Figure 1.
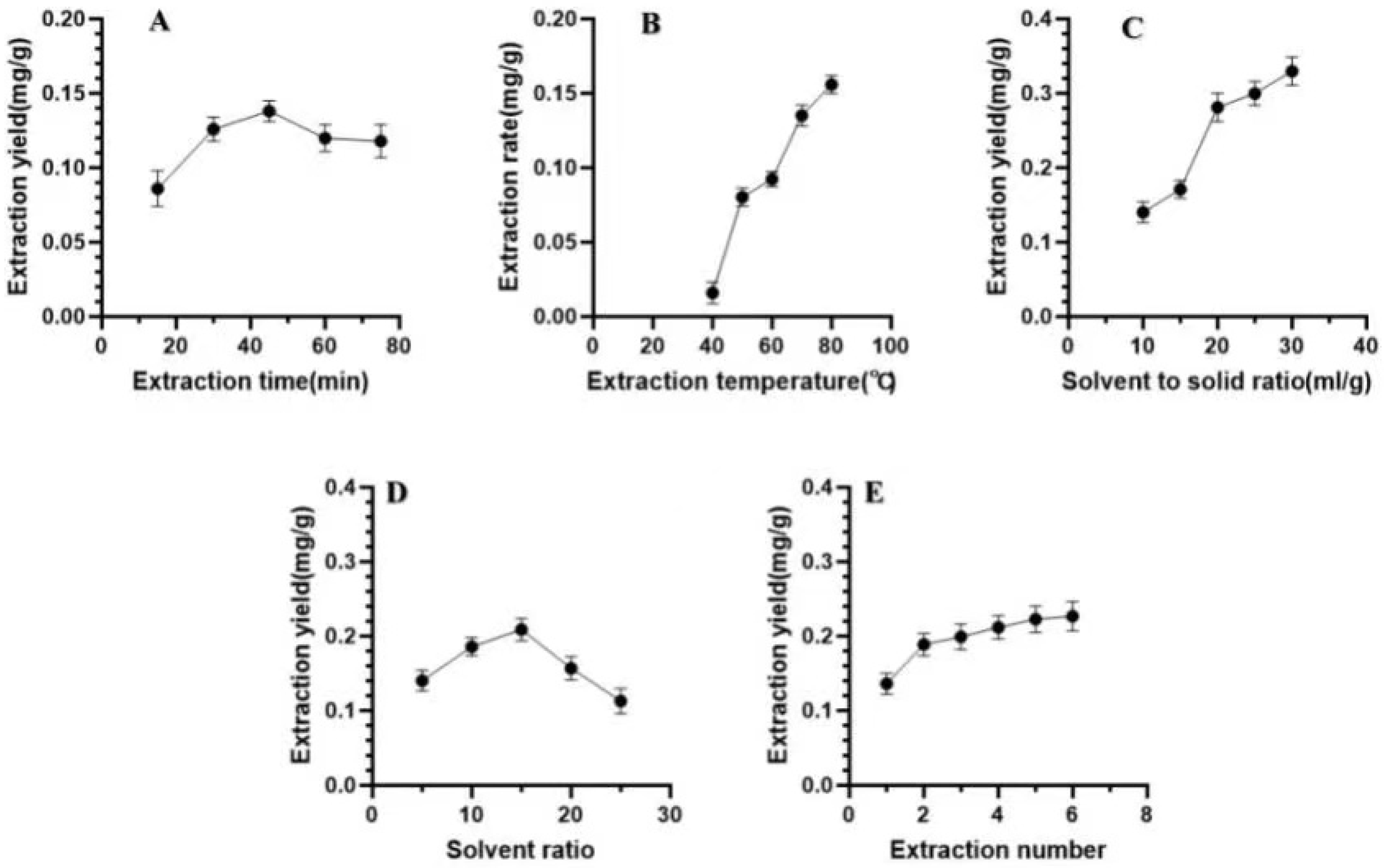
Effects of different extraction parameters on the extraction yield of luteolin. Single-factor experiments were conducted by varying the extraction time, extraction temperature, material/liquid ratio, methanol/hydrochloric acid solvent ratio, and number of extraction times to study the influence of these five factors on the extraction rate of luteolin. (A), the effect of extraction time on the extraction rate of Luteolin; (B), the effect of extraction temperature on the ex-traction rate of Luteolin; (C), the effect of liquid to material ratio on the extraction rate of Luteolin; (D), the influ-ence of solvent ratio (methanol: hydrochloric acid) on the extraction rate of Luteolin; (E) shows the effect of extrac-tion frequency on extraction rate.
Figure 1.
Effects of different extraction parameters on the extraction yield of luteolin. Single-factor experiments were conducted by varying the extraction time, extraction temperature, material/liquid ratio, methanol/hydrochloric acid solvent ratio, and number of extraction times to study the influence of these five factors on the extraction rate of luteolin. (A), the effect of extraction time on the extraction rate of Luteolin; (B), the effect of extraction temperature on the ex-traction rate of Luteolin; (C), the effect of liquid to material ratio on the extraction rate of Luteolin; (D), the influ-ence of solvent ratio (methanol: hydrochloric acid) on the extraction rate of Luteolin; (E) shows the effect of extrac-tion frequency on extraction rate.
Figure 2.
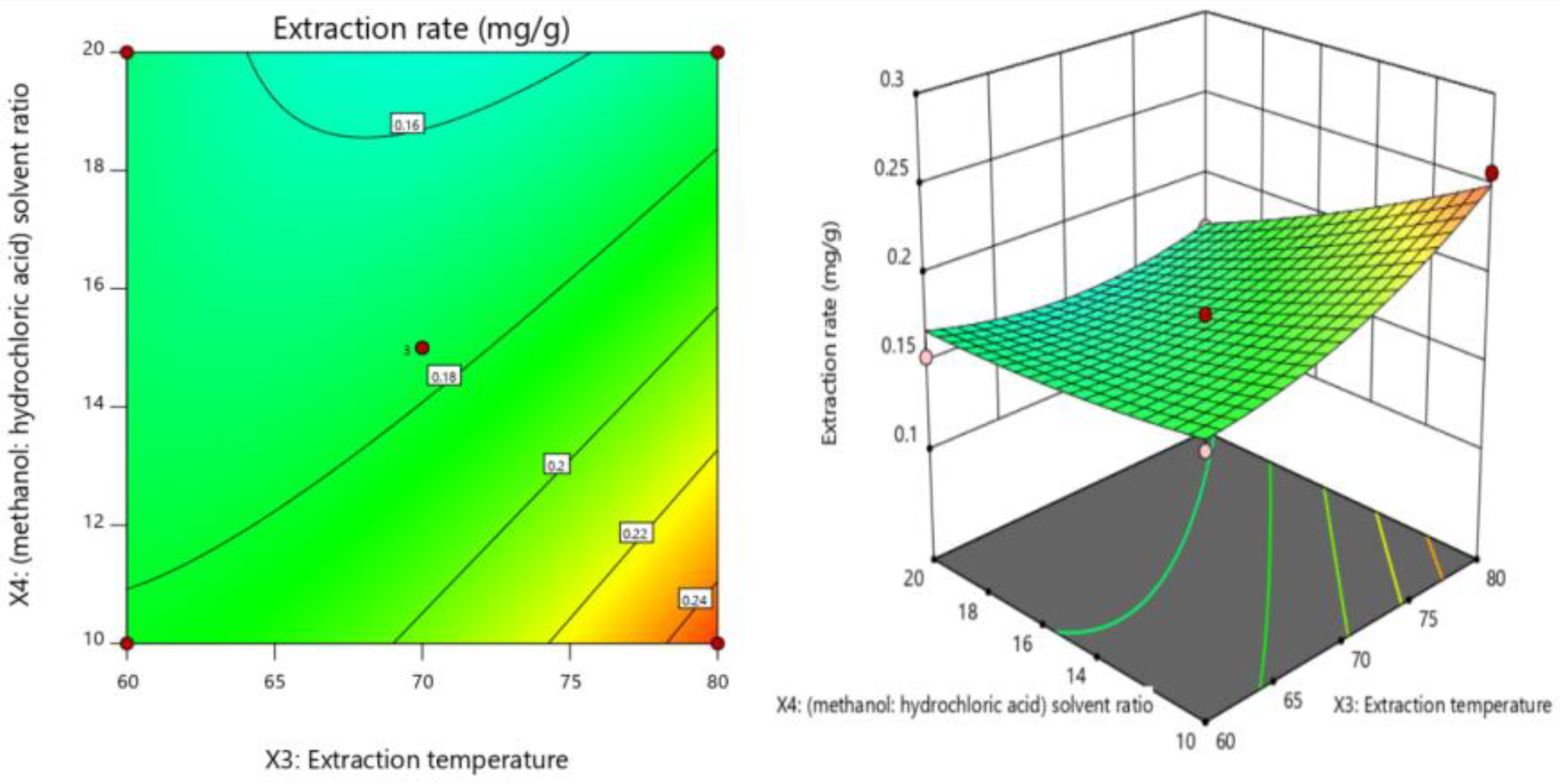
Contour map (2D) and response surface graph (3D) of the effect of extraction temperature and solvent concentration ratio on response value Y. The interaction effect between the extraction temperature (X3) and methanol/hydrochloric acid solvent ratio (X4) (X3X4) was the most significant. With increasing extraction temperature (X3) and decreasing methanol/hydrochloric acid solvent ratio (X4), the extraction rate of luteolin increased.
Figure 2.
Contour map (2D) and response surface graph (3D) of the effect of extraction temperature and solvent concentration ratio on response value Y. The interaction effect between the extraction temperature (X3) and methanol/hydrochloric acid solvent ratio (X4) (X3X4) was the most significant. With increasing extraction temperature (X3) and decreasing methanol/hydrochloric acid solvent ratio (X4), the extraction rate of luteolin increased.
Figure 3.
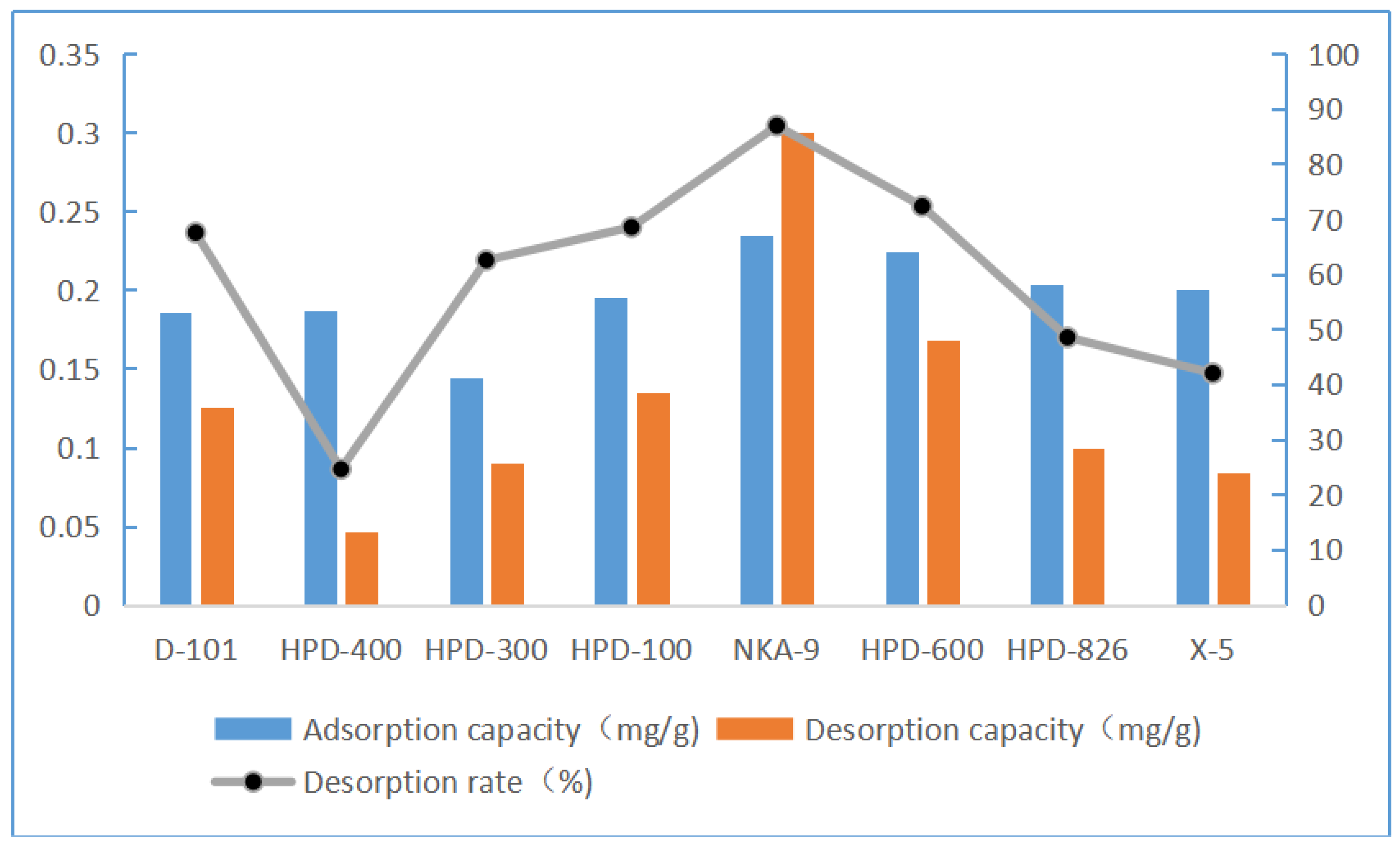
Static adsorption and desorption capacities and desorption rates of eight resins for luteolin. Resins NKA-9, HPD-600, X-5, and HPD-826 showed good adsorption capacity. NKA-9 resin had the best desorption capacity, while X-5 and HPD-826 exhibited poor desorption capacity. Therefore, only NKA-9 resin showed both good adsorption capacity and strong desorption capacity. The adsorption capacity of macroporous NKA-9 resin for luteolin was 0.24 mg/g, and the desorption rate was 86.90%. Thus, NKA-9 was selected for the adsorption kinetics experiment.
Figure 3.
Static adsorption and desorption capacities and desorption rates of eight resins for luteolin. Resins NKA-9, HPD-600, X-5, and HPD-826 showed good adsorption capacity. NKA-9 resin had the best desorption capacity, while X-5 and HPD-826 exhibited poor desorption capacity. Therefore, only NKA-9 resin showed both good adsorption capacity and strong desorption capacity. The adsorption capacity of macroporous NKA-9 resin for luteolin was 0.24 mg/g, and the desorption rate was 86.90%. Thus, NKA-9 was selected for the adsorption kinetics experiment.
Figure 4.
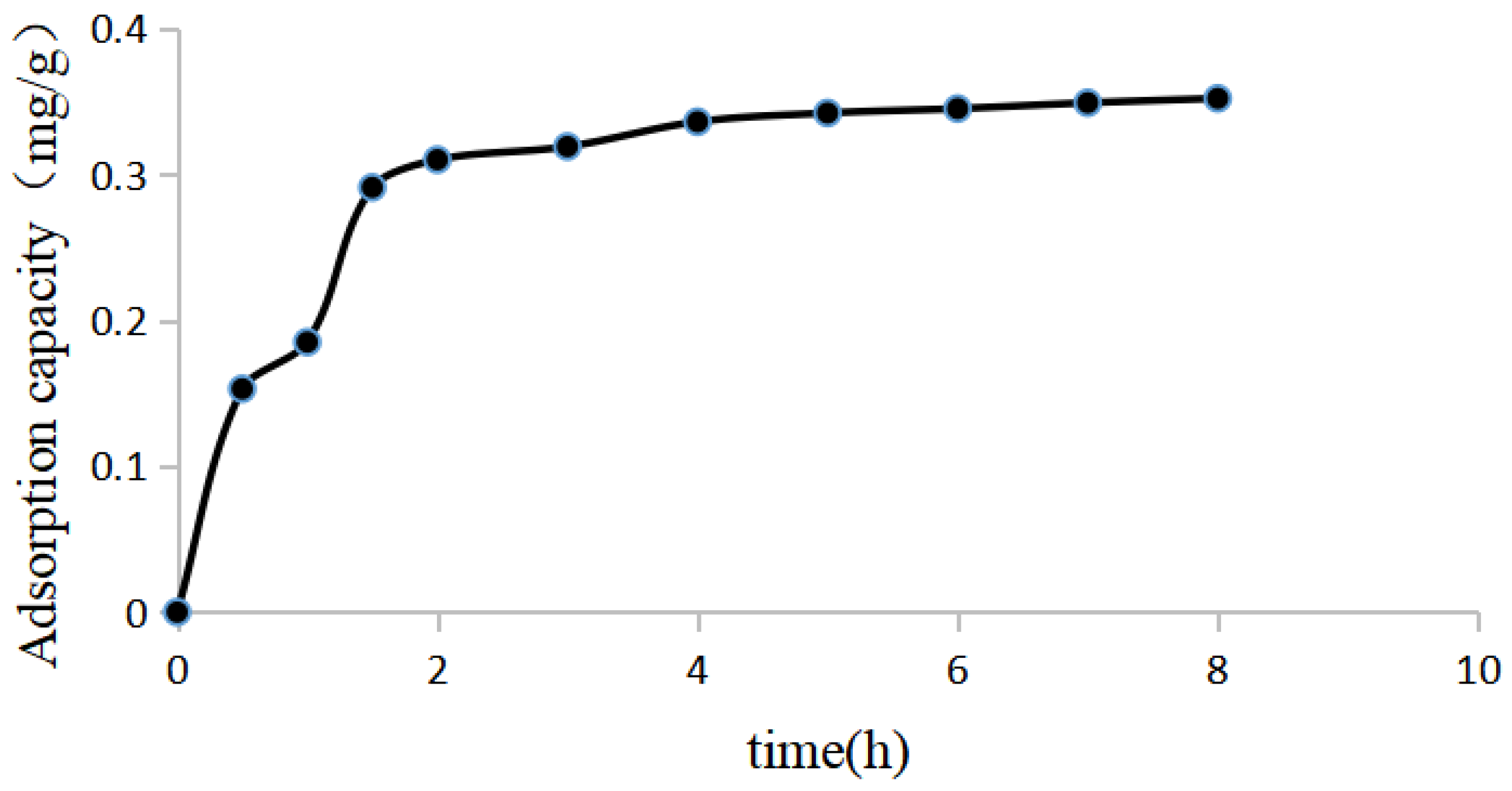
Adsorption kinetics curves of luteolin on NKA-9 resin. The results of the adsorption kinetics experiment showed that the adsorption of luteolin from P. villosa by NKA-9 resin reached equilibrium after 5 h of adsorption at 25 °C.
Figure 4.
Adsorption kinetics curves of luteolin on NKA-9 resin. The results of the adsorption kinetics experiment showed that the adsorption of luteolin from P. villosa by NKA-9 resin reached equilibrium after 5 h of adsorption at 25 °C.
Figure 5.
Adsorption isotherms of NKA-9 resin at different temperatures. The adsorption capacity of NKA-9 resin decreased with increasing extraction temperature, which demonstrates that the adsorption process was exothermic. This indicates that luteolin is more easily adsorbed at low temperature.
Figure 5.
Adsorption isotherms of NKA-9 resin at different temperatures. The adsorption capacity of NKA-9 resin decreased with increasing extraction temperature, which demonstrates that the adsorption process was exothermic. This indicates that luteolin is more easily adsorbed at low temperature.
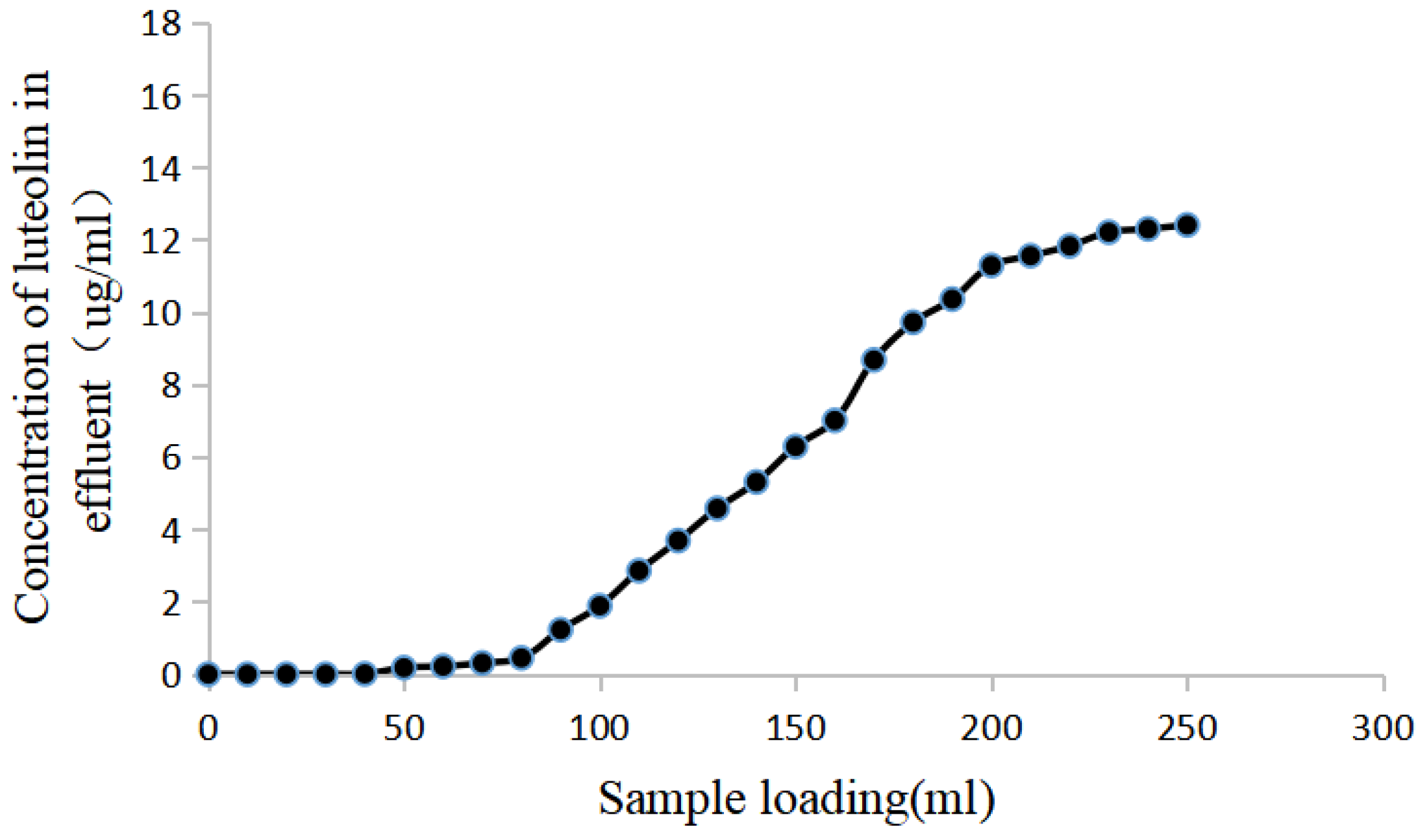
Figure 6.
Dynamic breakthrough curves of luteolin on NKA-9 resin. The leakage point, also known as the penetration point, refers to the point at which the concentration of a target component in the effluent is 10% of that of the concentration in the sample solution after the resin reaches adsorption saturation. In this study, the saturated loading amount of luteolin sample solution on NKA-9 resin was 90 mL (2.9 BV).
Figure 6.
Dynamic breakthrough curves of luteolin on NKA-9 resin. The leakage point, also known as the penetration point, refers to the point at which the concentration of a target component in the effluent is 10% of that of the concentration in the sample solution after the resin reaches adsorption saturation. In this study, the saturated loading amount of luteolin sample solution on NKA-9 resin was 90 mL (2.9 BV).
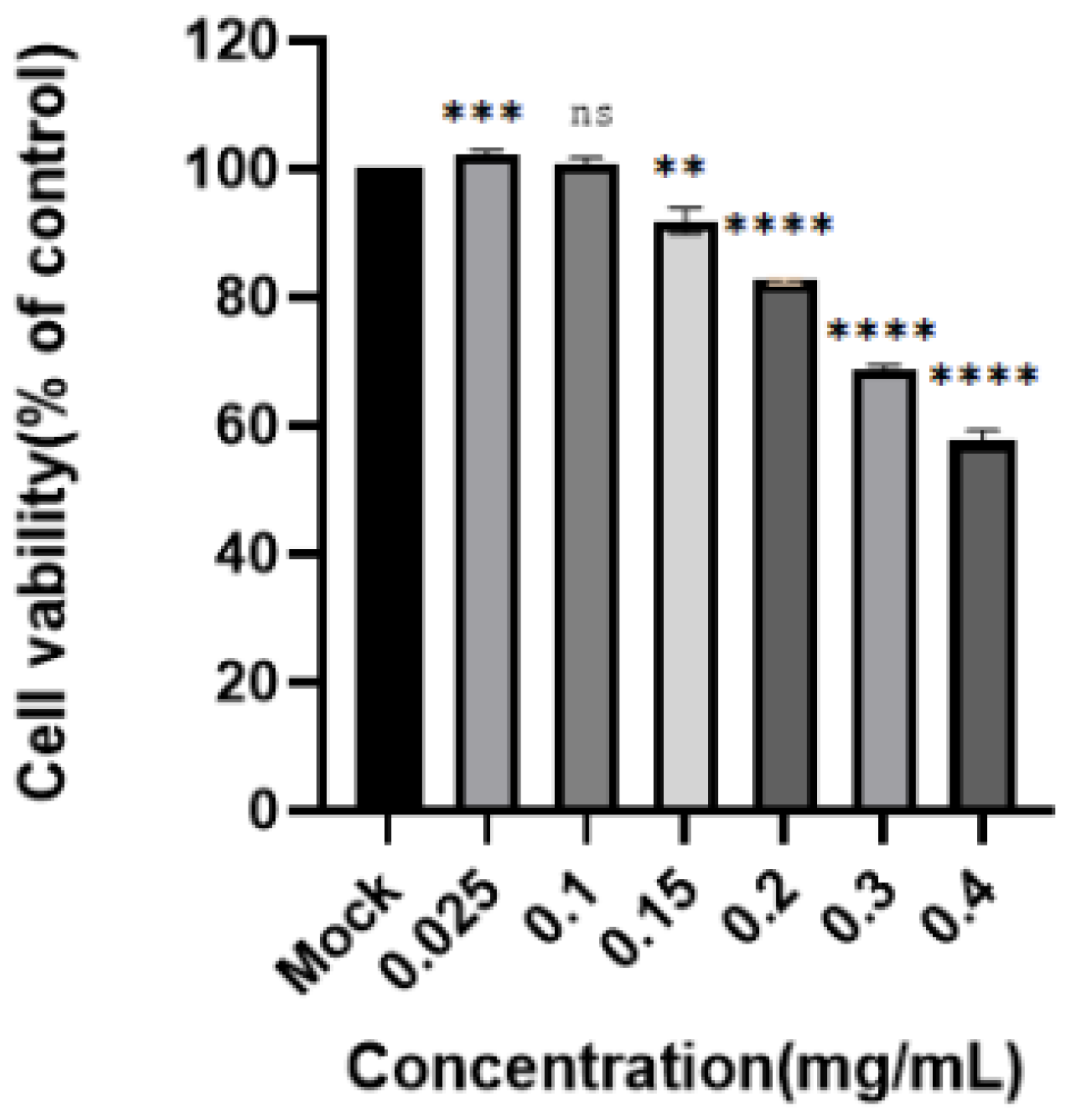
Figure 7.
Toxicity of the enriched products to PK-15 cells. A CCK8 assay was used to determine the toxicity of DAE to PK15 cells at concentrations of 0.4, 0.3, 0.2, 0.15, 0.1, and 0.025 mg/mL. Cell viability is expressed as a percentage of control cell viability (****: p < 0.0001, ***: p < 0.001, ** p < 0.01; ns: p > 0.05).
Figure 7.
Toxicity of the enriched products to PK-15 cells. A CCK8 assay was used to determine the toxicity of DAE to PK15 cells at concentrations of 0.4, 0.3, 0.2, 0.15, 0.1, and 0.025 mg/mL. Cell viability is expressed as a percentage of control cell viability (****: p < 0.0001, ***: p < 0.001, ** p < 0.01; ns: p > 0.05).
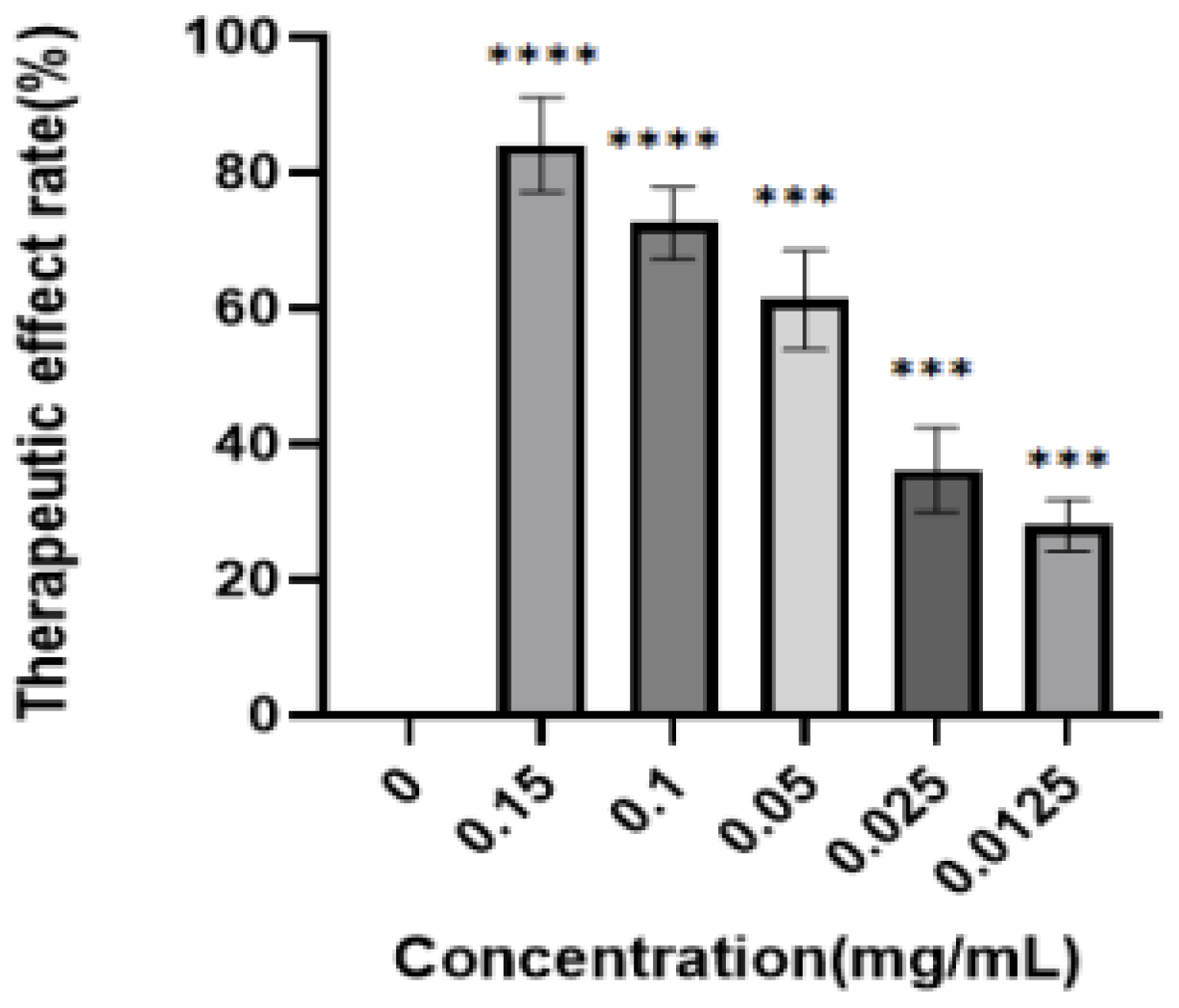
Figure 8.
Inhibition of PRV by the enriched products. PK-15 cells were first infected with PRV and then treated with the enriched products. After 48 h, the inhibition rate was determined by the CCK8 method. The analysis showed that the inhibition rate of the luteolin-enriched product was 84.13 ± 3.22%, the IC50 was 0.04 ± 0.012 mg/mL, and the SI was 3.5, indicating low toxicity and high efficiency (****: p < 0.0001, ***: p < 0.001).
Figure 8.
Inhibition of PRV by the enriched products. PK-15 cells were first infected with PRV and then treated with the enriched products. After 48 h, the inhibition rate was determined by the CCK8 method. The analysis showed that the inhibition rate of the luteolin-enriched product was 84.13 ± 3.22%, the IC50 was 0.04 ± 0.012 mg/mL, and the SI was 3.5, indicating low toxicity and high efficiency (****: p < 0.0001, ***: p < 0.001).
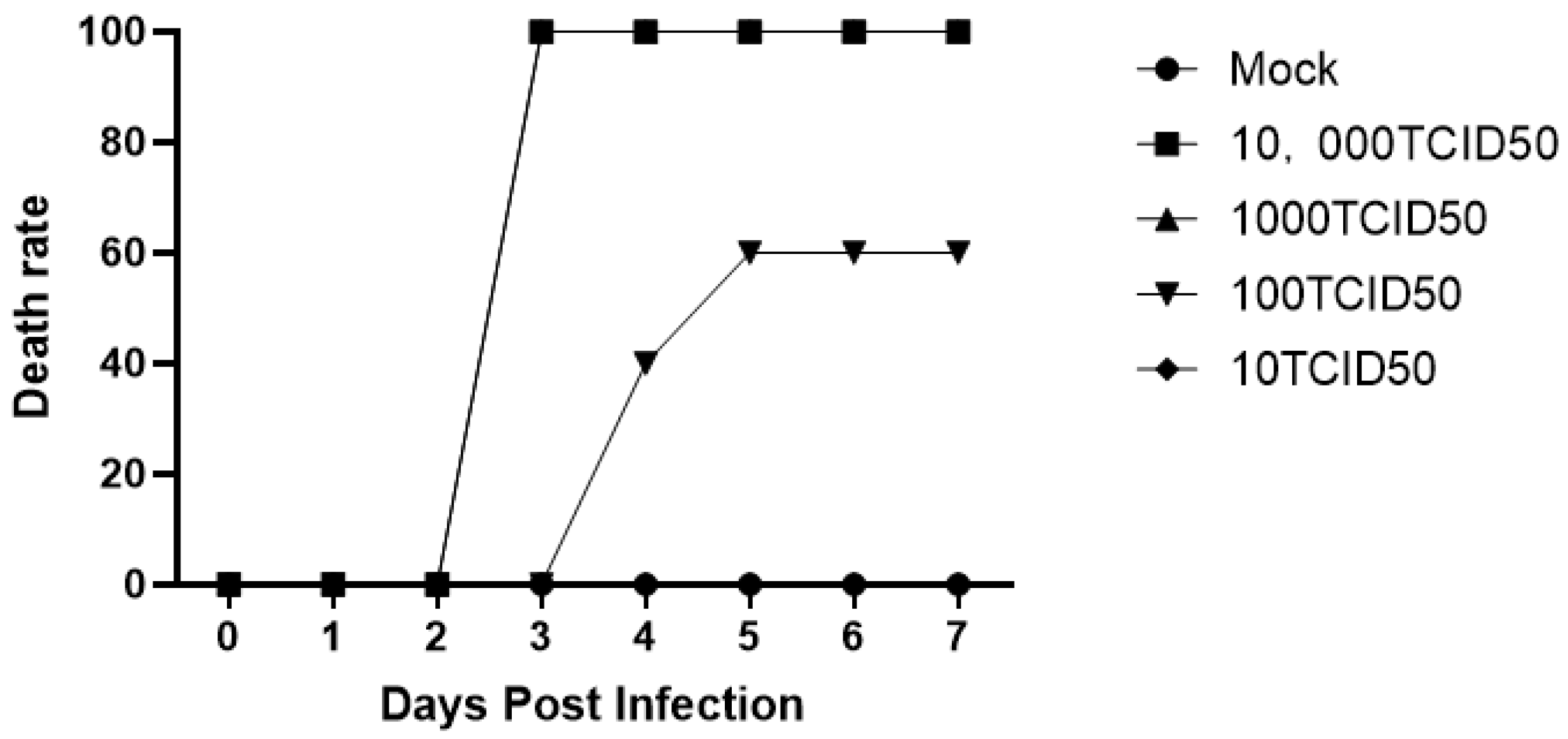
Figure 9.
Survival rate of challenged mice. Mice were injected intramuscularly with diluted virus. The 1000 TCID50 group began to itch and bite the injection site frantically on the third day. The survival rate was 0. The situation of mice in the 10,000 TCID50 group was identical to that of mice in the 1000 TCID50 group. An intramuscular injection of 100 μL of 1000 TCID50 virus was used as the challenge dose for the mice.
Figure 9.
Survival rate of challenged mice. Mice were injected intramuscularly with diluted virus. The 1000 TCID50 group began to itch and bite the injection site frantically on the third day. The survival rate was 0. The situation of mice in the 10,000 TCID50 group was identical to that of mice in the 1000 TCID50 group. An intramuscular injection of 100 μL of 1000 TCID50 virus was used as the challenge dose for the mice.
Figure 10.
Body weight changes in the mice after administration. Compared with the normal group, the weight gain of mice in the drug group was not significantly different after the drug was injected, and no mice died. Therefore, it was decided to conduct the next test at a drug dose of 300 mg/kg.
Figure 10.
Body weight changes in the mice after administration. Compared with the normal group, the weight gain of mice in the drug group was not significantly different after the drug was injected, and no mice died. Therefore, it was decided to conduct the next test at a drug dose of 300 mg/kg.
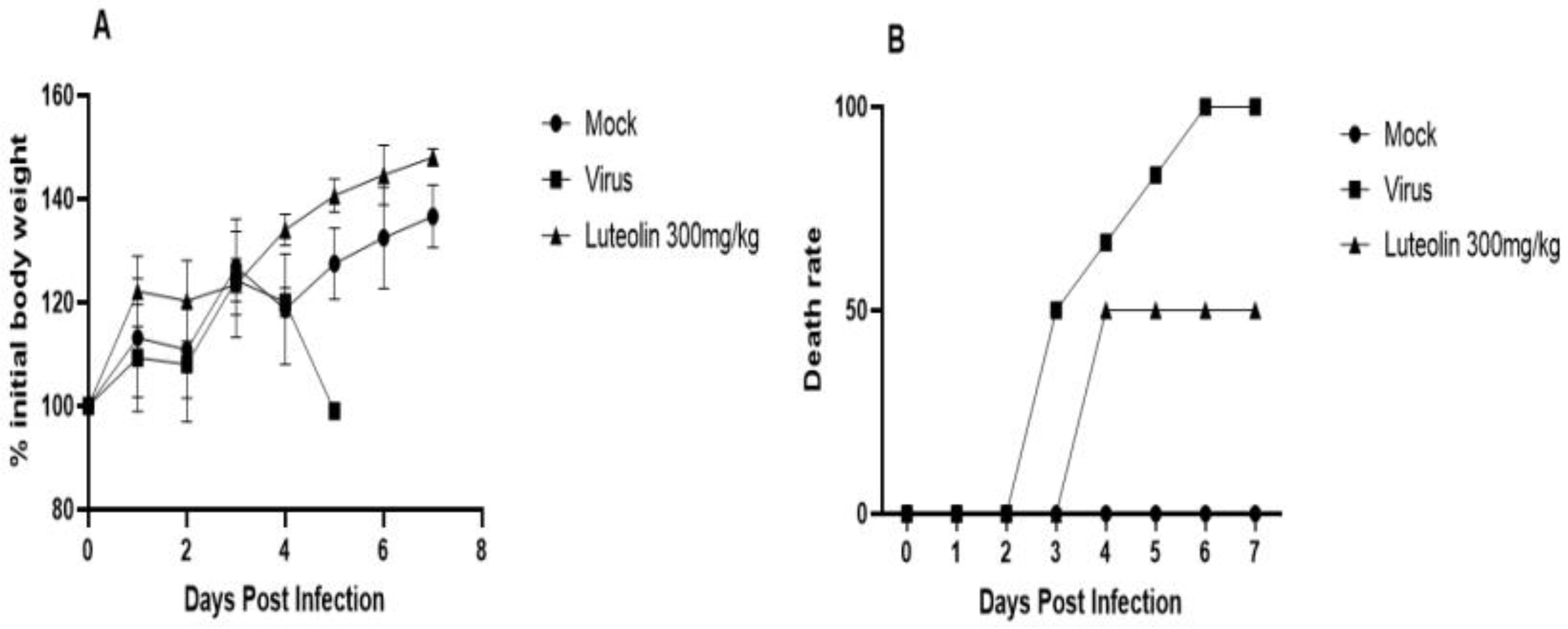
Figure 11.
Changes in body weight and death rate of the mice treated with the drug. All the subjects in the challenge group died on the 6th day. Obvious neurological symptoms appeared on the third day. The onset time for mice in the drug group (4 days) was significantly delayed compared with that in the challenge group, and the survival rate was 50%. Compared with the normal group, the weight gain of mice in the challenge group slowed, and the weight of mice in the drug group fluctuated significantly compared with that of the normal group. (A) Changes in mouse body weight; (B) Mouse mortality rate.
Figure 11.
Changes in body weight and death rate of the mice treated with the drug. All the subjects in the challenge group died on the 6th day. Obvious neurological symptoms appeared on the third day. The onset time for mice in the drug group (4 days) was significantly delayed compared with that in the challenge group, and the survival rate was 50%. Compared with the normal group, the weight gain of mice in the challenge group slowed, and the weight of mice in the drug group fluctuated significantly compared with that of the normal group. (A) Changes in mouse body weight; (B) Mouse mortality rate.
Figure 12.
Viral loads in the organs of mice after challenge. The viral loads in the brain, liver, heart, kidney, and lung of mice were detected by FQ–PCR.
Figure 12.
Viral loads in the organs of mice after challenge. The viral loads in the brain, liver, heart, kidney, and lung of mice were detected by FQ–PCR.
Figure 13.
Pathological sectioning of mouse brain tissue. The brains from mice in each group were dissected for pathological evaluations, and capillary congestion in the brain tissue was found to be slowed in the drug group. The pathological changes in brain tissue in the drug group were milder than those in the challenge group, no sieve-like softening regions or capillary congestion were observed, and only a small amount of lymphocyte infiltration occurred.
Figure 13.
Pathological sectioning of mouse brain tissue. The brains from mice in each group were dissected for pathological evaluations, and capillary congestion in the brain tissue was found to be slowed in the drug group. The pathological changes in brain tissue in the drug group were milder than those in the challenge group, no sieve-like softening regions or capillary congestion were observed, and only a small amount of lymphocyte infiltration occurred.
Table 1.
Experimental parameters of the Box–Behnken design and results.
Table 1.
Experimental parameters of the Box–Behnken design and results.
| Number | X1 (Time) | X2 (Liquid/Material Ratio) | (X3) (Temperature) | X4 (Solvent Ratio) | Y (Extraction Rate, mg/g) |
|---|
| 1 | 45 | 25 | 70 | 15 | 0.241 |
| 2 | 45 | 20 | 60 | 15 | 0.228 |
| 3 | 45 | 20 | 70 | 20 | 0.1936 |
| 4 | 45 | 20 | 80 | 15 | 0.2584 |
| 5 | 45 | 15 | 70 | 15 | 0.2001 |
| 6 | 45 | 20 | 70 | 10 | 0.2586 |
| 7 | 30 | 20 | 70 | 15 | 0.1712 |
| 8 | 30 | 20 | 70 | 15 | 0.1774 |
| 9 | 30 | 20 | 60 | 10 | 0.1766 |
| 10 | 30 | 20 | 60 | 20 | 0.1532 |
| 11 | 30 | 20 | 70 | 15 | 0.1778 |
| 12 | 30 | 20 | 80 | 20 | 0.1668 |
| 13 | 30 | 15 | 70 | 20 | 0.1622 |
| 14 | 30 | 25 | 70 | 10 | 0.2016 |
| 15 | 30 | 25 | 70 | 20 | 0.1795 |
| 16 | 30 | 20 | 80 | 10 | 0.257 |
| 17 | 30 | 15 | 80 | 15 | 0.1776 |
| 18 | 30 | 15 | 70 | 10 | 0.208 |
| 19 | 30 | 25 | 80 | 15 | 0.2272 |
| 20 | 30 | 25 | 60 | 15 | 0.199 |
| 21 | 30 | 15 | 60 | 15 | 0.1528 |
| 22 | 15 | 15 | 70 | 15 | 0.1238 |
| 23 | 15 | 20 | 80 | 15 | 0.1628 |
| 24 | 15 | 25 | 70 | 15 | 0.1378 |
| 25 | 15 | 20 | 60 | 15 | 0.1363 |
| 26 | 15 | 20 | 70 | 20 | 0.1128 |
| 27 | 15 | 20 | 70 | 10 | 0.1522 |
Table 2.
ANOVA of the response surface quadratic model analysis of variance.
Table 2.
ANOVA of the response surface quadratic model analysis of variance.
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value (Prob > F) | |
|---|
| Model | 0.0406 | 14 | 0.0029 | 17.98 | <0.0001 | significant |
| X1 | 0.0256 | 1 | 0.0256 | 158.6 | <0.0001 | |
| X2 | 0.0022 | 1 | 0.0022 | 13.5 | 0.0032 | |
| X3 | 0.0035 | 1 | 0.0035 | 21.48 | 0.0006 | |
| X4 | 0.0068 | 1 | 0.0068 | 42.24 | <0.0001 | |
| X1X2 | 0.0002 | 1 | 0.0002 | 1.12 | 0.3104 | |
| X1X3 | 3.80 × 10−6 | 1 | 3.80 × 10−6 | 0.0236 | 0.8805 | |
| X1X4 | 0.0002 | 1 | 0.0002 | 1.02 | 0.3334 | |
| X2X3 | 2.89 × 10−6 | 1 | 2.89 × 10−6 | 0.0179 | 0.8957 | |
| X2X4 | 0.0001 | 1 | 0.0001 | 0.8708 | 0.3691 | |
| X3X4 | 0.0011 | 1 | 0.0011 | 6.92 | 0.022 | |
| X12 | 0 | 1 | 0 | 0.1097 | 0.7463 | |
| X22 | 0 | 1 | 0 | 0.2019 | 0.6612 | |
| X32 | 0.0009 | 1 | 0.0009 | 5.68 | 0.0345 | |
| X42 | 0.0001 | 1 | 0.0001 | 0.5052 | 0.4908 | |
| R2 | 0.0019 | 12 | 0.0002 | | | |
| Adj-R2 | 0.0019 | 10 | 0.0002 | 13.93 | 0.0688 | not significant |
| Pred-R2 | 0.0002 | 2 | 0.0001 | | | |
| Adequate precision | 0.0425 | 26 | | | | |
Table 3.
Predicted and actual experimental values under the optimized conditions.
Table 3.
Predicted and actual experimental values under the optimized conditions.
| Optimum Condition | Extraction Rate (mg/g) |
|---|
| Extraction Time (min) | Extraction Temperature (°C) | Solvent Ratio (Methanol/Hydrochloric Acid) | Liquid/Material Ratio (mL/g) | Actual Value | Predicted Value |
|---|
| 43 | 77.6 | 13 | 22 | 0.265 ± 0.03 | 0.261 |
Table 4.
Effect of pH on the adsorption capacity of luteolin by NKA-9.
Table 4.
Effect of pH on the adsorption capacity of luteolin by NKA-9.
| pH Value | Luteolin Adsorption Capacity (mg/g) |
|---|
| 3 | 0.18 |
| 5 | 0.2 |
| 7 | 0.14 |
Table 5.
Langmuir and Freundlich adsorption parameters at 25, 35 and 45 °C.
Table 5.
Langmuir and Freundlich adsorption parameters at 25, 35 and 45 °C.
| Temperature | Langmuir Equation | R2 | Qm | Freundlich Equation | R2 | 1/n |
|---|
| (°C) | | | (mg/g) | | | |
|---|
| 25 | Ce/Qe = 2.1492Ce + 0.0049 | 0.99049 | 0.47 | Qe = 1.85Ce0.3765 | 0.97456 | 0.3765 |
| 35 | Ce/Qe = 2.2138Ce + 0.0056 | 0.99516 | 0.45 | Qe = 1.76Ce0.3366 | 0.94503 | 0.3366 |
| 45 | Ce/Qe = 2.3612Ce + 0.0071 | 0.994 | 0.42 | Qe = 1.69Ce0.3244 | 0.95114 | 0.3244 |
Table 6.
Effect of flow rate on the adsorption capacity of NKA-9 resin.
Table 6.
Effect of flow rate on the adsorption capacity of NKA-9 resin.
| Flow Rate (BV/h) | Luteolin Adsorption Rate (%) |
|---|
| 1 | 87.60% |
| 2 | 85.70% |
| 4 | 80.20% |
Table 7.
Results of gradient elution of luteolin from NKA-9 resin.
Table 7.
Results of gradient elution of luteolin from NKA-9 resin.
| Ethanol Concentration (%) | Luteolin Content (mg) |
|---|
| 0% | 0 |
| 10% | 0.0428 |
| 20% | 0.0756 |
| 40% | 0.0948 |
| 60% | 0.1789 |
| 80% | 0.2231 |
| 90% | 0.2393 |
Table 8.
Purification result of luteolin on column by NKA-9 resin under optimized conditions.
Table 8.
Purification result of luteolin on column by NKA-9 resin under optimized conditions.
| Treatment Condition | Solid Mass (g) | Luteolin Content (%) | Rate of Recovery (%) |
|---|
| Crude extract | 50 | 0.60% | 80.06% ± 1.16 |
| 80% ethanol concentrate | 11.62 ± 0.23 | 2.30% |
Table 9.
Variables and experimental levels of the response surface design.
Table 9.
Variables and experimental levels of the response surface design.
| | Level |
|---|
| Variable | Code | −1 | 0 | 1 |
|---|
| Extraction time (min) | X1 | 15 | 30 | 45 |
| Material/liquid ratio (mg/mL) | X2 | 15 | 20 | 25 |
| Extraction temperature (°C) | X3 | 60 | 70 | 80 |
| Solvent ratio (%) | X4 | 10:01 | 15:01 | 20:01 |
Table 10.
Physical properties of the 8 kinds of macroporous resins.
Table 10.
Physical properties of the 8 kinds of macroporous resins.
| Name | Polarity | Surface Area (m²/g) | Average Pore Diameter (nm) | Particle Diameter (mm) |
|---|
| NKA-9 | Polar | 250–290 | 15.5–16.5 | 0.30–1.25 |
| X-5 | Nonpolar | 500–600 | 29.0–30.0 | 0.30–1.25 |
| D-101 | Nonpolar | 480–520 | 25.0–28.0 | 0.30–1.25 |
| HPD-100 | Nonpolar | 650–700 | 8.5–9.0 | 0.30–1.25 |
| HPD-300 | Moderately polar | 800–870 | 5.0–5.5 | 0.30–1.20 |
| HPD-826 | Moderately polar | 500–600 | 9.0–10.0 | 0.30–1.25 |
| HPD-600 | Polar | 550–600 | 8 | 0.30–1.20 |
| HPD-400 | Moderately polar | 500–550 | 7.5–8.0 | 0.30–1.20 |