Abstract
For the creation of adaptable carbonyl compounds in organic synthesis, the oxidation of alcohols is a crucial step. As a sustainable alternative to the harmful traditional oxidation processes, transition-metal catalysts have recently attracted a lot of interest in acceptorless dehydrogenation reactions of alcohols. Here, using well-defined, air-stable palladium(II)–NHC catalysts (A–F), we demonstrate an effective method for the catalytic acceptorless dehydrogenation (CAD) reaction of secondary benzylic alcohols to produce the corresponding ketones and molecular hydrogen (H2). Catalytic acceptorless dehydrogenation (CAD) has been successfully used to convert a variety of alcohols, including electron-rich/electron-poor aromatic secondary alcohols, heteroaromatic secondary alcohols, and aliphatic cyclic alcohols, into their corresponding value-added ketones while only releasing molecular hydrogen as a byproduct.
1. Introduction
The conversion of alcohols to carbonyl compounds via oxidation/dehydrogenation is one of the most important processes in organic chemistry [1,2,3] Generally, these types of reactions have been performed by using the stoichiometric portion of metal-based oxidants, such as hypochlorite salts [4], permanganate salts [5,6], chromium/pyridinium dichromate salts [7], Dess-Martin reagents [8], and Swern reagents [9], by using H2O2 [10,11,12], manganese salts [13,14], and oxygen with transition-metal catalysts [15,16]. Traditional methods have several drawbacks in terms of the environment, economy, and energy use because they typically generate a significant amount of waste or undesirable byproducts. The “green” and “sustainable” catalytic acceptorless alcohol dehydrogenation (CAAD) reactions of organic compounds, which occur in tandem with H2 evolution, have recently attracted attention. For acceptorless dehydrogenation (AD) reactions, no stoichiometric waste is produced because they do not require conventional oxidants or sacrificial acceptors. These processes also result in gaseous H2, which is valuable and might be used as an energy source [17,18]. Utilizing pricey transition metals, such as Pt, Rh, Ru, Ir, Os, Au, and Ag, numerous catalytic systems have been developed [19,20,21,22,23,24,25,26,27,28,29,30,31], as shown in Figure 1a. Despite the fact that the oxidation process itself is simple and safe, the precious metals used for the catalytic dehydrogenation of alcohols have disadvantages in terms of their toxicity and high cost. Therefore, a lot of attention has been paid recently to the development of dehydrogenation catalysts based on earth-abundant base metals, such as Mn, Fe, Co, and Ni [32,33,34,35,36,37,38,39,40]. In the field of chemical catalysis, palladium is one of the frequently studied transition metals [41,42]. Ceri Hammond and coworkers and Simon J. Freakley and coworkers recently published research on Pd-supported catalysts for acceptorless dehydrogenation (AD) reactions of various alcohols [43,44]. Numerous advantageous characteristics of the palladium catalyst, including the relatively high thermal stability of the Pd–MIC bonds, have been attributed to the concurrent emergence of 1,2,3-triazolium-derived mesoionic carbene (tz–MIC) ligands in the various cross-coupling reactions. Because these electron-rich carbenes strongly bind to the metal center, the catalyst cannot be used repeatedly without suffering a significant loss in stability. The 1,2,3–triazolium-derived mesoionic carbene (tz–MIC) palladium complexes, on the other hand, have been crucial as catalysts in homogeneous catalysis [45,46,47,48,49,50,51,52,53,54,55]; their applications in catalytic acceptorless alcohol dehydrogenation (CAAD) reactions have surprisingly remained unexplored to date, and thus we were interested in pursuing the same by using Pd–NHC metal complexes as a catalyst, as shown in Figure 1b.

Figure 1.
Catalytic acceptorless dehydrogenation (CAD) reaction of various alcohols.
2. Results and Discussion
Catalytic Acceptorless Alcohol Dehydrogenation (CAAD) Using Pd(II)–NHC Catalysts (A–F)
The acceptorless dehydrogenation (AD) of different alcohols into their corresponding value-added carbonyl compounds using the catalyst palladium(II)–NHC complexes (A–F) was the main topic of this report. We started our research by screening different Pd(II)–NHC metal complexes as acceptorless dehydrogenation (AD) catalysts at 100 °C for 16 h, using 1a as the model substrate and toluene (0.2 M) as the solvent, as part of our ongoing efforts to develop sustainable chemistry under Pd(II)–NHC catalysis as shown in Scheme 1. It appears to be a very efficient catalyst among the Pd(II)–NHC (cat D), as evidenced by the 98% yield of the dehydrogenative product 2a (entries 1–7, Table 1). Potassium hydroxide (KOH) and potassium tert-butoxide (KOtBu) both produced comparable isolated yields of the dehydrogenative product 2a, despite the fact that the various bases were tested to determine the best reaction conditions (entries 8–12). After a quick solvent screening, such as TFE (2,2,2-Trifluoroethanol), DCE (1,2-Dichloroethane), TFT (Trifluorotoluene), acetone, H2O (water), CH3CN (acetonitrile), and DMF (Dimethylformamide), the toluene appeared to be the best option (entries 13–20). When the reaction was carried out, the control experiments without a Pd–NHC catalyst produced no product, showing that the direct formation of a dehydrogenative product from alcohols under optimal conditions is essentially a catalytic process (entry 21).

Scheme 1.
Catalytic acceptorless alcohol dehydrogenation (CAAD) using Pd(II)–NHC catalysts (A–F).

Table 1.
Reaction optimization for dehydrogenation of secondary alcohol catalyzed by Pd(II)–NHC catalysts (A–F) [a].
Figure 2 represents the results for synthesis of 2a via different Pd catalysts
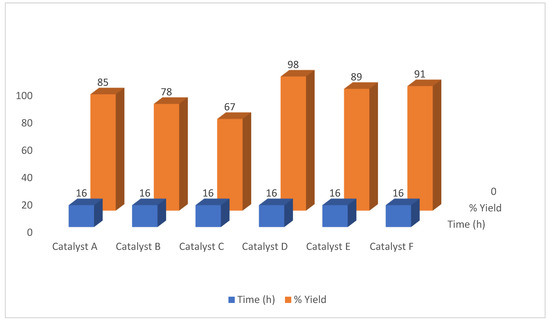
Figure 2.
Behavior of reaction for synthesis of 2a via different Pd catalysts.
We investigated the range of substrates for CAAD (catalytic acceptorless alcohol dehydrogenation) reactions, as depicted in Scheme 2. First, we investigated the extent of secondary alcohol acceptorless dehydrogenation. When the secondary alcohols had different electron-donating groups at the ortho, meta, and para positions of the arenes, they were tolerable and produced the corresponding dehydrogenated ketone products (2b–e) in good yields (68–86%, Scheme 2). The reaction was unaffected by the presence of an electron-withdrawing group at the C-2, C-3, and C-4 positions of the arenes (2f–k), such as a fluoro, chloro, bromo, or nitro group, and the yields of the dehydrogenated ketone products were very good to excellent (89–94%, Scheme 2). Strong yields of the dehydrogenated ketone products (88–89%) were produced by even more sterically hindered secondary alcohols with substituents at the orthopositions (2l–m). The oxidation of primary alcohols in the presence of minute amounts of dehydrogenated products is the subject of the following investigation (2n–o). To our surprise, hetero-aromatic secondary alcohols were reactive and produced the ketones (4a–c) in good yields of the dehydrogenated ketone products (63–68%, Scheme 2) even though they frequently poisoned the catalyst through strong coordination to the metal via heteroatoms. After that, we checked challenging α,β-unsaturated cyclic aliphatic secondary alcohol (4d), which produced a very trace amount of product. Last but not least, we investigated the more difficult cyclic aliphatic secondary alcohols for acceptorless dehydrogenation (AD) reactions. In moderate yields (63–68%, Scheme 2) of the dehydrogenated ketone products, we discovered that cyclopentanone, cyclohexanone, and cycloheptanone (4e–g) were produced.
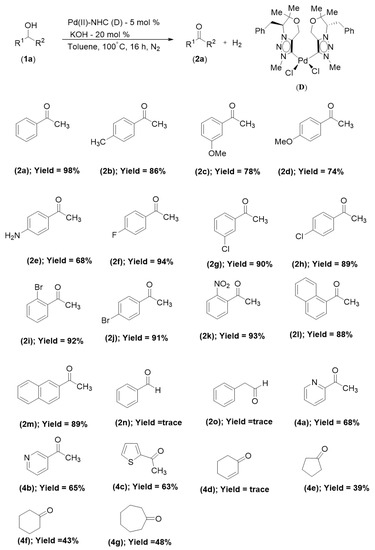
Scheme 2.
Selected results for the acceptorless dehydrogenation (AD) of secondary alcohol catalyzed using Pd(II)–NHC catalyst (D).
A plausible reaction mechanism for the Pd–NHC complex (D)-catalyzed acceptorless dehydrogenation (AD) reaction of secondary alcohol is proposed considering the experimental findings and previously reported findings (as shown in Scheme 3). The acceptorless dehydrogenation reaction’s mechanism might be comparable to some previously reported examples involving related catalytic systems [56,57,58]. First off, in the presence of KOH, the Pd–NHC catalyst D reaction with an alcohol molecule produces an intermediate called an alkoxide (I). Second, the intermediate (I) produces an aldehyde/ketone and one hydrido specie (II) by transferring the α–H to the Pd metal. The catalytic cycle is then produced by the intermediate (II) and H–Base, which evolves into H2 and the Pd–NHC complex (D) as shown in Scheme 3.
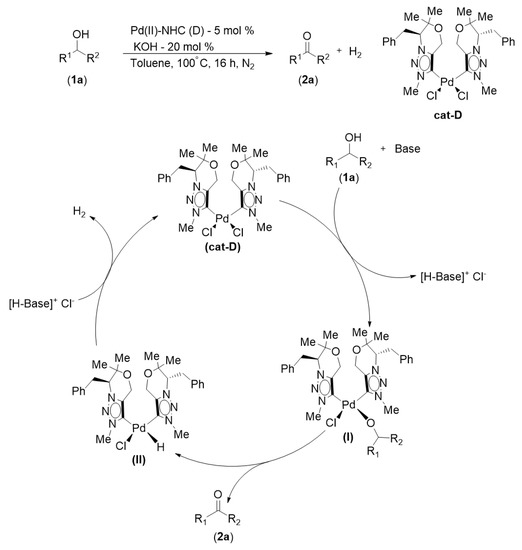
Scheme 3.
Proposed reaction mechanism for the acceptorless dehydrogenation (AD) of secondary alcohol catalyzed using Pd(II)–NHC catalyst (cat-D).
Experiments with intermolecular hydrogen transfer were carried out to verify the H2 evolution in the reactions (Scheme 4). In a closed system, 1-phenylethanol was hydrogenated to produce acetophenone (2a) 44% of the time and 1-(4-methoxyphenyl)ethan-1-ol (4a) 35% of the time when 1a was dehydrogenated in the presence of 1-(4-methoxyphenyl)ethan-1-one (3a) (Scheme 4a). The dehydrogenation and hydrogen transfer reactions were comparatively slowed down in an open system and an Ar atmosphere (Scheme 4b). A straightforward equation was used to calculate the hydrogen transfer efficiency: (yield of 4a)/(yield of 2a); 79% of the hydrogen transfer took place in a closed system (Scheme 4a). In contrast, the transfer efficiency significantly dropped to 51% in an open system (Scheme 4b). Additionally, we discovered H2 gas through gas chromatography (GC) analysis during the dehydrogenation of 1a (Figure S1) (cf. supporting information) According to the results, the catalytic reaction system operates in a dehydrogenative manner and generates H2 in a manner similar to other documented precious metal-based catalytic systems.
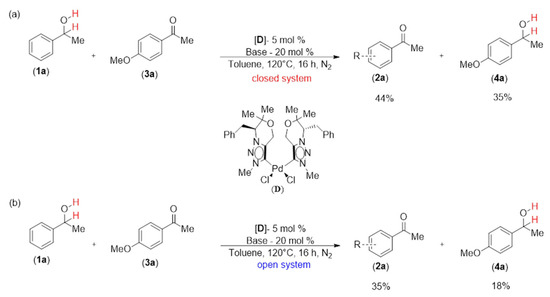
Scheme 4.
Experiments with intermolecular hydrogen transfer were carried out to verify H2 evolution in the reactions, (a) Closed system and (b) Open system.
3. Experimental Section
3.1. General Considerations
Unless otherwise noted, all commercially available substances were used exactly as they were given. Toluene, ethyl acetate, and hexane were used as such from the commercial sources as reagent-grade solvents. Using CDCl3 solvent, 1H and 13C{1H} NMR measurements were taken on Bruker 400 MHz and 500 MHz spectrometers. Relative to TMS, chemical shifts (δ) are given in ppm, and coupling constants (J) are given in Hz. The chemical shifts and solvent signals that were used as references were converted to the TMS scale (CDCl3, δC 77.0 ppm, δH 7.26 ppm). Using commercial aluminum sheets precoated with silica gel, analytical thin-layer chromatography (TLC) was used to track all the reactions. Silica gel (Merck, 200−400 mesh) was used for column chromatography. Singlet (s), doublet (d), triplet (t), quartet (q), doublet of doublet (dd), doublet of triplet (dt), triplet of triplet (tt), multiplet (m), etc., are the abbreviations used for 1H NMR spectra to denote the signal multiplicity. The palladium(II)–NHC catalysts used in this work (A–F) were prepared according to the literature procedure [59].
3.2. General Procedure for the Synthesis of NHC-Pd-I2(pyridine) (PEPPSI) Complexes (A−C)
NHC–Pd-I2(pyridine) (PEPPSI) complexes (A−C) were prepared according to the modified literature procedure [59]. A mixture of triazolium iodide ligands (1.0 mmol, 1.0 equiv), PdCl2 (1.0 mmol, 1.0 equiv), K2CO3 (8.0 mmol, 8.0 equiv), and NaI (5.0 mmol, 5.0 equiv) was refluxed in pyridine (5 mL, 63 mmol) for 16 h. The reaction mixture was cooled to room temperature, diluted with CHCl3 (ca. 100 mL), and subsequently washed with saturated aqueous CuSO4 solution (ca. 3 × 50 mL). The organic layer was separated and dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated under vacuum to give a sticky, brown residue. The residue thus obtained was further purified by column chromatography using silica gel as a stationary phase and eluted with EtOAc:petroleum ether (1:4 v/v) to give the PEPPSI complexes (A−C) as yellow solid products.
3.3. General Procedure for the Synthesis of Pd–bis-NHC (Cl2) Complexes (D−F)
Pd–bis-NHC (Cl2) complexes (D−F) were prepared according to the modified literature procedure [59]. A mixture of silver–NHC complexes (1.0 mmol, 1.0 equiv) and (COD)PdCl2 (0.50 mmol, 0.50 equiv) in CH3CN (ca. 50 mL) was stirred at room temperature, until the formation of an off-white AgCl precipitate were observed. The reaction mixture was filtered, and solvent was removed under vacuum to give the products (D−F) as light yellow solids.
3.4. General Procedure for the Preparation of Various Secondary Alcohols from Their Corresponding Ketones
All the secondary alcohols were prepared from the known procedure from the literature [60].
3.5. General Procedure (A) for Catalytic Acceptorless Dehydrogenation (CAD) of Secondary Alcohols into Their Corresponding Value-Added Ketones
To an oven-dried reaction tube equipped with magnetic stir bar, Pd(II)–NHC (cat-D), (17.2 mg, 0.025 mmol, 5 mol%), KOH (5.6 mg, 0.1 mmol, 20 mol%), and sec-aryl alcohol (61.1 mg, 0.5 mmol, 1 eq) were added followed by addition of 2 mL of toluene under nitrogen atmosphere. The closed reaction tube containing the reaction mixture was placed in a preheated oil bath and stirred at 100 °C for 16 h. After completion of the reaction time, the reaction mixture was cooled down to room temperature. The crude mixture was purified by flash column chromatography using silica gel as a stationary phase and hexane/ethyl acetate (95:5 v/v) as an eluent to afford the pure ketone product 2 as colorless oil in 98% (58.8 mg) yield.
3.6. Characterization of All Compounds
3.6.1. Synthesis of Acetophenone (2a)
Compound 2a was prepared according to the general procedure A from its corresponding secondary alcohol (61.1 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 2a as colorless oil in 98% (60.8 mg) yield. The NMR data of 2a are in accordance with the literature [61].
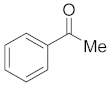
1H NMR (400 MHz, CDCl3): δ 7.95−7.90 (m, 2H), 7.51 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 7.3 Hz, 2H), 2.57 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 198.2, 137.2, 133.2, 128.6, 128.4, 26.6.
3.6.2. Synthesis of 1-p-Tolylethanone (2b)
Compound 2b was prepared according to the general procedure A from its corresponding secondary alcohol (68.1 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 2b as colorless oil in 86% (59.4 mg) yield. The NMR data of 2b are in accordance with the literature [61].
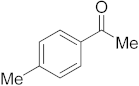
1H NMR (400 MHz, CDCl3): δ 7.83–7.81 (m, 2H), 7.21 (d, J = 8.0 Hz, 2H), 2.53 (s, 3H), 2.36 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 197.9, 143.9, 134.7, 129.3, 128.5, 26.6, 21.7.
3.6.3. Synthesis of 1-(3-Methoxyphenyl)ethanone (2c)
Compound 2c was prepared according to the general procedure A from its corresponding secondary alcohol (76.1 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 2c as colorless oil in 78% (60.1 mg) yield. The NMR data of 2c are in accordance with the literature [61].
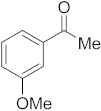
1H NMR (400 MHz, CDCl3): δ 7.50–7.48 (m, 1H), 7.45–7.44 (m, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.10–7.05 (m, 1H), 3.80 (s, 3H), 2.55 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 197.9, 159.7, 138.4, 129.5, 121.1, 119.5, 112.3, 55.3, 26.7.
3.6.4. Synthesis of 1-(4-Methoxyphenyl)ethanone (2d)
Compound 2d was prepared according to the general procedure A from its corresponding secondary alcohol (76.1 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 2d as colorless oil in 76% (57.0 mg) yield. The NMR data of 2d are in accordance with the literature [61].
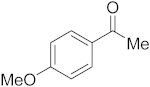
1H NMR (400 MHz, CDCl3): δ 7.95–7.90 (m, 2H), 6.94–6.90 (m, 2H), 3.86 (s, 3H), 2.55 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.8, 163.6, 130.7, 130.4, 113.8, 55.6, 26.4.
3.6.5. Synthesis of 1-(4-Aminophenyl)ethanone (2e)
Compound 2e was prepared according to the general procedure A from its corresponding secondary alcohol (68.5 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 2e as yellow solid in 68% (47.2 mg) yield. The NMR data of 2e are in accordance with the literature [62].
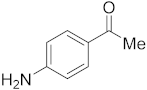
1H NMR (400 MHz, CDCl3): δ 7.78 (dd, J = 8.6 Hz, J = 1.5 Hz, 2H), 6.62 (dd, J = 8.6 Hz, J = 1.3 Hz, 2H), 4.20 (s, 2H), 2.50 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.7, 151.4, 130.9, 127.9, 113.8, 26.2.
3.6.6. Synthesis of 1-(4-Fluorophenyl)ethanone (2f)
Compound 2f was prepared according to the general procedure A from its corresponding secondary alcohol (77.0 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2f as colorless oil in 94% (73.4 mg) yield. The NMR data of 2f are in accordance with the literature [61].
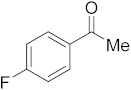
1H NMR (400 MHz, CDCl3): δ 7.92–7.90 (m, 2H), 7.05 (t, J = 8.7 Hz, 2H), 2.51 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.4, 167.0, 164.5, 133.6, 133.5, 131.0, 130.9, 115.7, 115.5, 26.5. 19F NMR (377 MHz, CDCl3): δ −105.5.
3.6.7. Synthesis of 1-(3-Chlorophenyl)ethanone (2g)
Compound 2g was prepared according to the general procedure A from its corresponding secondary alcohol (78.3 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2g as colorless oil in 90% (71.3 mg) yield. The NMR data of 2g are in accordance with the literature [62].
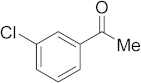
1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 1.6 Hz, 1H), 7.80–7.78 (m, 1H), 7.51–7.40 (m, 1H), 7.35 (t, J = 8.0 Hz, 1H), 2.56 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.7, 138.6, 134.9, 133.0, 130.0, 128.4, 126.5, 26.7.
3.6.8. Synthesis of 1-(4-Chlorophenyl)ethanone (2h)
Compound 2h was prepared according to the general procedure A from its corresponding secondary alcohol (78.0 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2h as yellow liquid in 89% (70.5 mg) yield. The NMR data of 2h are in accordance with the literature [62].
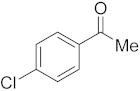
1H NMR (400 MHz, CDCl3): δ 7.82–7.80 (m, 2H), 7.36–7.33 (m, 2H), 2.51 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.7, 139.5, 135.5, 129.7, 128.9, 26.5.
3.6.9. Synthesis of 1-(2-Bromophenyl)ethanone (2i)
Compound 2i was prepared according to the general procedure A from its corresponding secondary alcohol (100 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2i as colorless oil in 92% (92.9 mg) yield. The NMR data of 2i are in accordance with the literature [61].
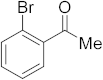
1H NMR (400 MHz, CDCl3): δ 7.56 (dd, J = 8 Hz, J = 1.0 Hz, 1H), 7.42 (dd, J = 7.7 Hz, J = 1.8 Hz, 1H), 7.34–7.30 (m, 1H), 7.25 (td, J = 7.6 Hz, J = 1.8 Hz, 1H), 2.60 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 199.9, 145.9, 137.9, 134.4, 130.8, 127.5, 124.4, 128.9, 30.2.
3.6.10. Synthesis of 1-(4-Bromophenyl)ethanone (2j)
Compound 2j was prepared according to the general procedure A from its corresponding secondary alcohol (100 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2j as colorless oil in 91% (91.9 mg) yield. The NMR data of 2j are in accordance with the literature [61].
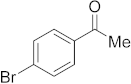
1H NMR (400 MHz, CDCl3): δ 7.83–7.80 (m, 2H), 7.63–7.59 (m, 2H), 2.59 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 197.1, 136.0, 132.0, 129.9, 128.4, 26.6.
3.6.11. Synthesis of 1-(2-Nitrophenyl)ethanone (2k)
Compound 2k was prepared according to the general procedure A from its corresponding secondary alcohol (83.5 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 97:3) to afford 2k as yellow liquid in 93% (78.5 mg) yield. The NMR data of 2k are in accordance with the literature [63].
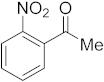
1H NMR (500 MHz, CDCl3): δ 8.02 (dd, J = 8.2, J = 0.9 Hz, 1H), 7.68 (td, J = 7.5, J = 1.1 Hz, 1H), 7.58–7.54 (m, 1H), 7.41 (dd, J = 7.6, J = 1.3 Hz, 1H), 2.52 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 199.8, 145.9, 137.9, 134.3, 130.8, 127.4, 124.4, 30.1.
3.6.12. Synthesis of 1-(Naphthalen-1-yl)ethanone (2l)
Compound 2l was prepared according to the general procedure A from its corresponding secondary alcohol (86.1 mg, 0.5 mmol, 1 eq), and the reaction mixture was purified by flash column chromatography (5% EtOAc/Hexane) to afford 2l as white solid in 88% (76.6 mg) yield. The NMR data of 2l are in accordance with the literature [64].
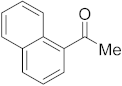
1H NMR (400 MHz, CDCl3): δ 8.76 (d, J = 8.6 Hz, 1H), 8.00–7.86 (m, 3H), 7.63–7.47 (m, 3H), 2.74 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 202.0, 135.5, 134.1, 133.2, 130.2, 128.8, 128.5, 128.2, 126.5, 126.1, 124.4, 30.1.
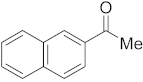
3.6.13. Synthesis of 1-(Naphthalen-2-yl)ethanone (2m)
Compound 2m was prepared according to the general procedure A from its corresponding secondary alcohol (86.1 mg, 0.5 mmol, 1 eq), and the reaction mixture was purified by flash column chromatography (5% EtOAc/Hexane) to afford 2m as colorless oil in 89% (77.5 mg) yield. The NMR data of 2m are in accordance with the literature [62].
1H NMR (400 MHz, CDCl3): δ 8.41 (s, 1H), 8.01 (dd, J = 8.7, J = 1.7 Hz, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.84–7.82 (m, 2H), 7.58–7.50 (m, 2H), 2.67 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 198.0, 135.6, 134.4, 132.5, 130.2, 129.5, 128.5, 128.4, 127.8, 126.8, 123.9, 26.7.
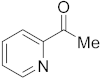
3.6.14. Synthesis of 1-(Pyridin-2-yl)ethanone (4a)
Compound 4a was prepared according to the general procedure A from its corresponding secondary alcohol (61.5 mg, 0.5 mmol, 1 eq), and the reaction mixture was purified by flash column chromatography (5% EtOAc/Hexane) to afford 4a as colorless oil in 68% (42.5 mg) yield. The NMR data of 4a are in accordance with the literature [63].
1H NMR (400 MHz, CDCl3): δ 8.61 (d, J = 4.8 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H), 7.77 (td, J = 7.8, J = 1.7 Hz, 1H), 7.42–7.37 (m, 1H), 2.66 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 201.1, 153.6, 149.0, 136.9, 127.2, 121.7, 25.8.
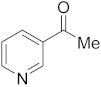
3.6.15. Synthesis of 1-(Pyridin-3-yl)ethanone (4b)
Compound 4b was prepared according to the general procedure A from its corresponding secondary alcohol (61.5 mg, 0.5 mmol, 1 eq), and the reaction mixture was purified by flash column chromatography (5% EtOAc/Hexane) to afford 4b as colorless oil in 65% (40.6 mg) yield. The NMR data of 4b are in accordance with the literature [61,63].
1H NMR (400 MHz, CDCl3): δ 9.05 (d, J = 1.9 Hz, 1H), 8.67 (dd, J = 4.8, J = 1.6 Hz, 1H), 8.14 (dt, J = 7.9, J = 1.9 Hz, 1H), 7.33 (dd, J = 7.9, J = 4.9 Hz, 1H), 2.54 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 196.8, 153.5, 149.9, 135.5, 132.2, 123.6, 26.7.
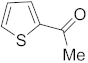
3.6.16. Synthesis of 1-(Thiophen-2-yl)ethanone (4c)
Compound 4c was prepared according to the general procedure A from its corresponding secondary alcohol (64.1 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 4c as colorless oil in 63% (41.0 mg) yield. The NMR data of 4c are in accordance with the literature [61].
1H NMR (400 MHz, CDCl3): δ 7.66 (dd, J = 3.7 Hz, J = 1.0 Hz, 1H), 7.62 (dd, J = 4.9 Hz, J = 1.0 Hz, 1H), 7.10 (dd, J = 4.8 Hz, J = 3.8 Hz, 1H), 2.54 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 190.8, 144.6, 133.9, 132.6, 128.2, 26.9.
3.6.17. Synthesis of Cyclopentanone (4e)
Compound 4e was prepared according to the general procedure A from its corresponding secondary alcohol (43.0 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 4e as colorless oil in 39% (17.1 mg) yield. The NMR data of 4e are in accordance with the literature [65].
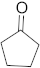
1H NMR (500 MHz, CDCl3): δ 2.10–1.92 (m, 4H), 1.91–1.85 (m, 4H). 13C NMR (125 MHz, CDCl3): δ 220.7, 38.3, 23.2.
3.6.18. Synthesis of Cyclohexanone (4f)
Compound 4f was prepared according to the general procedure A from its corresponding secondary alcohol (50.0 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 4f as colorless oil in 43% (21.9 mg) yield. The NMR data of 4f are in accordance with the literature [61,65].
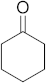
1H NMR (500 MHz, CDCl3): δ 2.22 (t, J = 6.7 Hz, 4H), 1.78–1.73 (m, 4H), 1.64–1.57 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 211.8, 41.9, 26.9, 24.9.
3.6.19. Synthesis of Cycloheptanone (4g)
Compound 4g was prepared according to the general procedure A from its corresponding secondary alcohol (57.0 mg, 0.5 mmol, 1 eq), and the crude product was further purified by column chromatography using silica as a stationary phase and eluting with petroleum ether/EtOAc (v/v 95:5) to afford 4g as colorless oil in 48% (27.8 mg) yield. The NMR data of 4g are in accordance with the literature [65].
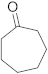
1H NMR (500 MHz, CDCl3): δ 2.42–2.40 (m, 4H), 1.64–1.58 (m, 8H). 13C NMR (125 MHz, CDCl3): δ 215.3, 43.9, 30.4, 24.4.
3.7. Mercury Drop Experiment Performed at Varying Time Intervals
3.7.1. Mercury Addition at the Start of the Reaction
A 10 mL vial was charged with a mixture of 1-phenylethan-1-ol (61.1 mg, 0.5 mmol, 1 eq) and KOH (5.6 mg, 0.1 mmol, 20 mol%) in molar ratio of 5:1, and mercury (0.121 g, 0.603 mmol) was added subsequently. The palladium complex D (17.2 mg, 0.025 mmol, 5 mol%) was added to the mixture, followed by toluene (ca. 2 mL) solvent, and closed reaction tube containing the reaction mixture was placed in a preheated oil bath and stirred at 100 °C for 6 h. The reaction mixture was cooled to room temperature, and water (ca. 12 mL) was added. The resulting mixture was extracted with EtOAc (ca. 50 mL). The water layer was further extracted with EtOAc (ca. 3 × 20 mL). The crude mixture was purified by flash column chromatography using silica gel as a stationary phase and hexane/ethyl acetate (95:5 v/v) as an eluent to afford the pure ketone product 2a as colorless oil in 84% (52.1 mg) yield.
3.7.2. Mercury Addition after 2 h of Reaction Time
A 10 mL vial was charged with a mixture of 1-phenylethan-1-ol (61.1 mg, 0.5 mmol, 1 eq) and KOH (5.6 mg, 0.1 mmol, 20 mol%) in molar ratio of 5:1. The palladium complex-D (17.2 mg, 0.025 mmol, 5 mol%) was added to the mixture, followed by toluene (ca. 2 mL), and then the reaction mixture was heated at 100 °C for 2 h. Mercury (0.126 g, 0.628 mmol) was added, and the reaction mixture was further heated at 100 °C for 4 h. The reaction mixture was cooled to room temperature, and water (ca. 12 mL) was added. The resulting mixture was extracted with EtOAc (ca. 50 mL). The water layer was further extracted with EtOAc (ca. 3 × 20 mL). The crude mixture was purified by flash column chromatography using silica gel as a stationary phase and hexane/ethyl acetate (95:5 v/v) as an eluent to afford the pure ketone product 2a as colorless oil in 72% (44.7 mg) yield.
3.8. Experimental Procedure for Detection of H2 Gas by GC
A 250 mL oven-dried Schlenk tube with a rubber septum was filled with 1-phenylethanol (10 mmol, 1.21 mL), palladium complex D (17.2 mg, 0.025 mmol, 5 mol%), KOH (5.6 mg, 0.1 mmol, 20 mol%), and toluene (10 mL). This was carried out in an atmosphere of argon. The reaction medium was stirred at 100 °C in a closed environment for 24 h. With the help of a Hamilton syringe, gas that had been filled to the top of a Schlenk tube was sampled in order to detect hydrogen.
4. Conclusions
In this report, we demonstrate an efficient protocol for the catalytic acceptorless dehydrogenation (CAD) reaction of secondary alcohols into their corresponding value-added ketones and the release of molecular hydrogen (H2) as the sole side product by using well-defined, air-stable, Pd(II)–NHC catalysts under mild reaction conditions. This protocol is a highly efficient, an economical, and an environmentally friendly alternative to all other methods, which require harsh reaction conditions/hazardous solvents and reagents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28134992/s1, Figure S1: (a) 1H NMR spectrum of 2a in CDCl3. (b) Crude 1H NMR spectrum of 2a in CDCl3. (c) GC traces after 24 hours reaction to confirm H2 evolution.; Figure S2: 13C{1H} NMR spectrum of 2a in CDCl3.; the 1H-NMR and 13C-NMR data for catalysis products from Figures S3–S39.
Author Contributions
Conceptualization, Formal analysis, Data curation, A.N.A.-R., R.H.A.-A., R.J.B. and A.V.; Methodology, Validation, Investigation, and Visualization, A.N.A.-R., A.V., M.K.G. and S.M.B.; Writing—original draft preparation, M.K.G., T.S.S., I.R.S. and M.M.M.M.; Writing—review and editing, all the authors, M.M.M.M., T.S.S., M.K.G. and A.N.A.-R.; Supervision, M.M.M.M., I.R.S. and M.K.G.; Funding acquisition, M.M.M.M., T.S.S. and A.N.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia and the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (IFPRC-026-247-2020).
Data Availability Statement
The catalytic product NMR data are contained within the supporting information of this article. Other data have been cited and listed in the bibliography.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number IFPRC-026-247-2020 and the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia. The authors, therefore, acknowledge with thanks the DSR for the technical and financial support. The authors also gratefully acknowledge the Department of Chemistry, University of Allahabad (UoA), Prayagraj, U. P., India. AK gratefully acknowledges UGC, New Delhi for the award of a Senior Research Fellowship (SRF) and Junior Research Fellowship (JRF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lombardo, M.; Trombini, C. α-Hydroxyallylation Reaction of Carbonyl Compounds. Chem. Rev. 2007, 107, 3843–3879. [Google Scholar] [CrossRef]
- Krief, A.; Laval, A.-M. Coupling of Organic Halides with Carbonyl Compounds Promoted by SmI2, the Kagan Reagent. Chem. Rev. 1999, 99, 745–778. [Google Scholar] [CrossRef]
- Kahn, B.E.; Rieke, R.D. Carbonyl coupling reactions using transition metals, lanthanides, and actinides. Chem. Rev. 1988, 88, 733–745. [Google Scholar] [CrossRef]
- Stevens, R.V.; Chapman, K.T.; Weller, H.N. Convenient and inexpensive procedure for oxidation of secondary alcohols to ketones. J. Org. Chem. 1980, 45, 2030–2032. [Google Scholar] [CrossRef]
- Fatiadi, A.J. The Classical Permanganate Ion: Still a Novel Oxidant in Organic Chemistry. Synthesis 1987, 2, 85–127. [Google Scholar] [CrossRef]
- Lee, D.G.; Ribagorda, M.; Adrio, J. “Potassium Permanganate” Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Highet, R.J.; Wildman, W.C. Solid Manganese Dioxide as an Oxidizing Agent. J. Am. Chem. Soc. 1955, 77, 4399–4401. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Omura, K.; Swern, D. Oxidation of alcohols by “activated” dimethyl sulfoxide: A preparative, steric and mechanistic study. Tetrahedron 1978, 34, 1651–1660. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jensen, D.R. Ligand-modulated palladium-catalyzed aerobic alcohol oxidations. Acc. Chem. Res. 2006, 39, 221–229. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Ten Brink, G.-J.; Dijksman, A. Green, catalytic oxidations of alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef]
- Csjernyik, G.; Éll, A.H.; Fadini, L.; Pugin, B.; Bäckvall, J.-E. Efficient ruthenium-catalyzed aerobic oxidation of alcohols using a biomimetic coupled catalytic system. J. Org. Chem. 2002, 67, 1657–1662. [Google Scholar] [CrossRef]
- Lee, D.G.; Spitzer, U.A. Aqueous dichromate oxidation of primary alcohols. J. Org. Chem. 1970, 35, 3589–3590. [Google Scholar] [CrossRef]
- Holum, J.R.J. Study of the Chromium(VI) Oxide-Pyridine Complex. Org. Chem. 1961, 26, 4814–4816. [Google Scholar] [CrossRef]
- Join, B.; Möller, K.; Ziebart, C.; Schröder, K.; Gördes, D.; Thurow, K.; Spannenberg, A.; Junge, K.; Beller, M. Selective Iron-Catalyzed Oxidation of Benzylic and Allylic Alcohols. Adv. Synth. Catal. 2011, 353, 3023–3030. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 1977–1986. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Development of Novel Photocatalyst and Cocatalyst Materials for Water Splitting under Visible Light. Bull. Chem. Soc. Jpn. 2016, 89, 627–648. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Arakawa, T.; Hocke, H.; Uozumi, Y. A Nanoplatinum Catalyst for Aerobic Oxidation of Alcohols in Water. Angew. Chem. Int. Ed. 2007, 46, 704–706. [Google Scholar] [CrossRef]
- Zeng, G.; Sakaki, S.; Fujita, K.-I.; Sano, H.; Yamaguchi, R. Efficient Catalyst for Acceptorless Alcohol Dehydrogenation: Interplay of Theoretical and Experimental Studies. ACS Catal. 2014, 4, 1010–1020. [Google Scholar] [CrossRef]
- Junge, H.; Loges, B.; Beller, M. Novel improved ruthenium catalysts for the generation of hydrogen from alcohols. Chem. Commun. 2007, 522–524. [Google Scholar] [CrossRef]
- Zweifel, T.; Naubron, J.V.; Grützmacher, H. Catalyzed dehydrogenative coupling of primary alcohols with water, methanol, or amines. Angew. Chem. Int. Ed. 2009, 48, 559–563. [Google Scholar] [CrossRef]
- Charman, H.B. Hydride Transfer Reactions catalysed by Metal Complexes. Nature 1966, 212, 278–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.-S.; Sim, D.S.B.; Pan, H.-J.; Zhao, Y. Catalytic Enantioselective Amination of Alcohols by the Use of Borrowing Hydrogen Methodology: Cooperative Catalysis by Iridium and a Chiral Phosphoric Acid. Angew. Chem. Int. Ed. 2014, 53, 1399–1403. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative Oxidation of Primary and Secondary Alcohols Catalyzed by a Cp*Ir Complex Having a Functional C,N-Chelate Ligand. Org. Lett. 2011, 13, 2278–2281. [Google Scholar] [CrossRef]
- Royer, A.M.; Rauchfuss, T.B.; Gray, D.L. Organoiridium Pyridonates and Their Role in the Dehydrogenation of Alcohols. Organometallics 2010, 29, 6763–6768. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Tanino, N.; Yamaguchi, R. Ligand-Promoted Dehydrogenation of Alcohols Catalyzed by Cp*Ir Complexes A New Catalytic System for Oxidant-Free Oxidation of Alcohols. Org. Lett. 2007, 9, 109–111. [Google Scholar] [CrossRef]
- Mena, I.; Casado, M.A.; Polo, V.; Garcia-Orduña, P.; Lahoz, F.J.; Oro, L.A. The Dehydrogenation of Alcohols through a Concerted Bimetallic Mechanism Involving an Amido-Bridged Diiridium Complex. Angew. Chem. Int. Ed. 2012, 51, 8259–8263. [Google Scholar] [CrossRef]
- Bertoli, M.; Choualeb, A.; Lough, A.J.; Moore, B.; Spasyuk, D.; Gusev, D.G. Osmium and Ruthenium Catalysts for Dehydrogenation of Alcohols. Organometallics 2011, 30, 3479–3482. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of Electronic Structures of Au Clusters Stabilized by Poly(N-vinyl-2-pyrrolidone) on Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [Google Scholar] [CrossRef]
- Mitsudome, T.; Mikami, Y.; Funai, H.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Oxidant-Free Alcohol Dehydrogenation Using a Reusable Hydrotalcite-Supported Silver Nanoparticle Catalyst. Angew. Chem. Int. Ed. 2008, 47, 138–141. [Google Scholar] [CrossRef]
- Waiba, S.; Barman, M.K.; Maji, B. Manganese-Catalyzed Acceptorless Dehydrogenative Coupling of Alcohols With Sulfones: A Tool To Access Highly Substituted Vinyl Sulfones. J. Org. Chem. 2019, 84, 973–982. [Google Scholar] [CrossRef]
- Zhihui, S.; Yujie, W.; Yaqian, L.; Qian, W.; Xiaoling, F.; Qiang, L. A general and efficient Mn-catalyzed acceptorless dehydrogenative coupling of alcohols with hydroxides into carboxylates. Org. Chem. Front. 2018, 5, 1248–1256. [Google Scholar]
- Nad, P.; Mukherjee, A. Acceptorless Dehydrogenative Coupling Reactions by Manganese Pincer Complexes. Asian J. Org. Chem. 2021, 10, 1958–1985. [Google Scholar] [CrossRef]
- Song, H.; Kang, B.; Hong, S.H. Fe-Catalyzed Acceptorless Dehydrogenation of Secondary Benzylic Alcohols. ACS Catal. 2014, 4, 2889–2895. [Google Scholar] [CrossRef]
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2014, 136, 8564–8567. [Google Scholar] [CrossRef]
- Alberico, E.; Sponholz, P.; Cordes, C.; Nielsen, M.; Drexler, H.-J.; Baumann, W.; Junge, H.; Beller, M. Selective Hydrogen Production from Methanol with a Defined Iron Pincer Catalyst under Mild Conditions. Angew. Chem. Int. Ed. 2013, 52, 14162–14166. [Google Scholar] [CrossRef]
- Zhang, G.; Hanson, S.K. Switchable Cobalt-Catalyzed α-Olefination and α-Alkylation of Nitriles with Primary Alcohols. Org. Lett. 2013, 15, 650–653. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kon, K.; Seto, M.; Shimura, K.; Yamazaki, H.; Kondo, J.N. Heterogeneous cobalt catalysts for the acceptorless dehydrogenation of alcohols. Green Chem. 2013, 15, 418–424. [Google Scholar] [CrossRef]
- Chakraborty, S.; Piszel, P.E.; Brennessel, W.W.; Jones, W.D. A Single Nickel Catalyst for the Acceptorless Dehydrogenation of Alcohols and Hydrogenation of Carbonyl Compounds. Organometallics 2015, 34, 5203–5206. [Google Scholar] [CrossRef]
- Bertrand, G.; Diez-Barra, E.; Fernandez-Baeza, J.; Gornitzka, H.; Moreno, A.; Otero, A.; Rodriguez-Curiel, R.I.; Tejeda, J. Synthesis, Characterization and Dynamic Behavior of Mono- and Dinuclear Palladium(II) Carbene Complexes Derived From 1,1′-Methylenebis(4-alkyl-1,2,4-triazolium) Diiodides. Eur. J. Inorg. Chem. 1999, 1999, 1965–1971. [Google Scholar] [CrossRef]
- Seitz, S.C.; Rominger, F.; Straub, B.F. Stepwise Deprotonation of a Thiol-Functionalized Bis(1,2,4-triazolium) Salt as a Selective Route to Heterometallic NHC Complexes. Organometallics 2013, 32, 2427–2434. [Google Scholar] [CrossRef]
- Rogers, H.; Daniel, I.T.; Freakley, S.J. Acceptorless dehydrogenation of 1-phenylethanol using Pd/TiO2 catalysts prepared by sol immobilization. Catal. Commun. 2022, 162, 106377. [Google Scholar] [CrossRef]
- Nicolau, G.; Tarantino, G.; Hammond, C. Acceptorless Alcohol Dehydrogenation Catalysed by Pd/C. ChemSusChem. 2019, 12, 4953–4961. [Google Scholar] [CrossRef]
- Hohloch, S.; Frey, W.; Su, C.-Y.; Sarkar, B. Abnormal carbenes derived from the 1,5-cycloaddition product between azides and alkynes: Structural characterization of Pd(ii) complexes and their catalytic properties. Dalton Trans. 2013, 42, 11355–11358. [Google Scholar] [CrossRef]
- Canseco-Gonzalez, D.; Gniewek, A.; Szulmanowicz, M.; Müeller-Bunz, H.; Trzeciak, A.M.; Albrecht, M. PEPPSI-Type Palladium Complexes Containing Basic 1,2,3-Triazolylidene Ligands and Their Role in Suzuki–Miyaura Catalysis. Chem. Eur. J. 2012, 18, 6055–6062. [Google Scholar] [CrossRef]
- Saravanakumar, R.; Ramkumar, V.; Sankararaman, S. Synthesis and Structure of 1,4-Diphenyl-3-methyl-1,2,3-triazol-5-ylidene Palladium Complexes and Application in Catalytic Hydroarylation of Alkynes. Organometallics 2011, 30, 1689–1694. [Google Scholar] [CrossRef]
- Nakamura, T.; Ogata, K.; Fukuzawa, S.-I. Synthesis of Dichlorobis(1,4-dimesityl-1H-1,2,3-triazol-5-ylidene)palladium [PdCl2(TMes)2] and Its Application to Suzuki–Miyaura Coupling Reaction. Chem. Lett. 2010, 39, 920–922. [Google Scholar] [CrossRef]
- Gangwar, M.K.; Kalita, A.C.; Ghosh, P. Palladium complexes of a new type of N-heterocyclic carbene ligand derived from a tricyclic triazolooxazine framework. J. Chem. Sci. 2014, 126, 1557–1563. [Google Scholar] [CrossRef]
- Yiğit, M.; Gök, Y.; Yiğit, B.; Celikal, O.O.; Yiğit, M. Palladium/Benzimidazolium Salt Catalyst Systems and N-Heterocyclic Carbene-Palladium(II)-Pyridine (PEPPSI) Complexes for Anti-Markovnikov Hydroaminations of Styrene in Ionic Liquid. Heterocycles 2019, 98, 403–415. [Google Scholar] [CrossRef]
- Prades, A.; Peris, E.; Albrecht, M. Oxidations and Oxidative Couplings Catalyzed by Triazolylidene Ruthenium Complexes. Organometallics 2011, 30, 1162–1167. [Google Scholar] [CrossRef]
- Keske, E.C.; Zenkina, O.V.; Wang, R.; Crudden, C.M. Synthesis and Structure of Palladium 1,2,3-Triazol-5-ylidene Mesoionic Carbene PEPPSI Complexes and Their Catalytic Applications in the Mizoroki–Heck Reaction. Organometallics 2012, 31, 6215–6221. [Google Scholar] [CrossRef]
- Gangwar, M.K.; Butcher, R.J. Chiral tricyclic triazolooxazine derived mesoionic carbene (MIC)-Pd(II) complexes of cyclohexene oxide scaffold: Synthesis, structure, and characterizations. J. Organomet. Chem. 2020, 930, 121598. [Google Scholar] [CrossRef]
- Gangwar, M.K.; Butcher, R.J. Axially Chiral bis-1,2,3-Triazol-4-ylidene-Ag(I)-MIC and, bis-Au(I)-MIC Complexes of (R)-BINOL and (-)-Menthol Scaffold: Synthesis, Structure, and Characterizations. J. Organomet. Chem. 2020, 932, 121626. [Google Scholar] [CrossRef]
- Anusha, G.; Reddy, M.V.K.; Reddy, P.V.G. Investigation of Pd-PEPPSI catalysts and coupling partners towards direct C2-arylation/heteroarylation of benzoxazole. Appl. Organomet Chem 2021, 35, e6296. [Google Scholar]
- Haack, K.J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. The Catalyst Precursor, Catalyst, and Intermediate in the RuII-Promoted Asymmetric Hydrogen Transfer between Alcohols and Ketones. Angew. Chem. Int. Ed. 1997, 36, 285–288. [Google Scholar] [CrossRef]
- Alonso, D.A.; Brandt, P.; Nordin, S.J.M.; Andersson, P.G. Ru(arene)(amino alcohol)-Catalyzed Transfer Hydrogenation of Ketones: Mechanism and Origin of Enantioselectivity. J. Am. Chem. Soc. 1999, 121, 9580–9588. [Google Scholar] [CrossRef]
- Weismann, J.; Gessner, V.H. Catalytic Transfer Hydrogenation with a Methandiide-Based Carbene Complex: An Experimental and Computational Study. Chem. Eur. J. 2015, 21, 16103–16112. [Google Scholar] [CrossRef]
- Gangwar, M.K.; Dey, S.; Prakasham, A.P.; Ghosh, P. Palladium(II), silver(I), and gold(I) complexes of a new class of chiral bicyclic [1,2,3]-triazolooxazine derived N-heterocyclic carbenes (NHCs): Synthesis, structure and application studies. Polyhedron 2021, 197, 115011. [Google Scholar] [CrossRef]
- Hu, A.; Ngo, H.L.; Lin, W. Chiral Porous Hybrid Solids for Practical Heterogeneous Asymmetric Hydrogenation of Aromatic Ketones. J. Am. Chem. Soc. 2003, 125, 11490–11491. [Google Scholar] [CrossRef]
- Tan, D.-W.; Li, H.-X.; Zhang, M.-J.; Yao, J.-L.; Lang, J.-P. Acceptorless Dehydrogenation of Alcohols Catalysed by Cu(I) Nheterocycle Thiolate Complexes. ChemCatChem. 2017, 9, 1113–1118. [Google Scholar] [CrossRef]
- Polukeev, A.V.; Abdelaziz, O.Y.; Wendt, O.F. Combined Experimental and Computational Study of the Mechanism of Acceptorless Alcohol Dehydrogenation by POCOP Iridium Pincer Complexes. Organometallics 2022, 41, 859–873. [Google Scholar] [CrossRef]
- Alabau, R.G.; Esteruelas, M.A.; Martínez, A.; Oliván, M.; Oñate, E. Base-Free and Acceptorless Dehydrogenation of Alcohols Catalyzed by an Iridium Complex Stabilized by a N,N,N-Osmaligand. Organometallics 2018, 37, 2732–2740. [Google Scholar] [CrossRef]
- Jayaprakash, H.; Guo, L.; Wang, S.; Bruneau, C.; Fischmeister, C. Acceptorless and Base-Free Dehydrogenation of Alcohols Mediated by a Dipyridylamine-Iridium(III) Catalyst. Eur. J. Org. Chem. 2020, 2020, 4326–4330. [Google Scholar] [CrossRef]
- Fuse, H.; Mitsunuma, H.; Kanai, M. Catalytic Acceptorless Dehydrogenation of Aliphatic Alcohols. J. Am. Chem. Soc. 2020, 142, 4493–4499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).