Mechanism of the Photochemical Isomerization and Oxidation of 2-Butenedial: A Theoretical Study
Abstract
1. Introduction
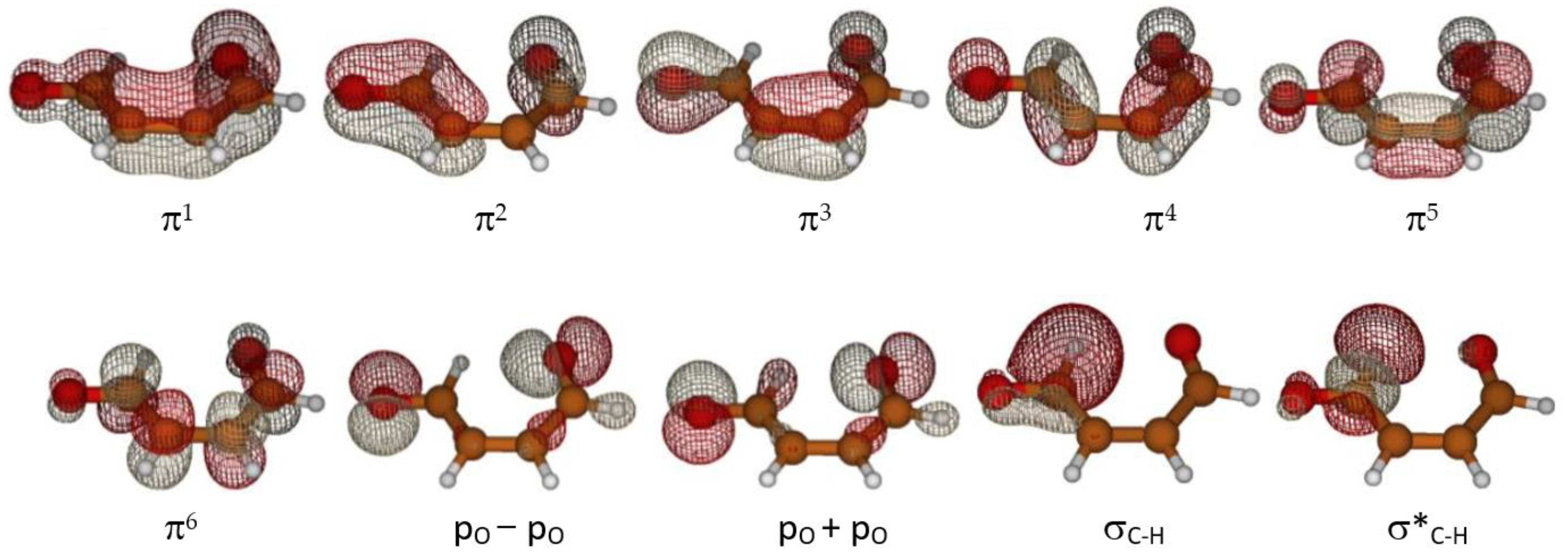
2. Results
2.1. Formation of Ketene-Enol
2.2. Formation of Furanones
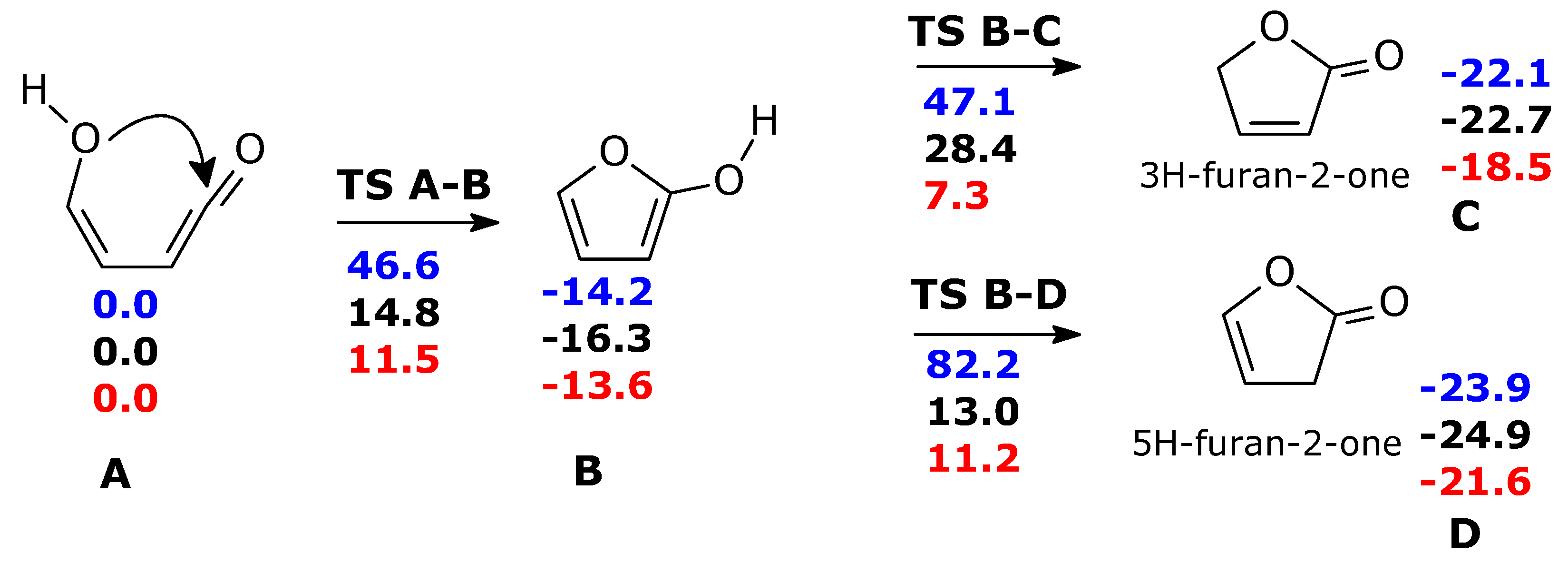
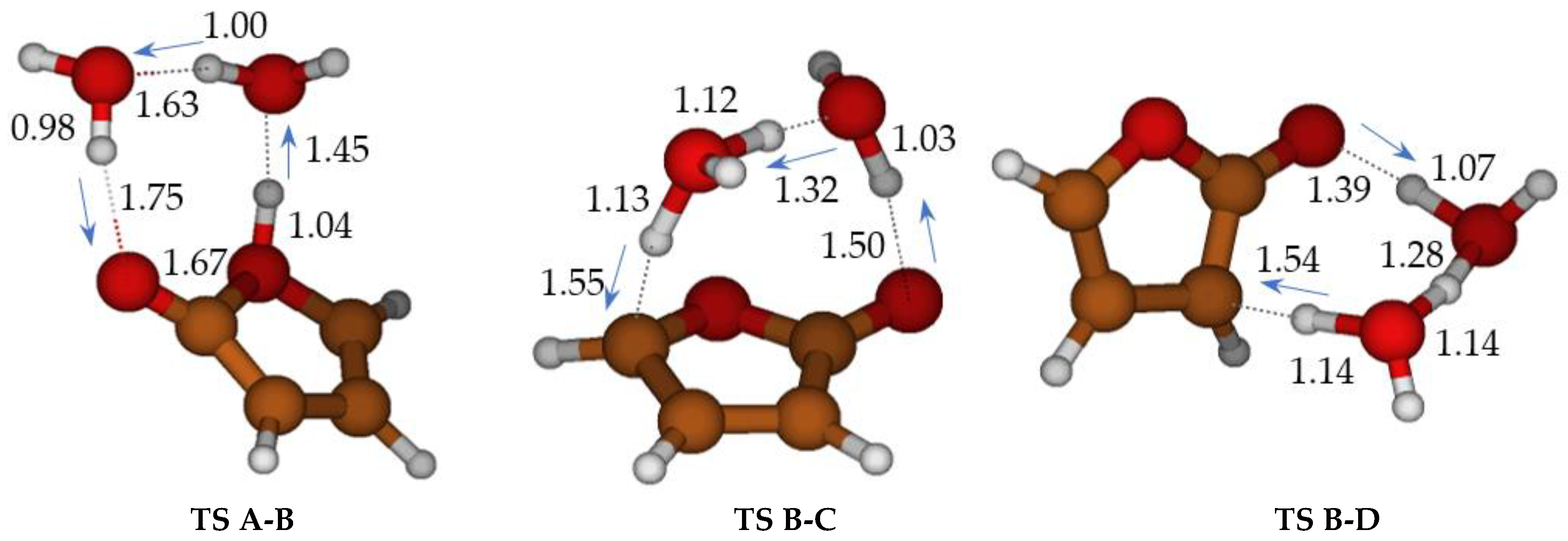
2.3. Formation of Maleic Anhydride
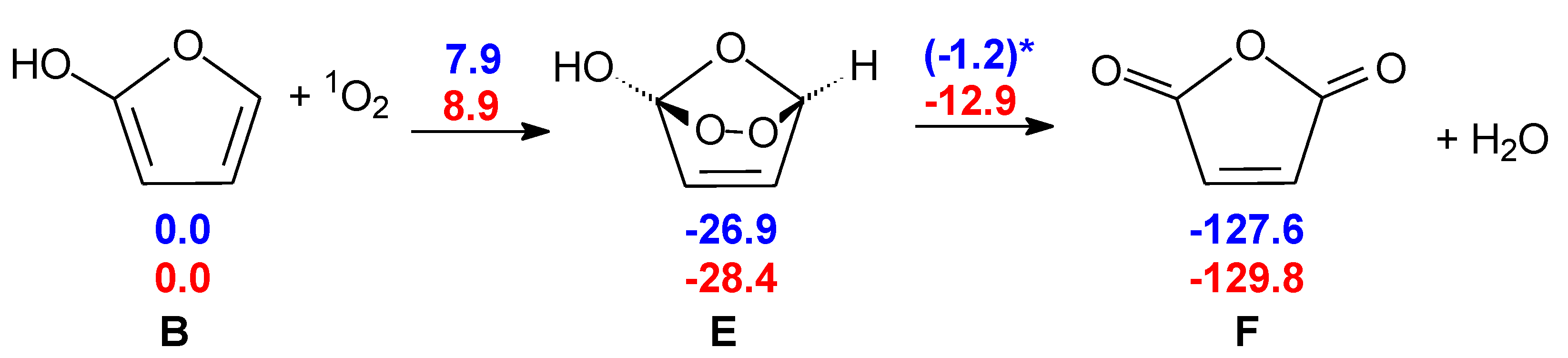
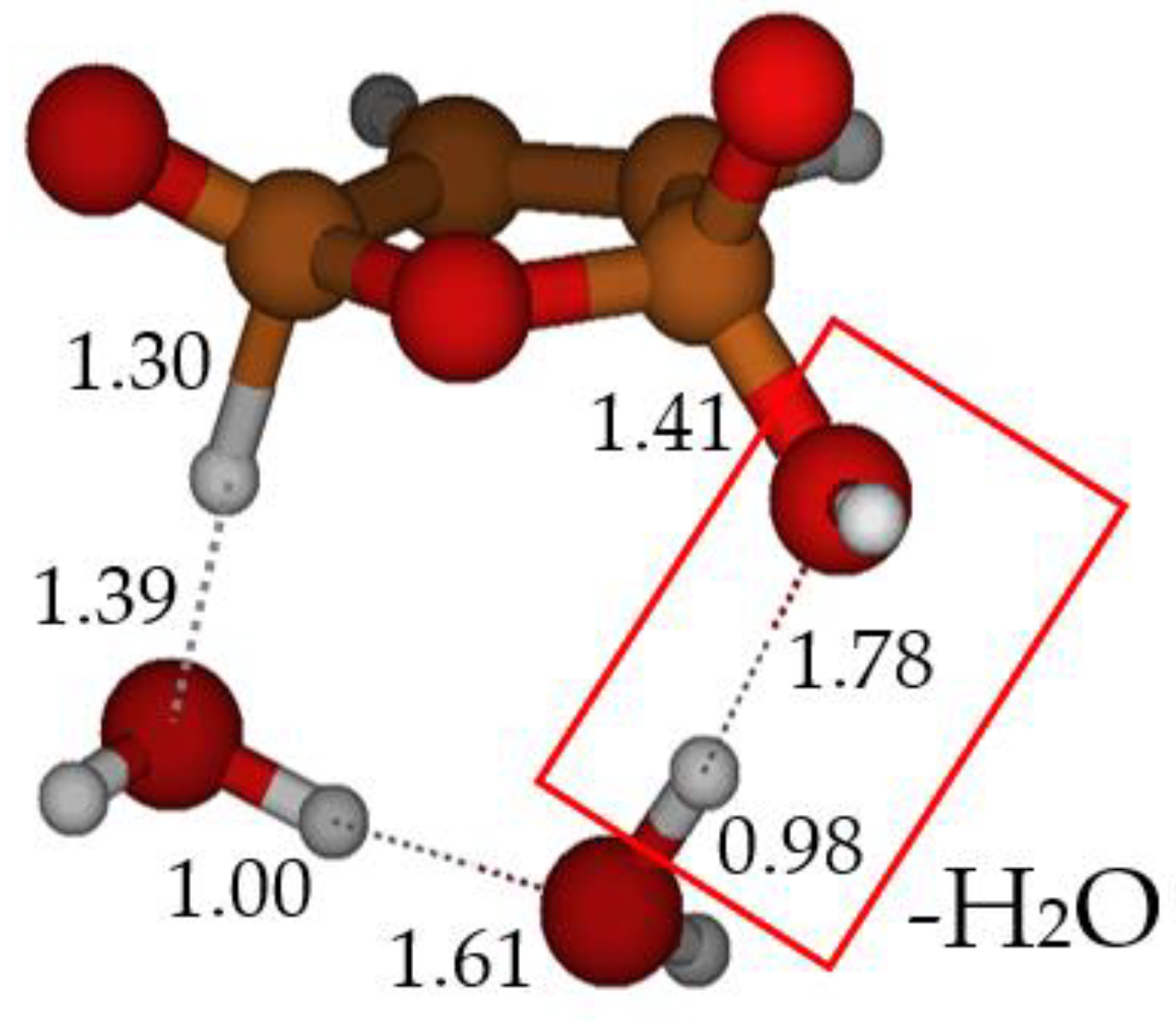
3. Materials and Methods
3.1. CASSCF Method
3.2. Density Functional Theory Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Method | Final State x | λ/nm | f1x | Transition 1 |
|---|---|---|---|---|
| TD-DFT(M06-2X/cc-pVTZ) | S1 | 430.09 | 0.0003 | n→π4 |
| S2 | 360.83 | 0.0000 | n′→π4 | |
| S3 | 222.44 | 0.3421 | π3→π4 | |
| EOM-CCSD/cc-pVTZ | S1 | 377.68 | 0.0003 | n→π4 |
| S2 | 328.94 | 0.0000 | n′→π4 | |
| S3 | 204.31 | 0.3935 | π3→π4 |
References
- Obermeyer, G.; Aschmann, S.M.; Atkinson, R.; Arey, J. Carbonyl atmospheric reaction products of aromatic hydrocarbons in ambient air. Atmos. Environ. 2009, 43, 3736–3744. [Google Scholar] [CrossRef]
- Gómez Alvarez, E.; Viidanoja, J.; Muñoz, A.; Wirtz, K.; Hjorth, J. Experimental Confirmation of the Dicarbonyl Route in the Photo-oxidation of Toluene and Benzene. Environ. Sci. Technol. 2007, 41, 8362–8369. [Google Scholar] [CrossRef]
- Yokelson, R.J.; Karl, T.; Artaxo, P.; Blake, D.R.; Christian, T.J.; Griffith, D.W.T.; Guenther, A.; Hao, W.M. The Tropical Forest and Fire Emissions Experiment: Overview and airborne fire emission factor measurements. Atmos. Chem. Phys. 2007, 7, 5175–5196. [Google Scholar] [CrossRef]
- Andino, J.M.; Smith, J.N.; Flagan, R.C.; Goddard, W.A.; Seinfeld, J.H. Mechanism of Atmospheric Photooxidation of Aromatics: A Theoretical Study. J. Phys. Chem. 1996, 100, 10967–10980. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Klotz, B.; Barnes, I.; Becker, K.-H. Kinetic study of the gas-phase photolysis and OH radical reaction of E,Z- and E,E-2,4-Hexadienedial. Int. J. Chem. Kinet. 1999, 31, 689–697. [Google Scholar] [CrossRef]
- Newland, M.J.; Rea, G.J.; Thuner, L.P.; Henderson, A.P.; Golding, B.T.; Rickard, A.R.; Barnes, I.; Wenger, J. Photochemistry of 2-butenedial and 4-oxo-2-pentenal under atmospheric boundary layer conditions. Phys. Chem. Chem. Phys. 2019, 21, 1160–1171. [Google Scholar] [CrossRef]
- Marshall, P.; Papadimitriou, V.C.; Papanastasiou, D.K.; Roberts, J.M.; Burkholder, J.B. UV and infrared absorption spectra and 248 nm photolysis of maleic anhydride (C4H2O3). J. Photochem. Photobiol. A Chem. 2019, 382, 111953. [Google Scholar] [CrossRef]
- Back, R.A.; Parsons, J.M. The thermal and photochemical decomposition of maleic anhydride in the gas phase. Can. J. Chem. 1981, 59, 1342–1346. [Google Scholar] [CrossRef]
- Röhrl, A.; Lammel, G. Determination of malic acid and other C4 dicarboxylic acids in atmospheric aerosol samples. Chemosphere 2002, 46, 1195–1199. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, L. Photolysis of butenedial at 193, 248, 280, 308, 351, 400, and 450 nm. Chem. Phys. Lett. 2005, 409, 151–156. [Google Scholar] [CrossRef]
- Bierbach, A.; Barnes, I.; Becker, K.H.; Wiesen, E. Atmospheric Chemistry of Unsaturated Carbonyls: Butenedial, 4-Oxo-2-pentenal, 3-Hexene-2,5-dione, Maleic Anhydride, 3H-Furan-2-one, and 5-Methyl-3H-furan-2-one. Environ. Sci. Technol. 1994, 28, 715–729. [Google Scholar] [CrossRef]
- Calvert, J.; Mellouki, A.; Orlando, J.; Pilling, M.; Wallington, T. Mechanisms of Atmospheric Oxidation of the Oxygenates; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Tadic, J.M.; Moortgat, G.K.; Bera, P.P.; Loewenstein, M.; Yates, E.L.; Lee, T.J. Photochemistry and photophysics of n-butanal, 3-methylbutanal, and 3,3-dimethylbutanal: Experimental and theoretical study. J. Phys. Chem. A 2012, 116, 5830–5839. [Google Scholar] [CrossRef]
- Shemesh, D.; Nizkorodov, S.A.; Gerber, R.B. Photochemical Reactions of Cyclohexanone: Mechanisms and Dynamics. J. Phys. Chem. A 2016, 120, 7112–7120. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Encinas, M.V.; George, M.V. Photochemistry of o-phthalaldehyde. J. Chem. Soc. Perkin Trans. 2 1980, 7, 724–730. [Google Scholar] [CrossRef]
- Li, Q.; Migani, A.; Blancafort, L. Irreversible phototautomerization of o-phthalaldehyde through electronic relocation. Phys. Chem. Chem. Phys. 2012, 14, 6561–6568. [Google Scholar] [CrossRef]
- Fröbel, S.; Buschhaus, L.; Villnow, T.; Weingart, O.; Gilch, P. The photoformation of a phthalide: A ketene intermediate traced by FSRS. Phys. Chem. Chem. Phys. 2015, 17, 376–386. [Google Scholar] [CrossRef]
- Gebicki, J.; Kuberski, S.; Kamiński, R. Structure and photochemistry of matrix-isolated o-phthalaldehyde. J. Chem. Soc., Perkin Trans. 2 1990, 765–769. [Google Scholar] [CrossRef]
- He, H.-Y.; Fang, W.-H.; Phillips, D.L. Photochemistry of Butyrophenone: Combined Complete-Active-Space Self-Consistent Field and Density Functional Theory Study of Norrish Type I and II Reactions. J. Phys. Chem. A 2004, 108, 5386–5392. [Google Scholar] [CrossRef]
- Rowell, K.N.; Kable, S.H.; Jordan, M.J.T. An assessment of the tropospherically accessible photo-initiated ground state chemistry of organic carbonyls. Atmos. Chem. Phys. 2022, 22, 929–949. [Google Scholar] [CrossRef]
- Rowell, K.; Kable, S.; Jordan, M.J.T. Structural Causes of Singlet/triplet Preferences of Norrish Type II Reactions in Carbonyls. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Rowell, K.N.; Kable, S.H.; Jordan, M.J.T. Structural Effects on the Norrish Type I α-Bond Cleavage of Tropospherically Important Carbonyls. J. Phys. Chem. A 2019, 123, 10381–10396. [Google Scholar] [CrossRef]
- Liu, X.; Jeffries, H.E.; Sexton, K.G. Atmospheric Photochemical Degradation of 1,4-Unsaturated Dicarbonyls. Environ. Sci. Technol. 1999, 33, 4212–4220. [Google Scholar] [CrossRef]
- Martín, P.; Cabañas, B.; Colmenar, I.; Salgado, M.S.; Villanueva, F.; Tapia, A. Reactivity of E-butenedial with the major atmospheric oxidants. Atmos. Environ. 2013, 70, 351–360. [Google Scholar] [CrossRef]
- Goings, J.J.; Caricato, M.; Frisch, M.J.; Li, X. Assessment of low-scaling approximations to the equation of motion coupled-cluster singles and doubles equations. J. Chem. Phys. 2014, 141, 164116. [Google Scholar] [CrossRef]
- Caricato, M. A corrected-linear response formalism for the calculation of electronic excitation energies of solvated molecules with the CCSD-PCM method. Comput. Theor. Chem. 2014, 1040–1041, 99–105. [Google Scholar] [CrossRef]
- Kállay, M.; Gauss, J. Calculation of excited-state properties using general coupled-cluster and configuration-interaction models. J. Chem. Phys. 2004, 121, 9257–9269. [Google Scholar] [CrossRef]
- Weingart, O.; Reschke, B.; Marian, C.M. Solvent mediated catalysis and proton-shuttling in the formation of 3-methylphthalide from a ketene intermediate. Chem. Phys. 2018, 515, 750–756. [Google Scholar] [CrossRef]
- Lewis, M.; Glaser, R. Synergism of Catalysis and Reaction Center Rehybridization. An ab Initio Study of the Hydrolysis of the Parent Carbodiimide. J. Am. Chem. Soc. 1998, 120, 8541–8542. [Google Scholar] [CrossRef]
- Wei, X.-G.; Sun, X.-M.; Wu, X.-P.; Geng, S.; Ren, Y.; Wong, N.-B.; Li, W.-K. Cooperative effect of water molecules in the self-catalyzed neutral hydrolysis of isocyanic acid: A comprehensive theoretical study. J. Mol. Model. 2010, 17, 2069–2082. [Google Scholar] [CrossRef]
- Newland, M.J.; Nelson, B.S.; Muñoz, A.; Ródenas, M.; Vera, T.; Tárrega, J.; Rickard, A.R. Trends in stabilisation of Criegee intermediates from alkene ozonolysis. Phys. Chem. Chem. Phys. 2020, 22, 13698–13706. [Google Scholar] [CrossRef]
- Schurath, U. Metastable Oxygen Molecules in the Troposphere. Free. Radic. Res. Commun. 2009, 3, 173–184. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J. A second order multiconfiguration SCF procedure with optimum convergence. J. Chem. Phys. 1985, 82, 5053–5063. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N. Spectroscopy and Photochemistry. In Chemistry of the Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 2000; pp. 43–85. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knizia, P.J.K.G.; Manby, F.R.; Celani, M.S.P.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G.; Shamasundar, K.R.; Amos, R.D.; et al. MOLPRO, version 2010.1; A Package of Ab Initio Programs; Molpro: Stuttgart, Germany, 2010. [Google Scholar]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar] [CrossRef]
- McQuarrie, D.A.; Simon, J.D. Molecular Thermodynamics; University Science Books: Melville, NY, USA, 1999. [Google Scholar]
- Thermochemistry in Gaussian. Available online: https://gaussian.com/thermo/ (accessed on 19 April 2000).
- Yamanaka, S.; Kawakami, T.; Nagao, H.; Yamaguchi, K. Effective exchange integrals for open-shell species by density functional methods. Chem. Phys. Lett. 1994, 231, 25–33. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Jensen, F.; Dorigo, A.; Houk, K.N. A spin correction procedure for unrestricted Hartree-Fock and Møller-Plesset wavefunctions for singlet diradicals and polyradicals. Chem. Phys. Lett. 1988, 149, 537–542. [Google Scholar] [CrossRef]
- Scuseria, G.E.; Schaefer, H.F. Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration interaction (QCISD)? J. Chem. Phys. 1989, 90, 3700–3703. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
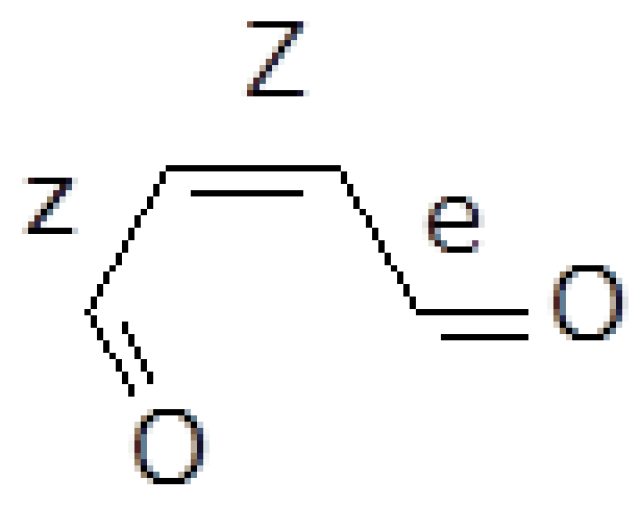

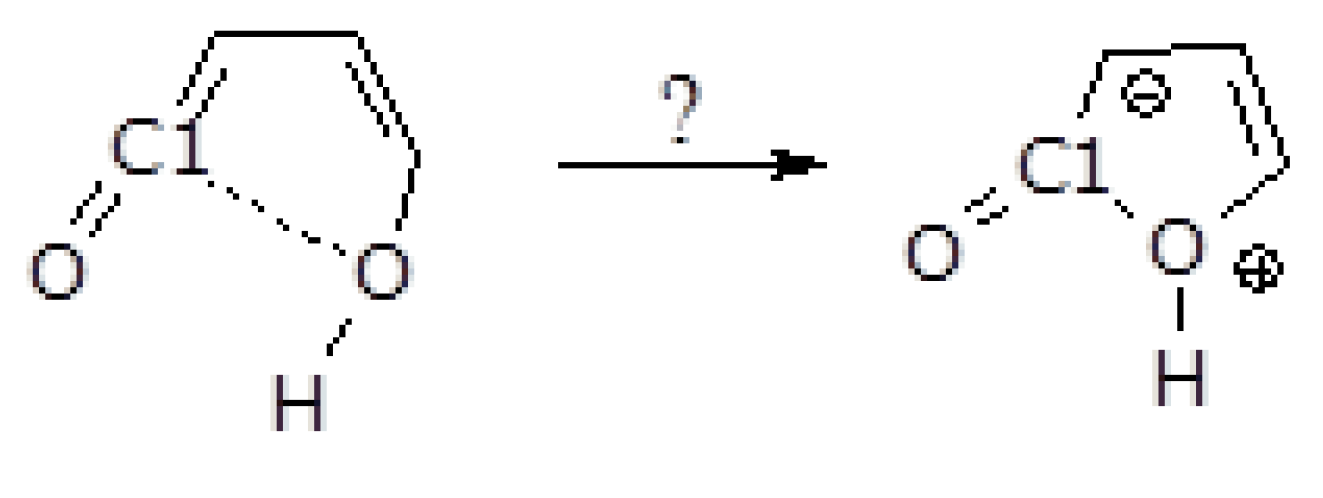
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranzana, A.; Tonachini, G. Mechanism of the Photochemical Isomerization and Oxidation of 2-Butenedial: A Theoretical Study. Molecules 2023, 28, 4994. https://doi.org/10.3390/molecules28134994
Maranzana A, Tonachini G. Mechanism of the Photochemical Isomerization and Oxidation of 2-Butenedial: A Theoretical Study. Molecules. 2023; 28(13):4994. https://doi.org/10.3390/molecules28134994
Chicago/Turabian StyleMaranzana, Andrea, and Glauco Tonachini. 2023. "Mechanism of the Photochemical Isomerization and Oxidation of 2-Butenedial: A Theoretical Study" Molecules 28, no. 13: 4994. https://doi.org/10.3390/molecules28134994
APA StyleMaranzana, A., & Tonachini, G. (2023). Mechanism of the Photochemical Isomerization and Oxidation of 2-Butenedial: A Theoretical Study. Molecules, 28(13), 4994. https://doi.org/10.3390/molecules28134994






