and significantly reduced the NO and leukocyte levels in the exudate. The extract also inhibited acetic acid-induced vascular permeability in a dose-dependent manner in acetic acid-induced abdominal peristalsis in mice. In summary, medicinal dandelion has anti-angiogenic, anti-inflammatory, and anti-nociceptive properties by inhibiting NO production and cyclooxygenase-2 (COX-2) expression and/or its anti-oxidant activity.
Mouse macrophages (RAW 264.7) were used to study the anti-inflammatory effects and mechanism of the methanolic extract of T. officinale leaves on LPS induction. The methanolic extract and its components inhibited LPS-induced production of NO, pro-inflammatory cytokines, and prostaglandin (PG) E2 in a dose-dependent manner. However, the chloroform soluble fraction significantly inhibited the production of NO, PG E2, and two pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β)) in a dose-dependent manner, with MIC values of 66.51, 90.96, 114.76, and 171.06 μg·mL−1, respectively. Hence, the anti-inflammatory effects of leaf extract may be due to the down-regulation of NO, PG, E2, and pro-inflammatory cytokines along with the inactivation of the MAP kinase signaling pathway, thereby reducing the expression of inducible NO synthase (iNOS) and COX-2 [107]. Therefore, in the anti-inflammatory mechanism study, the aqueous extract of T. mongolicum exerted certain protective effects on acute lung injury-induced inflammation in mice [69].
6. Other Effects
Baek examined the methanolic extract of T. mongolicum and its fraction for their scavenging effects on DPPH and superoxide radicals and also their hepatoprotective effects on tacrine-induced cytotoxicity in the human hepatoma cell line, HepG2 cells. The extract had free radical scavenging and hepatoprotective effects [129]. The novel homogenous polysaccharide DPSW-A was obtained from T. mongolicum and its derivative demonstrated limited anti-coagulant function [55]. Furthermore, the newly isolated compound 1β,3β-dihydroxy-eudesman-11(13)-en-6α from T. mongolicum inhibited the NO production, with an IC50 of 38.9 μM [36].
In the study of its hypolipidemic action and mechanism, T. mongolicum was extracted separately with water, 50% ethanol, and 95% ethanol. The 50% ethanolic extract was the most effective among the 13 extracts. Prolonged administration of the 50% ethanolic extract significantly reduced the body weight of rats and the serum levels of triglyceride LDL-C and total cholesterol. Hence, T. mongolicum helps in lowering blood lipid levels [68]. Moreover, the T. mongolicum methanol extract strongly inhibited monoamine oxidase. Therefore, the extract can potentially affect diseases, such as depression, dementia, and Alzheimer’s disease [95].
Skin whitening is becoming popular among people. Melanin is an important factor that determines skin color. In the study of melanin synthesis inhibition by T. mongolicum extract, reverse-transcriptase polymerase chain reaction and Western blot were used to analyze the protein and mRNA levels of tyrosinase-related protein (TRP)-1, TRP-2, tyrosinase, MITF, ERK, and PKA, and it was found to inhibit melanin synthesis [110].
To verify the antiviral effect of T. mongolicum on the hepatitis B virus, researchers found that 50–100 g·mL−1 T. mongolicum extract could protect the rat hepatocytes as compared with D-galactosamine (D-GalN), thioacetamide (TAA), and t-butyl hydroperoxide (t-BHP). The protective effect of 100 g·mL−1 T. mongolicum extract on rat hepatocytes was enhanced. Furthermore, the T. mongolicum extract significantly inhibited DNA replication at 1–100 g·mL−1, and reduced the levels of HBsAg and HBeAg at 25–100 g·mL−1, with inhibition rates of 91.39% and 91.72% at 100 g·mL−1, respectively. The T. mongolicum extract significantly inhibited DNA replication at 25–100 g·mL−1, thus exerting a strong antiviral effect on HBV. The protective effect of T. mongolicum extract on hepatocytes may be achieved by inhibiting oxidative stress. However, the antiviral properties of the T. mongolicum extract may help block protein synthesis and DNA replication. The main components of the T. mongolicum extract were quantitatively analyzed to provide a scientific basis for its use in the treatment of hepatitis [71].
7. Toxicity
When a plant or a compound isolated from a plant has no significant toxicity or side effects, its potential therapeutic effect should be studied further. This is particularly important for dandelion [130]. In daily life, the recommended dosage of dandelion is 10–15 g [1]. In 1974, Râcz–Kotilla et al. [114] studied the diuretic effect of a 4% aqueous extract of dandelion. Firstly, they performed acute toxicity tests on different parts of dandelion and fluid extracts of grass (DL50 = 27.2 g·kg−1 body weight). In the diuretic experiment, the aqueous extract of dandelion was administered at a dose of 8 g·kg−1 body weight for one month, and the body weight of the mice and rats were found to be reduced by ~30%. In the study of the protective effect of renal oxidative damage caused by CCl4, the oral administration of 100, 250, 300, 500, and 750 mg·kg−1 dandelion aqueous extract for the duration of the test was considered safe. In addition, the corresponding lesions in mice showed a good prognosis, thus indicating that these dosages are within the normal range [115,122,131]. In terms of cytotoxicity, HepG2, HeLa, HL60, and Vero E6 cells had different IC50 values (0.015 ± 0.001, 0.023 ± 0.002, >0.25) [92].
8. Conclusions and Future Prospects
As a well-known complementary and alternative medicine, the whole dandelion herb, including its roots, stem, leaf, flower, and seed is rich in diverse bioactive ingredients including sesquiterpenes, phenolic compounds, phytosterols, triterpenes, etc. However, previous studies have mainly focused on extracting and identifying active ingredient structures from different kinds of dandelion. At present, most research focuses on studying the biological activity of partial extracts such as the root extracts, while the research on the biological activity of other effective active ingredients of dandelion are relatively fewer. Moreover, the pharmacological research of the effective active ingredients of dandelion mostly focuses on the basic pharmacological mechanism, and the form of mechanism research is relatively simple. For example, in order to clarify the specific anti-cancer mechanism of dandelion, more advanced strategies including network pharmacology, molecular pharmacology, and metabolomics methods can be flexibly used to comprehensively demonstrate the multi-target anti-cancer action mechanism of dandelion, which will provide new insights for further accurate search, and confirmation and optimization of the relationship between the active ingredient of dandelion and the target. Additionally, most of the current studies focus on in vitro cell experiments, and the research results lack clinical applicability. In the future, a large number of in vivo animal models are needed to deeply study the pharmacological mechanisms and targets of active ingredients of dandelion, so that they can realize clinical application as soon as possible and offer new ideas and methods for precise treatment.
To be more specific, T. mongolicum, T. borealisinense, T. coreanum Nakai, and T. officinale are the most frequently utilized species for complementary and alternative medicine. Additionally, in terms of pharmacological effects, dandelion could exert potent anti-bacterial, anti-oxidant, anti-cancer, and anti-rheumatic activities. Moreover, the extracts from different parts all displayed excellent aforementioned activities, which provided strong evidence regarding the use of the traditional medicinal herb as an anti-bacterial drug. However, the anti-bacterial effect differed between the different types of dandelion. Although dandelion is a traditional medicinal plant used for different treatments, its mechanism of action and its corresponding biological activity and safety should be further studied. Furthermore, when dandelion is clinically applied, in-depth research and investigation should be conducted regarding its distribution and metabolism. Therefore, we believe that with further developments in science and technology, novel drug technologies can be combined with traditional therapeutic medicinal plants, such as dandelion, to achieve better treatment outcomes.
Funding
This research was supported by the Innovation Ability Improvement Project of Colleges and Universities of Gansu Province (Project No.: 2020B-262), Higher Education Teaching Achievements Cultivation Project of Gansu Province in 2021 (Project No.: JXCGPY2021145), and College Students’ Innovation and Entrepreneurship Project of Gansu Province in 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Abbreviations
COX-2: cyclooxygenase-2; D-GalN: D-galactosamine; HPLC: high-performance liquid chromatography; HSCCC: high-speed counter-current chromatography; IL-1β: interleukin-1β; iNOS: inducible NO synthase; LPS: lipopolysaccharide; MIC: minimum inhibitory concentration; MTT: tetrazolium salt colorimetric; NMR: nuclear magnetic resonance; PG: prostaglandin; PHPLC: preparative HPLC; TAA: thioacetamide; t-BHP: t-butyl hydroperoxide; TCM: traditional Chinese medicine; TNBC: triple-negative breast cancer; TNF-α: tumor necrosis factor-α; UPLC: ultra-performance liquid chromatography.
References
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Gu, W.; Qiu, R.; Chao, J.; Pei, L.; Ma, L.; Guo, Y.; Tian, R. Metabolomics analysis of dandelions from different geographical regions in China. Phytochem. Anal. 2021, 32, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Y.; Zhao, C.; Li, S.; Wang, T.; Qiao, B.; Fu, Y. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). R. Soc. Open Sci. 2021, 8, 210614. [Google Scholar] [CrossRef]
- Olas, B. New Perspectives on the Effect of Dandelion, Its Food Products and Other Preparations on the Cardiovascular System and Its Diseases. Nutrients 2022, 14, 1350. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, P.; Chen, X.D. Mechanistic insights into the influence of flavonoids from dandelion on physicochemical properties and in vitro digestibility of cooked potato starch. Food Hydrocoll. 2022, 130, 107714. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia—Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Dokos, C.; Hadjicosta, C.; Dokou, K.; Stephanou, N. Ethnopharmacological survey of endemic medicinal plants in Paphos district of Cyprus. Ethnobot. Leafl. 2009, 13, 1060–1068. [Google Scholar]
- Chaudhary, M.I.; He, Q.; Cheng, Y.Y.; Xiao, P.G. Ethnobotany of medicinal plants from tian mu Shan biosphere reserve, Zhejiang-province, China. Asian J. Plant Sci. 2006, 5, 646–653. [Google Scholar]
- Trak, T.H.; Giri, R.A. Inventory of the plants used by the tribals (Gujjar and bakarwal) of district kishtwar, Jammu and Kashmir (India). Indian J. Sci. Res. 2017, 13, 104–115. [Google Scholar]
- Gheno-Heredia, Y.A.; Nava-Bernal, G.; Martínez-Campos, Á.R.; Sánchez-Vera, E. Las plantas medicinales de la organización de parteras y médicos indígenas tradicionales de Ixhuatlancillo, Veracruz, México y su significancia cultural. Polibotánica 2011, 31, 199–251. [Google Scholar]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.; Kumar, K. Traditional phytoremedies for the treatment of menstrual disorders in district Udhampur, J&K, India. J. Ethnopharmacol. 2015, 160, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Mahmood, A.; Mujtaba, G.; Mumtaz, M.S.; Kayani, W.K.; Khan, M.A. Indigenous medicinal knowledge of common plants from district Kotli Azad Jammu and Kashmir Pakistan. J. Med. Plant. Res. 2012, 6, 4961–4967. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A.; Pajazita, Q.; Syla, B.; Quave, C.; Pieroni, A. An ethnobotanical survey of the Gollak region, Kosovo. Genet. Resour. Crop. Evol. 2011, 59, 739–754. [Google Scholar] [CrossRef]
- Pieroni, A.; Nedelcheva, A.; Dogan, Y. Local knowledge of medicinal plants and wild food plants among Tatars and Romanians in Dobruja (South-East Romania). Genet. Resour. Crop. Evol. 2014, 62, 605–620. [Google Scholar] [CrossRef]
- Rahman, I.U.; Ijaz, F.; Iqbal, Z.; Afzal, A.; Ali, N.; Afzal, M.; Khan, M.A.; Muhammad, S.; Qadir, G.; Asif, M. A novel survey of the ethno medicinal knowledge of dental problems in Manoor Valley (Northern Himalaya), Pakistan. J. Ethnopharmacol. 2016, 194, 877–894. [Google Scholar] [CrossRef]
- Kozuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Napier, J. Descriptive study of contemporary status of the traditional knowledge on medicinal plants in Bulgaria. Afr. J. Pharm. Pharmacol. 2013, 7, 185–198. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnopharmacol. 2014, 151, 1005–1018. [Google Scholar] [CrossRef]
- Ummara, U.; Bokhari, T.Z.; Altaf, A.; Younis, U.; Dasti, A.A. Pharmacological study of Shogran valley flora, Pakistan. Int. J. Sci. Eng. Res. 2013, 4, 1–9. [Google Scholar]
- Trillo, C.; Arias Toledo, B.; Galetto, L.; Colantonio, S. Persistence of the use of medicinal plants in rural communities of the Western Arid Chaco [Córdoba, Argentina]. Open Complement. Med. J. 2010, 2, 80–89. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Giusti, M.E.; Quave, C.L. Cross-cultural ethnobiology in the Western Balkans: Medical ethnobotany and ethnozoology among Albanians and Serbs in the Pešter Plateau, Sandžak, South-Western Serbia. Hum. Ecol. 2011, 39, 333–349. [Google Scholar] [CrossRef]
- Macía, M.J.; García, E.; Vidaurre, P.J. An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol. 2005, 97, 337–350. [Google Scholar] [CrossRef]
- Dangwal, L.R.; Sharma, A.; Rana, C.S. Ethnomedicinal plants of the Garhwal Himalaya used to cure various diseases: A case study. N. Y. Sci. J. 2010, 3, 28–31. [Google Scholar]
- Bhatt, D.; Kumar, R.; Joshi, G.; Tewari, L. Indigenous uses of medicinal plants by the Vanraji tribes of Kumaun Himalaya, India. J. Med. Plant Res. 2013, 7, 2747–2754. [Google Scholar]
- Bussmann, R.W.; Paniagua Zambrana, N.Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Hart, R.E. Medicinal and food plants of Svaneti and Lechkhumi, Sakartvelo (republic of Georgia), Caucasus. Med. Aromat. Plants. 2016, 5, 1–18. [Google Scholar]
- Rana, P.K.; Kumar, P.; Singhal, V.K.; Rana, J.C. Uses of Local Plant Biodiversity among the Tribal Communities of Pangi Valley of District Chamba in Cold Desert Himalaya, India. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterrenean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 33–43. [Google Scholar]
- Kim, H.; Song, M.-J.; Potter, D. Medicinal efficacy of plants utilized as temple food in traditional Korean Buddhism. J. Ethnopharmacol. 2006, 104, 32–46. [Google Scholar] [CrossRef]
- Özdemir, E.; Alpınar, K. An ethnobotanical survey of medicinal plants in western part of central Taurus Mountains: Aladaglar (Nigde-Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Alves, R.C.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Nutritional composition, antioxidant activity and phenolic compounds of wild Taraxacum sect. Ruderalia. Food Res. Int. 2014, 56, 266–271. [Google Scholar] [CrossRef]
- Hänsel, R.; Kartarahardja, M.; Huang, J.-T.; Bohlmann, F. Sesquiterpenlacton-β-d-glucopyranoside sowie ein neues eudesmanolid aus Taraxacum officinale. Phytochemistry 1980, 19, 857–861. [Google Scholar] [CrossRef]
- Zielińska, K.; Kisiel, W. Sesquiterpenoids from roots of Taraxacum laevigatum and Taraxacum disseminatum. Phytochemistry 2000, 54, 791–794. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Yasmeen, S.; Ali, Z.; Khan, M.A.; Choudhary, M.I.; Akhtar, F.; Zahid, M. Taraxacin, a new guaianolide from Taraxacum wallichii. J. Nat. Prod. 2000, 63, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Choo, S.-J.; Ryoo, I.-J.; Ahn, J.-S.; Yoo, I.-D. Eudesmanolides from Taraxacum mongolicum and their inhibitory effects on the production of nitric oxide. Arch. Pharmacal Res. 2011, 34, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, C.; Kim, Y.H.; Ma, J.Y.; Shim, S.H. Chemical constituents of the aerial part of Taraxacum mongolicum and their chemotaxonomic significance. Nat. Prod. Res. 2017, 31, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Zhou, Q.; Peng, H.; Zhou, C.X.; Hu, M.H.; Tao, Q.F.; Hao, X.J.; Stöckigt, J.; Zhao, Y. Four new constituents from Taraxacum mongolicum. Chin. Chem. Lett. 2007, 18, 1367–1370. [Google Scholar] [CrossRef]
- Kisiel, W.; Barszcz, B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia 2000, 71, 269–273. [Google Scholar] [CrossRef]
- Kisiel, W.; Michalska, K. Sesquiterpenoids and phenolics from Taraxacum hondoense. Fitoterapia 2005, 76, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Kisiel, W. Sesquiterpene Lactones from Taraxacum obovatum. Planta Medica 2003, 69, 181–183. [Google Scholar] [CrossRef]
- Michalska, K.; Kisiel, W. A Guaianolide Sulfate Conjugate and Other Constituents from Taraxacum alpinum. Pol. J. Chem. 2007, 81, 515. [Google Scholar] [CrossRef]
- Michalska, K.; Kisiel, W. Sesquiterpene lactones from Taraxacum erythrospermum. Biochem. Syst. Ecol. 2008, 36, 444–446. [Google Scholar] [CrossRef]
- Michalska, K.; Kisiel, W. Sesquiterpenoids from Taraxacum serotinum. Biochem. Syst. Ecol. 2009, 37, 519–521. [Google Scholar] [CrossRef]
- Michalska, K.; Marciniuk, J.; Kisiel, W. Sesquiterpenoids and phenolics from roots of Taraxacum udum. Fitoterapia 2010, 81, 434–436. [Google Scholar] [CrossRef]
- Zidorn, C.; Ellmerermuller, E.; Stuppner, H. Eudesmanolides and inositol derivatives from Taraxacum linearisquameum. Phytochemistry 1999, 51, 991–994. [Google Scholar] [CrossRef]
- Warashina, T.; Umehara, K.; Miyase, T. Constituents from the Roots of Taraxacum platycarpum and Their Effect on Proliferation of Human Skin Fibroblasts. Chem. Pharm. Bull. 2012, 60, 205–212. [Google Scholar] [CrossRef]
- Ho, C.; Choi, E.J.; Yoo, G.S.; Kim, K.M.; Ryu, S.Y. Desacetylmatricarin, an anti-allergic component from Taraxacum platycarpum. Planta Med. 1998, 64, 577–578. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Takanaka, K.; Tsukada, H.; Miwa, Y.; Taga, T.; Tanaka, S.; Ikeshiro, Y. Sesquiterpene glucosides from anti-leukotriene B4 release fraction of Taraxacum officinale. J. Asian Nat. Prod. Res. 2001, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 19, 179–186. [Google Scholar] [CrossRef]
- Chen, H.-J.; Inbaraj, B.S.; Chen, B.-H. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci. 2011, 13, 260–285. [Google Scholar] [CrossRef]
- Mo, E.J.; Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum. Molecule 2017, 22, 1349. [Google Scholar] [CrossRef]
- Bylka, W.; Matlawska, I.; Frański, R. Essential oil composition of Taraxacum officinale. Acta Physiol. Plant. 2009, 32, 231–234. [Google Scholar] [CrossRef]
- Hook, I.; Sheridan, H.; Wilson, G. Volatile metabolites from suspension cultures of Taraxacum officinale. Phytochemistry 1991, 30, 3977–3979. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Shi, S.; Chen, Y.; Wang, H.; Fan, H.; Wang, S. Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr. Polym. 2016, 152, 241–252. [Google Scholar] [CrossRef]
- Schütz, K.; Muks, E.; Carle, R.; Schieber, A. Separation and quantification of inulin in selected artichoke (Cynara Scolymus L.) cultivars and dandelion (Taraxacum officinale WEB. ex WIGG.) roots by high-performance anion exchange chromatography with pulsed amperometric detection. Biomed. Chromatogr. 2006, 20, 1295–1303. [Google Scholar] [CrossRef]
- Li, X.-H.; He, X.-R.; Zhou, Y.-Y.; Zhao, H.-Y.; Zheng, W.-X.; Jiang, S.-T.; Zhou, Q.; Li, P.-P.; Han, S.-Y. Taraxacum mongolicum extract induced endoplasmic reticulum stress associated-apoptosis in triple-negative breast cancer cells. J. Ethnopharmacol. 2017, 206, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, K.; Zhang, Y.; Liu, S. Preparative isolation and purification of two flavonoid glycosides from Taraxacum mongolicum by high-speed counter-current chromatography. Sep. Purif. Technol. 2008, 60, 81–85. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Y.; Zhou, H.; Zhang, Y.; Jiang, X.; Huang, K. Identification of antioxidants from Taraxacum mongolicum by high-performance liquid chromatography–diode array detection–radical-scavenging detection–electrospray ionization mass spectrometry and nuclear magnetic resonance experiments. J. Chromatogr. A 2008, 1209, 145–152. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Huang, K.; Liu, S.; Zhao, Y. Application of preparative high-speed counter-current chromatography for separation and purification of lignans from Taraxacum mongolicum. Food Chem. 2008, 108, 402–406. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Zhao, Y.; Huang, K. Preparative isolation and purification of three flavonoid glycosides from Taraxacum mongolicum by high-speed counter-current chromatography. J. Sep. Sci. 2008, 31, 683–688. [Google Scholar] [CrossRef]
- Shi, S.; Honghao, Z.; Zhang, Y.; Zhao, Y.; Kelong, H.; Liu, S. A High-Speed Counter-Current Chromatography-- HPLC--DAD Method for Preparative Isolation and Purification of Two Polymethoxylated Flavones From Taraxacum mongolicum. J. Chromatogr. Sci. 2009, 47, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tanaka, A.; Uriuda, M.; Yamada, T.; Tanaka, R. Three Novel Triterpenoids from Taraxacum officinale Roots. Molecules 2016, 21, 1121. [Google Scholar] [CrossRef] [PubMed]
- Komissarenko, N.; Derkach, A. Coumarins of taraxacum-officinale. Khimiya Prir. Soedin. 1981, 4, 519. [Google Scholar]
- Peng, S.; Dai, W.; Yu, H.; Wang, Y.; Wang, X.; Wang, X.; Peng, S. Anti-bacterial activity of aqueous and ethanolic extracts of Portulaca oleracea L. and Taraxacum mongolicum against pathogenic bacteria of cow mastitis. Int. J. Appl. Res. Vet. Med. 2014, 12, 210–213. [Google Scholar]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, N.; Liu, M. A new inositol triester from Taraxacum mongolicum. Nat. Prod. Res. 2014, 28, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.R.; Park, Y.J.; Lee, Y.H.; Chung, K.H. Ethanolic extract of dandelion (Taraxacum mongolicum) induces estrogenic activity in MCF-7 cells and immature rats. Chin. J. Nat. Med. 2015, 13, 808–814. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Guan, R.-F.; Wu, Y.-H.; Yu, X.-P.; Lin, W.-Y.; Zhang, Y.-Y.; Liu, T.; Zhao, J.; Shi, S.-Y.; Zhao, Y. Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol. Med. Rep. 2014, 9, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Dong, Z.; Tian, G.; Zhu, M.; Li, C.; Bu, W.; Feng, L. Protective effects of organic acid component from Taraxacum mongolicum Hand.-Mazz. against LPS-induced inflammation: Regulating the TLR4/IKK/NF-κB signal pathway. J. Ethnopharmacol. 2016, 194, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, C.; Tian, G.; Zhu, M.; Bu, W.; Chen, J.; Feng, L. Organic acid component from Taraxacum mongolicum Hand.-Mazz alleviates inflammatory injury in lipopolysaccharide-induced acute tracheobronchitis of ICR mice through TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 34, 92–100. [Google Scholar] [CrossRef]
- Kao, T.; Loh, C.; Inbaraj, B.S.; Chen, B. Determination of carotenoids in Taraxacum formosanum by HPLC–DAD–APCI-MS and preparation by column chromatography. J. Pharm. Biomed. Anal. 2012, 66, 144–153. [Google Scholar] [CrossRef]
- Kenny, O.; Brunton, N.P.; Walsh, D.; Hewage, C.M.; McLoughlin, P.; Smyth, T.J. Characterisation of Antimicrobial Extracts from Dandelion Root (Taraxacum officinale) Using LC-SPE-NMR. Phytotherapy Res. 2015, 29, 526–532. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.; Hewage, C.; Brunton, N.; McLoughlin, P. 4-Hydroxyphenylacetic acid derivatives of inositol from dandelion (Taraxacum officinale) root characterised using LC–SPE–NMR and LC–MS techniques. Phytochemistry 2013, 98, 197–203. [Google Scholar] [CrossRef]
- Melendezmartinez, A.; Britton, G.; Vicario, I.M.; Heredia, F.J. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F. Weber ex Wiggers). Phytochemistry 2006, 67, 771–777. [Google Scholar] [CrossRef]
- Demin, G.A.O. Analysis of nutritional components of Taraxacum mongolicum and its antibacterial activity. Pharmacogn. J. 2010, 2, 502–505. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, T.J. Antibacterial activity of ethanol extract and fractions obtained from Taraxacum mongolicum flower. Res. J. Pharmacogn. 2014, 1, 35–39. [Google Scholar]
- Zhu, M.; Wong, P.Y.; Li, R.C. Effects of Taraxacum mongolicum on the bioavailability and disposition of ciprofloxacin in rats. J. Pharm. Sci. 1999, 88, 632–634. [Google Scholar] [CrossRef]
- Iqbal, Z.; Afzal, M.; Afzal, A.; Rahman, I.U.; Ahmed, B.; Anjum, N.; Bibi, A. In vitro antibacterial study of Taraxacum officinale leaves extracts against different bacterial pathogenic strains. J. Pharmacogn. Phytochem. 2014, 3, 15–17. [Google Scholar]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. In-vitro antidiabetic studies of various extracts of Taraxacum officinale. Pharma Innov. 2015, 4, 61. [Google Scholar]
- Tettey, C.; Ocloo, A.; Nagajyothi, P.; Lee, K. An in vitro analysis of antiproliferative and antimicrobial activities of solvent fractions of Taraxacum officinale (Dandelion) leaf. J. Appl. Pharm. Sci. 2014, 4, 41–45. [Google Scholar] [CrossRef]
- Astafieva, A.A.; Rogozhin, E.I.; Odintsova, T.I.; Khadeeva, N.V.; Grishin, E.V.; Egorov, T.A. Discovery of novel antimicrobial peptides with unusual cysteine motifs in dandelion Taraxacum officinale Wigg. flowers. Peptides 2012, 36, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.; Predan, G.; Rizea, G.D.; Mihele, D.; Dune, A.; Ivopol, G.; Ionita, C. Antimicrobial Activity of some Hydroalcoholic Extracts of Artichoke (Cynara Scolymus), Burdock (Arctium Lappa) and Dandelion (Taraxacum officinale). Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2013, 6, 113–120. [Google Scholar]
- Paul, N.C.; Kim, W.K.; Woo, S.K.; Park, M.S.; Yu, S.H. Diversity of endophytic fungi associated with Taraxacum coreanum and their antifungal activity. Mycobiology 2006, 34, 185–190. [Google Scholar] [CrossRef]
- Chon, S.-U. Antioxidant Activity and Cytotoxicity of Different Taraxacum Species in Korea. Korean J. Crop. Sci. 2012, 57, 51–59. [Google Scholar] [CrossRef]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef]
- Chon, S.U.; Bae, C.H.; Lee, S.C. Antioxidant and cytotoxic potentials of methanol extracts from Taraxacum officinale FH Wigg. at different plant parts. Korean J. Plant Resour. 2012, 25, 232–239. [Google Scholar] [CrossRef]
- Petkova, N.T.; Ivanov, I.; Topchieva, S.; Denev, P.; Pavlov, A. Biologically active substances and in vitro antioxidant activity of different extracts from dandelion (Taraxacum officinale) roots. Sci. Bulletin. Ser. F. Biotechnol. 2015, 19, 190–197. [Google Scholar] [CrossRef]
- Modaresi, M.; Resalatpour, N. The Effect of Taraxacum officinale Hydroalcoholic Extract on Blood Cells in Mice. Adv. Hematol. 2012, 2012, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mingarro, D.M.; Plaza, A.; Galán, A.; Vicente, J.A.; Martínez, M.P.; Acero, N. The effect of five Taraxacum species on in vitro and in vivo antioxidant and antiproliferative activity. Food Funct. 2015, 6, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- Seo, J.-E.; Kim, G.-H. Antioxidant Activity and Differentiation Effect of Taraxacum mongolicum Extracts against Hydrogen Peroxide-induced Oxidative Damage of MC3T3-E1 Osteoblast Cells. Korean J. Food Cook. Sci. 2012, 28, 311–318. [Google Scholar] [CrossRef]
- Hwang, K.H.; Park, T.K. The inhibitory activity of the Taraxacum mongolicum on monoamine oxidase. Korean J. Pharmacogn. 2006, 37, 229–234. [Google Scholar]
- García-Carrasco, B.; Fernandez-Dacosta, R.; Dávalos, A.; Ordovás, J.M.; Rodriguez-Casado, A. In vitro Hypolipidemic and Antioxidant Effects of Leaf and Root Extracts of Taraxacum Officinale. Med. Sci. 2015, 3, 38–54. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Rauber, R.; de Mattos, S.E.C.; Rocha, J.B.T.D.; Nogueira, C.W.; Soares, F.A.A. Antioxidant properties of Taraxacum officinale fruit extract are involved in the protective effect against cellular death induced by sodium nitroprusside in brain of rats. Pharm. Biol. 2012, 50, 883–891. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Gubert, P.; da Luz, S.C.A.; Athayde, M.L.; Rocha, J.B.T.; Soares, F.A.A. Antioxidant Properties of Taraxacum officinale Leaf Extract Are Involved in the Protective Effect Against Hepatoxicity Induced by Acetaminophen in Mice. J. Med. Food 2012, 15, 549–556. [Google Scholar] [CrossRef]
- Choi, U.-K.; Lee, O.-H.; Yim, J.H.; Cho, C.-W.; Rhee, Y.K.; Lim, S.-I.; Kim, Y.-C. Hypolipidemic and Antioxidant Effects of Dandelion (Taraxacum officinale) Root and Leaf on Cholesterol-Fed Rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-carcinogenic Activity of Taraxacum Plant. I. Biol. Pharm. Bull. 1999, 22, 602–605. [Google Scholar] [CrossRef]
- Sun, Z.; Su, R.; Qiao, J.; Zhao, Z.; Wang, X. Flavonoids Extraction from Taraxacum officinale (Dandelion): Optimisation Using Response Surface Methodology and Antioxidant Activity. J. Chem. 2014, 2014, 956278. [Google Scholar] [CrossRef]
- Koo, H.-N.; Hong, S.-H.; Song, B.-K.; Kim, C.-H.; Yoo, Y.-H.; Kim, H.-M. Taraxacum officinale induces cytotoxicity through TNF-α and IL-1α secretion in Hep G2 cells. Life Sci. 2004, 74, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Kang, H.-J.; Jung, H.-J.; Kang, Y.-S.; Lim, C.-J.; Kim, Y.-M.; Park, E.-H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Youn, H.J.; Chang, H.K.; Song, Y.S. TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem. Toxicol. 2010, 48, 1255–1261. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Choi, H.Y. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef]
- Koh, Y.-J.; Cha, D.-S.; Ko, J.-S.; Park, H.-J.; Choi, H.-D. Anti-Inflammatory Effect of Taraxacum officinale Leaves on Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Cells. J. Med. Food 2010, 13, 870–878. [Google Scholar] [CrossRef]
- Lee, K.H.; Hsu, K.C.; Wang, Y.S.; Yeh, C.C.; Chen, J.Y.; Chang, C.L.; Chi, C.H. Effects of Taraxacum mongolicum Extract on Lipopolysaccharide-Induced Nitric Oxide and Cytokines Production by Bovine Mammary Epithelial Cells. Intern. J. Appl. Res. Vet. Med. 2013, 11, 144–152. [Google Scholar]
- Lee, M.H.; Song, S.H.; Ham, I.H.; Bu, Y.M.; Kim, H.C.; Choi, H.Y. Anti-inflammatory effect and contents from the aerial part and root of the various Taraxacum spp. distributed in Korea. Korea J. Herbol. 2010, 25, 77–84. [Google Scholar]
- Jang, M.-H.; Ahn, T.-W. Inhibitory effects of Taraxacum mongolicum with phreatic water on melanin synthesis. Integr. Med. Res. 2014, 4, 76–93. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Shieh, P.-C.; Lee, J.-C.; Chen, F.-A.; Lee, C.-H.; Kuo, S.-C.; Ho, C.-T.; Kuo, D.-H.; Huang, L.-J.; Way, T.-D. Hypolipidemic activity of Taraxacum mongolicum associated with the activation of AMP-activated protein kinase in human HepG2 cells. Food Funct. 2014, 5, 1755–1762. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, L.; Xu, Y.; Cheng, H.; Wang, W.; Liu, Z.; Ning, W. In vitro evaluation of hydroxycinnamoyl CoA: Quinate hydroxycinnamoyl transferase expression and regulation in Taraxacum antungense in relation to 5-caffeoylquinic acid production. Phytochemistry 2019, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Romić, Ž; Rahelić, D.; Tadić, Ž. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J. Ethnopharmacol. 2010, 130, 569–577. [Google Scholar] [CrossRef]
- Râcz–Kotilla, E.; Râcz, G.; Solomon, A. The action of Taraxacum officinale extracts on the body weight and diuresis of laboratory animaLS. Planta Medica 1974, 26, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, A.; Değer, Y.; Yıldırım, S. Protective effect of Silybum marianum and Taraxacum officinale extracts against oxidative kidney injuries induced by carbon tetrachloride in rats. Ren. Fail. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L.; Abo-Golayel, M.K.; Abo-Elnaga, G.; Al-Beshri, H. Hepatoprotective effect of dandelion (Taraxacum officinale) against induced chronic liver cirrhosis. Med. Plants Res. 2013, 7, 1494–1505. [Google Scholar]
- Choi, J.H.; Shin, K.M.; Kim, N.Y.; Hong, J.P.; Lee, Y.S.; Kim, H.J.; Lee, K.T. Taraxinic Acid, a Hydrolysate of Sesquiterpene Lactone Glycoside from the Taraxacum coreanum N AKAI, Induces the Differentiation of Human Acute Promyelocytic Leukemia HL-60 Cells. Biol. Pharm. Bull. 2002, 25, 1446–1450. [Google Scholar] [CrossRef]
- Li, Y.C.; Shen, J.D.; Li, Y.Y.; Huang, Q. Antidepressant effects of the water extract from Taraxacum officinale leaves and roots in mice. Pharm. Biol. 2014, 52, 1028–1032. [Google Scholar] [CrossRef]
- Ahmad, D.; Gulfraz, M.; Ahmad, M.S.; Nazir, H.; Gul, H.; Asif, S. Protective action of Taraxacum officinale on CCl4 induced hepatotoxicity in rats. Afr. J. Pharm. Pharmacol. 2014, 8, 775–780. [Google Scholar]
- Clare, B.A.; Conroy, R.S.; Spelman, K.; Cand, P.; Zhang, N.; Pang, L.; Dong, N.; Xu, D. The Diuretic Effect in Human Subjects of an Extract of Taraxacum officinale Folium over a Single Day. J. Altern. Complement. Med. 2009, 15, 929–934. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.-T.; Park, J.H.; Kim, H.-J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- Fallah, H.H.; Zareei, A.M.; SA, Z.; SM, A. The effects of Taraxacum officinale L. and Berberis vulgaris L. root extracts on carbon tetrachloride induced liver toxicity in rats. J. Med. Plants 2010, 1, 45–52. [Google Scholar]
- Jin, Y.-R.; Jin, J.; Piao, X.-X.; Jin, N.G. The effect of Taraxacum officinale on gastric emptying and smooth muscle motility in Rodents. Neurogastroenterol. Motil. 2011, 23, 766-e333. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, H.; Ping, J.; Ju, Y.; Zhang, X. Taraxacum officinale protects against lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2010, 130, 392–397. [Google Scholar] [CrossRef]
- Mahesh, A.; Jeyachandran, R.; Cindrella, L.; Thangadurai, D.; Veerapur, V.; Muralidhara Rao, D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. Acta Biol. Hung. 2010, 61, 175–190. [Google Scholar] [CrossRef]
- Lee, B.-R.; Lee, J.-H.; An, H.-J. Effects of Taraxacum officinale on Fatigue and Immunological Parameters in Mice. Molecules 2012, 17, 13253–13265. [Google Scholar] [CrossRef] [PubMed]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and identification of compounds from bioactive extracts of Taraxacum officinale Weber ex FH Wigg.(Dandelion) as a potential source of antibacterial agents. Evid.-Based Complement. Altern. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef]
- Qu, J.; Ke, F.; Liu, Z.; Yang, X.; Li, X.; Xu, H.; Li, Q.; Bi, K. Uncovering the mechanisms of dandelion against triple-negative breast cancer using a combined network pharmacology, molecular pharmacology and metabolomics approach. Phytomedicine 2022, 99, 153986. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.Y. In vitro free radical scavenging and hepatoprotective activities of Taraxacum mongolicum. Korean J. Pharmacogn. 2003, 34, 324–326. [Google Scholar]
- Akhtar, W.; Ali, G.; Ashraf, N.; Fatima, I.; Kayani, W.K.; Shaheen, H.; Khames, A. Efficiency of Multiple Extraction Solvents on Antioxidant, Cytotoxic, and Phytotoxic Potential of Taraxacum officinale (L.) Weber ex FH Wigg. from Poonch Valley, Azad Kashmir, Pakistan. Evid.-Based Complement. Altern. Med. 2022, 2022, 5118553. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.H.; Zaree, A.M.; Naghdi, H.B.; SM, A.; Mohammadi, R.S. The protective effect of medicinal herbs extracts including Cynara Scolymus L.; cichorium intybus L. Taraxacum officinal L. and Berberis vulgaris L. in single and in combination form in CCI4 induced rat liver toxicity. J. Med. Plants 2012, 1, 78–85. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).