Multi-Residue Method for Pesticides Determination in Dried Hops by Liquid Chromatography Tandem Mass Spectrometry
Abstract
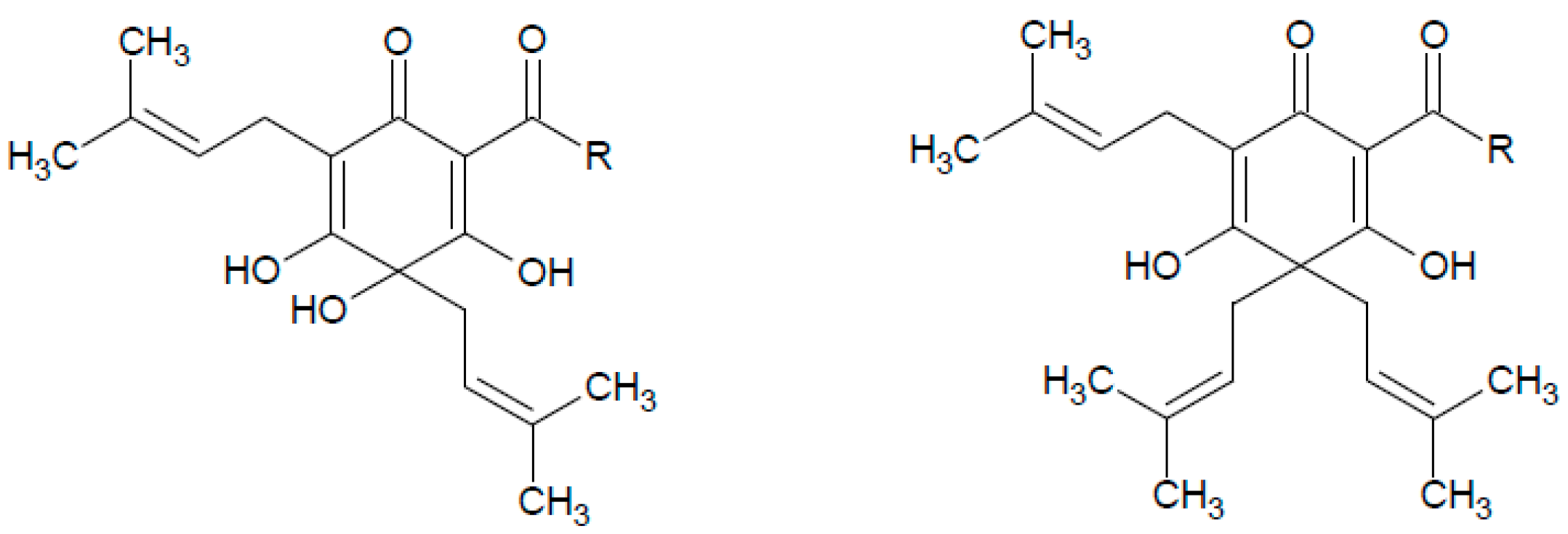
1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Preparation
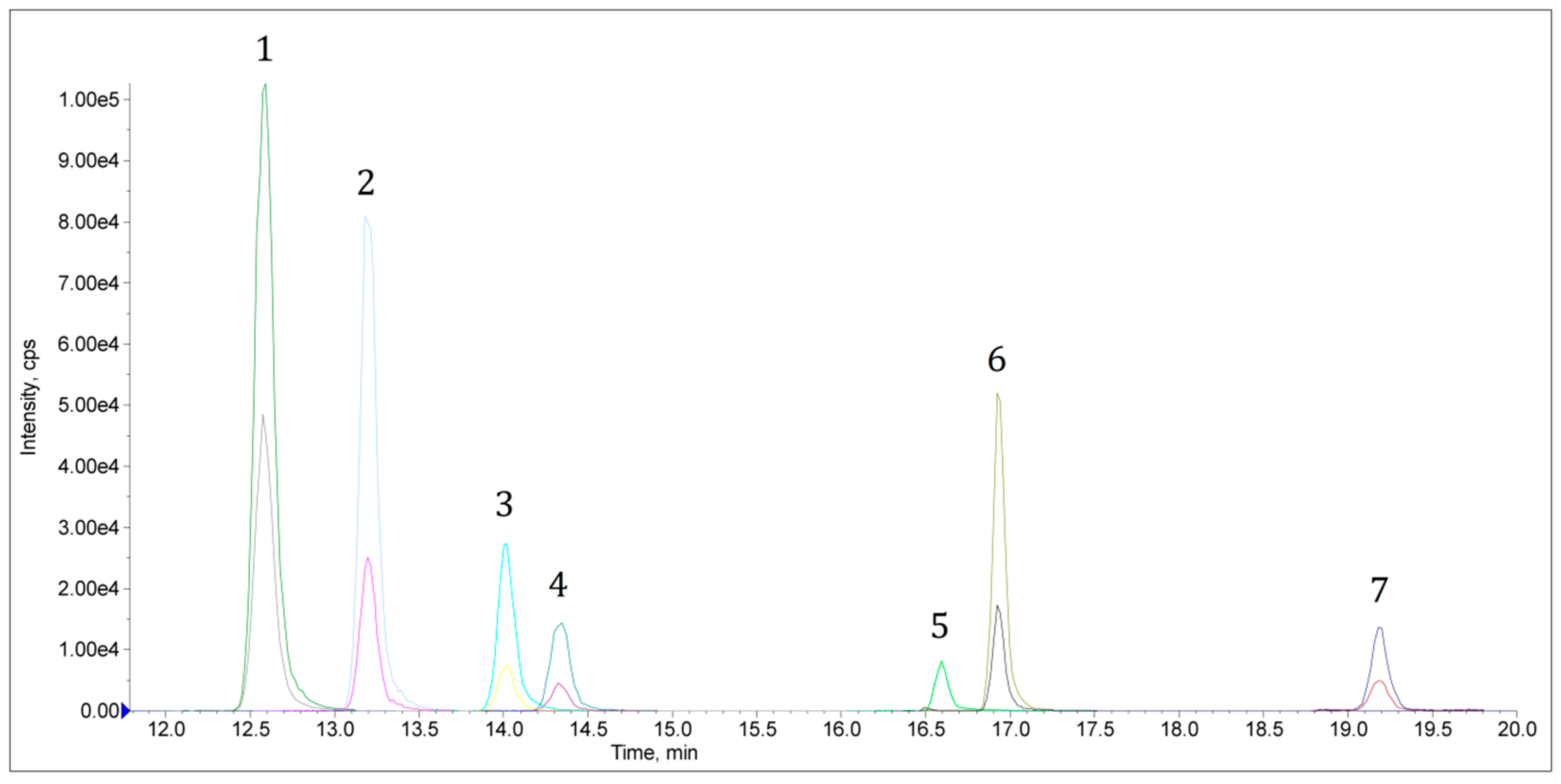
2.2. Validation of Analytical Procedure
| Analyte | Category | MRL (mg/kg) | LOQ (mg/kg) | ME (%) w/o IS | Linearity (mg/kg) | R2 | Recovery, % (RSDr, %) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.02 mg/kg | 0.05 mg/kg | 0.1 mg/kg | 1 mg/kg | |||||||
| Acetamiprid | I | 0.05 | 0.02 | −53 | 0.02–4 | 0.999 | 100 (8) | 98 (5) | 101 (9) | 92 (3) |
| Azoxystrobin | F | 30 | 0.02 | −22 | 0.02–4 | 0.998 | 84 (3) | 90 (6) | 91 (4) | 90 (3) |
| Bifenazate | I | 20 | 0.05 | −60 | 0.05–4 | 0.996 | 108 (10) | 113 (5) | 104 (9) | |
| Boscalid | F | 80 | 0.1 | −56 | 0.1–4 | 0.995 | 108 (7) | 98 (10) | ||
| Carbofuran | I | 0.05 | 0.02 | −28 | 0.02–4 | 0.998 | 110 (8) | 105 (4) | 105 (2) | 94 (2) |
| Chloridazon | H | 0.1 | 0.05 | −39 | 0.05–4 | 0.999 | 114 (2) | 106 (5) | 90 (4) | |
| Chlorotoluron | H | 0.05 | 0.02 | −33 | 0.02–4 | 0.999 | 112 (4) | 107 (1) | 109 (1) | 101 (1) |
| Diazinon | I | 0.5 | 0.05 | −58 | 0.05–4 | 0.996 | 98 (7) | 99 (3) | 89 (6) | |
| Difenoconazole | F | 0.05 | 0.05 | −40 | 0.05–4 | 0.999 | 111 (7) | 110 (5) | 96 (4) | |
| Dimethoate | I | 0.05 | 0.02 | −25 | 0.02–4 | 0.999 | 119 (4) | 108 (2) | 105 (3) | 89 (4) |
| Dimethomorph | F | 80 | 0.05 | −9 | 0.05–4 | 0.998 | 116 (5) | 106 (6) | 89 (4) | |
| Epoxiconazole | F | 0.1 | 0.05 | −33 | 0.05–4 | 0.995 | 112 (7) | 110 (6) | 92 (8) | |
| Etoxazole | I | 15 | 0.05 | −54 | 0.05–4 | 0.999 | 85 (5) | 92 (3) | 84 (2) | |
| Fenamidone | F | 0.05 | 0.05 | −34 | 0.05–4 | 0.994 | 102 (8) | 97 (7) | 85 (9) | |
| Fenhexamid | F | 0.05 | 0.05 | −45 | 0.05–4 | 0.997 | 100 (7) | 96 (8) | 81 (7) | |
| Fenpyroximate | A | 15 | 0.05 | −65 | 0.05–4 | 0.998 | 107 (7) | 99 (4) | 85 (3) | |
| Flufenoxuron | I | 0.05 | 0.05 | −70 | 0.05–4 | 0.991 | 111 (5) | 108 (7) | 92 (1) | |
| Flusilazole | F | 0.05 | 0.05 | −48 | 0.05–4 | 0.994 | 96 (13) | 100 (7) | 92 (6) | |
| Hexythiazox | A | 20 | 0.1 | −86 | 0.1–4 | 0.989 | 104 (3) | 102 (5) | ||
| Imidacloprid | I | 10 | 0.02 | −18 | 0.02–4 | 0.999 | 114 (6) | 108 (6) | 101 (8) | 93 (5) |
| Isoproturon | H | 0.05 | 0.02 | −14 | 0.02–4 | 0.999 | 95 (1) | 101 (4) | 103 (4) | 96 (1) |
| Linuron | H | 0.05 | 0.05 | −50 | 0.05–4 | 0.997 | 117 (4) | 111 (5) | 102 (3) | |
| Mandipropamid | F | 90 | 0.05 | −26 | 0.05–4 | 0.998 | 110 (4) | 107 (4) | 91 (3) | |
| Metalaxyl | F | 15 | 0.02 | −43 | 0.02–4 | 0.998 | 89 (10) | 94 (9) | 95 (4) | 89 (4) |
| Myclobutanil | F | 6 | 0.05 | −45 | 0.05–4 | 0.998 | 106 (10) | 95 (5) | 80 (7) | |
| Picoxystrobin | F | 0.05 | 0.02 | −24 | 0.02–4 | 0.995 | 99 (9) | 107 (7) | 110 (5) | 101 (4) |
| Propargite | A | 0.05 | 0.05 | −69 | 0.05–4 | 0.997 | 92 (12) | 97 (7) | 82 (5) | |
| Pyraclostrobin | F | 15 | 0.05 | −58 | 0.05–4 | 0.998 | 105 (6) | 111 (7) | 99 (4) | |
| Pyrimethanil | F | 0.05 | 0.05 | −18 | 0.05–4 | 0.995 | 114 (3) | 109 (4) | 93 (5) | |
| Quinoxyfen | F | 2 | 0.1 | −75 | 0.1–4 | 0.994 | 103 (7) | 83 (5) | ||
| Spirodiclofen | A | 40 | 0.1 | −58 | 0.1–4 | 0.997 | 116 (9) | 99 (4) | ||
| Spirotetramat | I | 15 | 0.05 | −2 | 0.05–4 | 0.998 | 104 (8) | 108 (7) | 98 (6) | |
| Tebuconazole | F | 40 | 0.05 | −50 | 0.05–4 | 0.996 | 79 (12) | 89 (5) | 92 (7) | |
| Thiabendazole | F | 0.05 | 0.02 | −27 | 0.02–4 | 0.993 | 97 (11) | 106 (3) | 107 (8) | 100 (4) |
| Thiacloprid | I | 0.05 | 0.02 | −21 | 0.02–4 | 0.999 | 105 (2) | 107 (3) | 104 (3) | 95 (3) |
| Trifloxystrobin | F | 40 | 0.02 | −47 | 0.02–4 | 0.999 | 113 (6) | 106 (2) | 103 (3) | 97 (4) |
2.3. Real Samples
3. Materials and Methods
3.1. Standards and Reagents
3.2. Instrumentation
3.3. Sample Preparation
3.4. Validation of the Analytical Procedure
3.5. Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Briendl, M.; Engelhard, B.; Forster, A.; Gahr, A.; Lutz, A.; Mitter, W.; Schmidt, R.; Schönberger, C. Hops: Their Cultivation, Composition and Usage; Fachverlag Hans Carl: Nuremberg, Germany, 2014. [Google Scholar]
- European Commission (EC). Hop Report for the Harvest Year 2019. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/plants_and_plant_products/documents/hop-report-2019_en.pdf (accessed on 15 October 2020).
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ciric, A.; Sokovic, M.; Calhelha, R.C.; Torija-Isasa, E.; Sanchez-Mata, M.C.; Ferreira, I.C.F.R. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crop. Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.-H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and Cytotoxic Effects of Prenylated Flavonoids from Hops (Humulus lupulus) in Human Cancer Cell Lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 440–447. [Google Scholar] [CrossRef]
- Kowalska, G.; Bouchentouf, S.; Kowalski, R.; Wyrostek, J.; Pankiewicz, U.; Mazurek, A.; Sujka, M.; Włodarczyk-Stasiak, M. The hop cones (Humulus lupulus L.) Chemical composition, antioxidant properties and molecular docking simulations. J. Herb. Med. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Regulation (EC). No 396/2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, L 70, 1–16. [Google Scholar]
- Dusek, M.; Jandovska, V.; Olsovska, J. Analysis of multiresidue pesticides in dried hops by LC-MS/MS using QuEChERS extraction together with dSPE clean-up. J. Inst. Brew. 2018, 124, 222–229. [Google Scholar] [CrossRef]
- Dusek, M.; Jandovska, V.; Kalachova, K.; Olsovska, J. Comparative Study of Three Sample Preparation Methods for Multi-residue Extraction of Pesticide Residues in Hop Samples. Food Anal. Method. 2020, 13, 503–515. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruit and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multi-residue method employing acetonictrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Scherbaum, E.; Tasdelen, B.; Stajnbaher, D. Pesticide Chemistry: Crop protection, Public Health, Environmental Safety; Ohkava, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 439–458. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Mastovska, K.; Lightfield, A.R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruit and vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Rajski, Ł.; Lozano, A.; Ucles, A.; Ferrer, C.; Fernandez-Alba, A.R. Detremination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikova, Y.; Lehotay, S.J. Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography-tandem mass spectrometry. Anal. Chim. Acta 2013, 758, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kiljanek, T.; Niewiadomska, A.; Semeniuk, S.; Gaweł, M.; Borzęcka, M.; Posyniak, A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry-Honeybee poisoning incidents. J. Chromatogr. A 2016, 1435, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Lehotay, S.J.; Mastovska, K.; Fernandes, J.O.; Beatriz, M.; Oliveira, P.P. Evaluation of the QuEChERS sample preparation approach for the analysis of pesticide residues in olives. J. Sep. Sci. 2007, 30, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, C.; Miliadis, G.E. Development and validation of an easy multiresidue method for the determination of multiclass pesticide residues uning GC-MS/MS and LC-MS/MS in olive oil and olives. Talanta 2013, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hajslova, J.; Zrostlikova, J. Matrix effects in (ultra)trace analysis of pesticide residues in food and biotic matrices. J. Chromatogr. A 2003, 1000, 181–197. [Google Scholar] [CrossRef] [PubMed]
- PPDB, Pesticide Properties DataBase, University of Hertfordshire, Haltfield, UK. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed on 14 December 2022).
- Skomra, U. Metodyka Integrowanej Ochrony Chmielu (Methodology of Integrated Hop Protection), 1st ed.; Institute of Soil Science and Plant Cultivation, State Research Institute: Pulawy, Poland, 2015; Available online: http://pw.iung.pl/pdf/IUNG_Metodyka_Integrowanej_ochrony_chmielu.pdf (accessed on 20 January 2023).
- SANTE/11813/2017; Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. European Commission: Brussels, Belgium, 2017. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11813_2017-fin.pdf (accessed on 29 August 2019).
- Patzak, J.; Krofta, K.; Henychova, A.; Nesvadba, V. Number and size of lupulin glands, grandular trichomes of hop (Humulus lupulus L.), play a key role in contents of bitter acids and polyphenols in hop cone. Int. J. Food Sci. Technol. 2015, 50, 1864–1872. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Technoques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
| Clean-Up Mixtures | Content of Matrix Co-Extractives (mg/mL) |
|---|---|
| No clean-up | 12.32 ± 0.07 |
| (A) 50 mg MgSO4, 50 mg PSA, 50 mg C18 | 4.50 ± 0.09 |
| (B) 50 mg MgSO4, 50 mg PSA, 50 mg Z-Sep | 4.00 ± 0.14 |
| (C) 50 mg MgSO4, 50 mg PSA, 50 mg Z-Sep+ | 2.62 ± 0.12 |
| (D) 50 mg MgSO4, 50 mg PSA, 50 mg Z-Sep+, 5 mg GCB | 2.37 ± 0.05 |
| Analyte | Number of Samples Containing | Range of Detected Residues (mg/kg) | MRL (mg/kg) |
|---|---|---|---|
| Azoxystrobin | 9 | 0.030–2.2 | 30 |
| Boscalid | 7 | 0.16–9.4 | 80 |
| Pyraclostrobin | 6 | 0.065–2.1 | 15 |
| Trifloxystrobin | 6 | 0.020–0.76 | 40 |
| Spirodiclofen | 3 | 0.29–1.2 | 40 |
| Metalaxyl | 3 | 0.040–0.052 | 15 |
| Mandipropamid | 2 | 0.84–7.3 | 90 |
| Dimethomorph | 1 | 3.4 | 80 |
| Myclobutanil | 1 | 0.98 | 6 |
| Quinoxyfen | 1 | 0.67 | 2 |
| Imidacloprid | 1 | 0.093 | 10 |
| Fenpyroximate | 1 | 0.064 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruba, M.; Jóźwik, E.; Chmiel, M.; Tyśkiewicz, K.; Konkol, M.; Watros, A.; Skalicka-Woźniak, K.; Woźniakowski, G. Multi-Residue Method for Pesticides Determination in Dried Hops by Liquid Chromatography Tandem Mass Spectrometry. Molecules 2023, 28, 4989. https://doi.org/10.3390/molecules28134989
Gruba M, Jóźwik E, Chmiel M, Tyśkiewicz K, Konkol M, Watros A, Skalicka-Woźniak K, Woźniakowski G. Multi-Residue Method for Pesticides Determination in Dried Hops by Liquid Chromatography Tandem Mass Spectrometry. Molecules. 2023; 28(13):4989. https://doi.org/10.3390/molecules28134989
Chicago/Turabian StyleGruba, Marcin, Emilia Jóźwik, Mariusz Chmiel, Katarzyna Tyśkiewicz, Marcin Konkol, Anna Watros, Krystyna Skalicka-Woźniak, and Grzegorz Woźniakowski. 2023. "Multi-Residue Method for Pesticides Determination in Dried Hops by Liquid Chromatography Tandem Mass Spectrometry" Molecules 28, no. 13: 4989. https://doi.org/10.3390/molecules28134989
APA StyleGruba, M., Jóźwik, E., Chmiel, M., Tyśkiewicz, K., Konkol, M., Watros, A., Skalicka-Woźniak, K., & Woźniakowski, G. (2023). Multi-Residue Method for Pesticides Determination in Dried Hops by Liquid Chromatography Tandem Mass Spectrometry. Molecules, 28(13), 4989. https://doi.org/10.3390/molecules28134989







