Understanding the Mechanisms of Action and Effects of Drugs of Abuse
Abstract
1. Introduction
2. Results
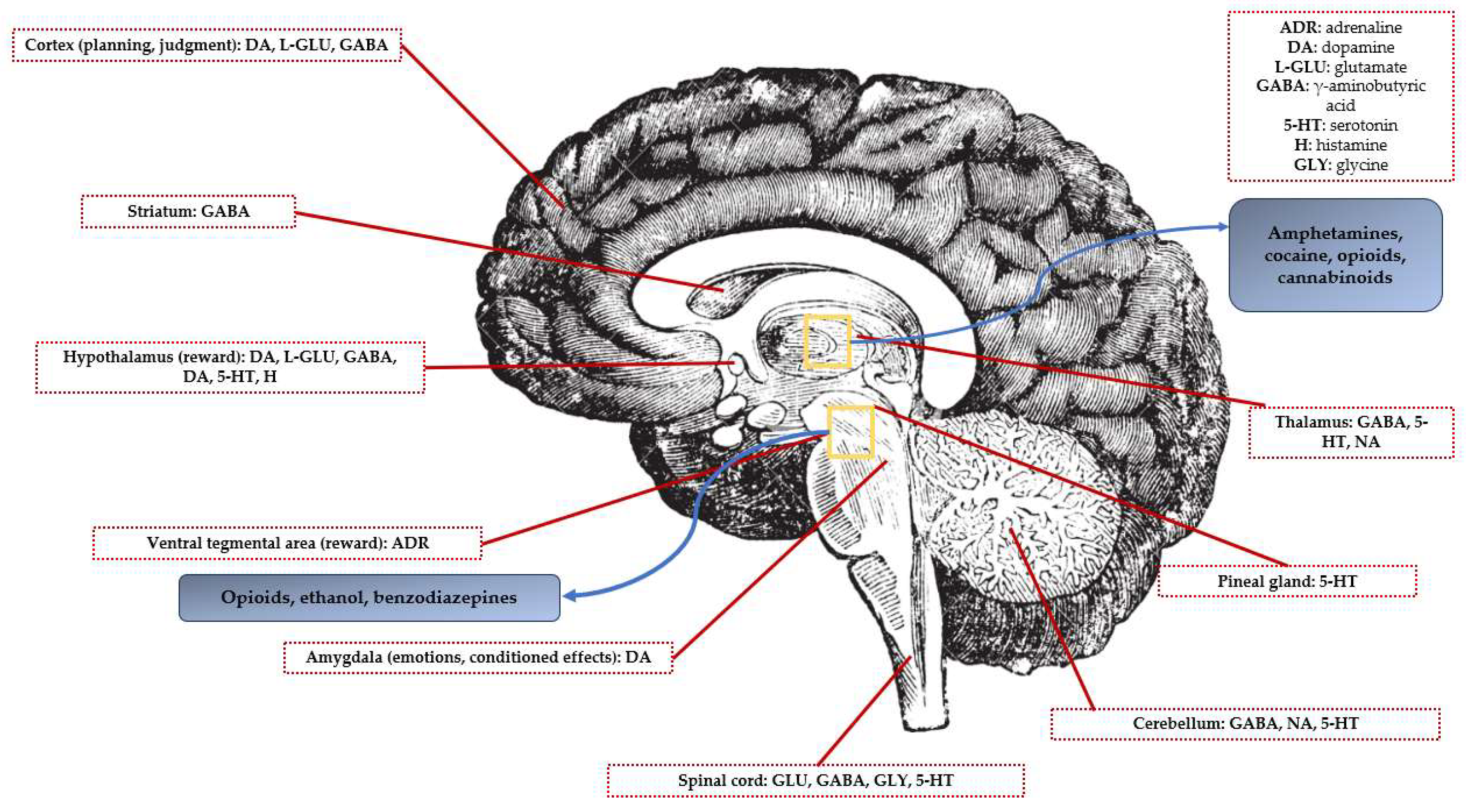
2.1. Targeted Neurotransmitters
2.2. Effects of Drugs of Abuse on the Brain
2.2.1. Behavioral Effects
Depressants
Stimulants
Particular Mechanism
2.2.2. Biochemical Effects
Depressants
Stimulants
Particular Mechanism
2.2.3. Toxic Organic and Functional Effects
2.3. Genetic Susceptibility and Addiction
2.4. Mechanisms of Action and Effects of Drugs of Abuse
2.4.1. Mechanisms of Action
Depressants
Stimulants
Particular Mechanism
2.4.2. Effects of Drugs of Abuse
Acute Effects
- Depressants
- 2.
- Stimulants
- 3.
- Particular mechanism
Chronic Effects
- Depressants
- 2.
- Stimulants
- 3.
- Particular mechanism
Withdrawal Syndrome
- Depressants
- 2.
- Stimulants
- 3.
- Particular mechanism
2.5. Cardiovascular Effects of Drugs of Abuse
3. Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinz, A.; Gül Halil, M.; Gutwinski, S.; Beck, A.; Liu, S. ICD-11: Änderungen der diagnostischen Kriterien der Substanzabhängigkeit [ICD-11: Changes in the diagnostic criteria of substance dependence]. Der Nervenarzt 2022, 93, 51–58. [Google Scholar] [CrossRef]
- WHO Expert Committee on Drug Dependence; Technical Reports Series, No. 407, Sixteenth Report; WHO: Geneva, Switzerland, 1969.
- Rysztak, L.G.; Jutkiewicz, E.M. The role of enkephalinergic systems in substance use disorders. Front. Neurosci. 2022, 16, 932546. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Baranyi, G.; Fazel, S.; Langerfeldt, S.D.; Mundt, A.P. The prevalence of comorbid serious mental illnesses and substance use disorders in prison populations: A systematic review and meta-analysis. Lancet Public Health 2022, 7, e557–e568. [Google Scholar] [CrossRef]
- Dini, G.; Bragazzi, N.L.; Montecucco, A.; Rahmani, A.; Durando, P. Psychoactive drug consumption among truck-drivers: A systematic review of the literature with meta-analysis and meta-regression. J. Prev. Med. Hyg. 2019, 60, E124–E139. [Google Scholar]
- Li, M.C.; Brady, J.E.; DiMaggio, C.J.; Lusardi, A.R.; Tzong, K.Y.; Li, G. Marijuana use and motor vehicle crashes. Epidemiol. Rev. 2012, 34, 65–72. [Google Scholar] [CrossRef]
- Platt, B.; O’Driscoll, C.; Curran, V.H.; Rendell, P.G.; Kamboj, S.K. The effects of licit and illicit recreational drugs on prospective memory: A meta-analytic review. Psychopharmacology 2019, 236, 1131–1143. [Google Scholar] [CrossRef]
- Graham, R.M. Adrenergic receptors: Structure and function. Clevel. Clin. J. Med. 1990, 57, 481–491. [Google Scholar]
- Zhang, H.; Cui, M.; Cao, J.L.; Han, M.H. The Role of Beta-Adrenergic Receptors in Depression and Resilience. Biomedicines 2022, 10, 2378. [Google Scholar] [CrossRef]
- Alhayek, S.; Preuss, C.V. Beta 1 Receptors. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Taylor, B.N.; Cassagnol, M. Alpha Adrenergic Receptors. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Bhatia, A.; Lenchner, J.R.; Saadabadi, A. Biochemistry, Dopamine Receptors. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, Z.; Piao, J.; Cui, R.; Li, B. Dopamine Receptors: Is It Possible to Become a Therapeutic Target for Depression? Front. Pharmacol. 2022, 13, 947785. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Wróbel, T.M.; Bartuzi, D. Allosteric Modulators of Dopamine D2 Receptors for Fine-Tuning of Dopaminergic Neurotransmission in CNS Diseases: Overview, Pharmacology, Structural Aspects and Synthesis. Molecules 2022, 28, 178. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Henter, I.D.; de Sousa, R.T.; Zarate, C.A., Jr. Glutamatergic Modulators in Depression. Harv. Rev. Psychiatry 2018, 26, 307–319. [Google Scholar] [CrossRef]
- Stallard, C.N.; Anoruo, M.; Saadabadi, A. Biochemistry, Glutamate. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Zhu, D.; Wang, X.; Fang, L.; Zhong, J.; Mao, Q.; Sun, L.; Gong, X.; Xia, J.; et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry 2016, 16, 319. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Filizola, M. Opioid receptors: Structural and mechanistic insights into pharmacology and signaling. Eur. J. Pharmacol. 2015, 763 Pt B, 206–213. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Rego, A.C.; Carvalho, F.; Oliveira, C. Chapter 17—Medical Toxicology of Drugs of Abuse. In Principles of Addiction; Miller, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 159–175. [Google Scholar]
- Devoto, F.; Zapparoli, L.; Spinelli, G.; Scotti, G.; Paulesu, E. How the harm of drugs and their availability affect brain reactions to drug cues: A meta-analysis of 64 neuroimaging activation studies. Transl. Psychiatry 2020, 10, 429. [Google Scholar] [CrossRef]
- Chou, T.; D’Orsogna, M.R. A mathematical model of reward-mediated learning in drug addiction. Chaos 2022, 32, 021102. [Google Scholar] [CrossRef]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef]
- Egenrieder, L.; Mitricheva, E.; Spanagel, R.; Noori, H.R. No basal or drug-induced sex differences in striatal dopaminergic levels: A cluster and meta-analysis of rat microdialysis studies. J. Neurochem. 2020, 152, 482–492. [Google Scholar] [CrossRef]
- Long, Y.; Pan, N.; Ji, S.; Qin, K.; Chen, Y.; Zhang, X.; He, M.; Suo, X.; Yu, Y.; Wang, S.; et al. Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: A comparative meta-analysis. Transl. Psychiatry 2022, 12, 368. [Google Scholar] [CrossRef]
- Spindler, C.; Mallien, L.; Trautmann, S.; Alexander, N.; Muehlhan, M. A coordinate-based meta-analysis of white matter alterations in patients with alcohol use disorder. Transl. Psychiatry 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.A.; Jones, A.; Montgomery, C. Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users. Neurosci. Biobehav. Rev. 2016, 63, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Duperrouzel, J.C.; Granja, K.; Pacheco-Colón, I.; Gonzalez, R. Adverse Effects of Cannabis Use on Neurocognitive Functioning: A Systematic Review of Meta- Analytic Studies. J. Dual Diagn. 2020, 16, 43–57. [Google Scholar] [CrossRef]
- Rocchetti, M.; Crescini, A.; Borgwardt, S.; Caverzasi, E.; Politi, P.; Atakan, Z.; Fusar-Poli, P. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin. Neurosci. 2013, 67, 483–492. [Google Scholar] [CrossRef]
- Chen, Y.I.; Choi, J.K.; Xu, H.; Ren, J.; Andersen, S.L.; Jenkins, B.G. Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev. Neurosci. 2010, 32, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Bonnet, U.; Thomasius, R.; Ganzer, F.; Havemann-Reinecke, U.; Preuss, U.W. Risks associated with the non-medicinal use of cannabis. Dtsch. Arztebl. Int. 2015, 112, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gowin, J.L.; Mackey, S.; Paulus, M.P. Altered risk-related processing in substance users: Imbalance of pain and gain. Drug Alcohol Depend. 2013, 132, 13–21. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Perlman, G.; Moeller, S.J. Meta-analysis of aberrant post-error slowing in substance use disorder: Implications for behavioral adaptation and self-control. Eur. J. Neurosci. 2019, 50, 2467–2476. [Google Scholar] [CrossRef]
- Thoma, P.; Daum, I. Comorbid substance use disorder in schizophrenia: A selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin. Neurosci. 2013, 67, 367–383. [Google Scholar] [CrossRef]
- Viola, T.W.; Orso, R.; Florian, L.F.; Garcia, M.G.; Gomes, M.G.S.; Mardini, E.M.; Niederauer, J.P.O.; Zaparte, A.; Grassi-Oliveira, R. Effects of substance use disorder on oxidative and antioxidative stress markers: A systematic review and meta-analysis. Addict. Biol. 2023, 28, e13254. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhou, W.Z.; Zhang, P.W.; Johnson, C.; Wei, L.; Uhl, G.R. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genom. 2011, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, H.; Gelernter, J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol. Psychiatry 2011, 70, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sulovari, A.; Cheng, C.; Zhao, H.; Kranzler, H.R.; Gelernter, J. Association of gamma-aminobutyric acid A receptor α2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology 2014, 39, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Stringer, S.; Minică, C.C.; Verweij, K.J.; Mbarek, H.; Bernard, M.; Derringer, J.; van Eijk, K.R.; Isen, J.D.; Loukola, A.; Maciejewski, D.F.; et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl. Psychiatry 2016, 6, e769. [Google Scholar] [CrossRef]
- Clarke, T.K.; Bloch, P.J.; Ambrose-Lanci, L.M.; Ferraro, T.N.; Berrettini, W.H.; Kampman, K.M.; Dackis, C.A.; Pettinati, H.M.; O’Brien, C.P.; Oslin, D.W.; et al. Further evidence for association of polymorphisms in the CNR1 gene with cocaine addiction: Confirmation in an independent sample and meta-analysis. Addict. Biol. 2013, 18, 702–708. [Google Scholar] [CrossRef]
- Cao, J.; LaRocque, E.; Li, D. Associations of the 5-hydroxytryptamine (serotonin) receptor 1B gene (HTR1B) with alcohol, cocaine, and heroin abuse. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162, 169–176. [Google Scholar] [CrossRef]
- Cao, J.; Liu, X.; Han, S.; Zhang, C.K.; Liu, Z.; Li, D. Association of the HTR2A gene with alcohol and heroin abuse. Hum. Genet. 2014, 133, 357–365. [Google Scholar] [CrossRef]
- Ciucă-Anghel, M.D.; Stan, M.; Bălălău, C.; Anghel, E.E.; Bălălău, D.; Paunica, I.; Dimitriu, A.S.; Paunica, S.; Baconi, D.L. Adverse effects of new psychoactive drug use. Psychological insights of addiction. Mediterr. J. Clin. Psychol. 2022, 10, 1–22. [Google Scholar]
- Honkalampi, K.; Jokela, M.; Lehto, S.M.; Kivimäki, M.; Virtanen, M. Association between alexithymia and substance use: A systematic review and meta-analysis. Scand. J. Psychol. 2022, 63, 427–438. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report; Sales No. E.15.XI; United Nations Publication: New York, NY, USA, 2015. [Google Scholar]
- Cristea, A.N. Tratat de Farmacologie, 1st ed.; Editura Medicală: Bucharest, Romania, 2011. [Google Scholar]
- Cong, X.; Maurel, D.; Déméné, H.; Vasiliauskaité-Brooks, I.; Hagelberger, J.; Peysson, F.; Saint-Paul, J.; Golebiowski, J.; Granier, S.; Sounier, R. Molecular insights into the biased signaling mechanism of the μ-opioid receptor. Mol. Cell 2021, 81, 4165–4175.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Historical Review: Opiate Addiction and Opioid Receptors. Cell. Transplant. 2019, 28, 233–238. [Google Scholar] [CrossRef]
- Costardi, J.V.; Nampo, R.A.; Silva, G.L.; Ribeiro, M.A.; Stella, H.J.; Stella, M.B.; Malheiros, S.V. A review on alcohol: From the central action mechanism to chemical dependency. Rev. Assoc. Med. Bras. 2015, 61, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.T.; Bajo, M.; Schweitzer, P.; Roberto, M. Shared Mechanisms of Alcohol and Other Drugs. Alcohol Res. Health 2008, 31, 137–147. [Google Scholar]
- Tasnim, S.; Tang, C.; Musini, V.M.; Wright, J.M. Effect of alcohol on blood pressure. Cochrane Database Syst. Rev. 2020, 7, CD012787. [Google Scholar] [CrossRef]
- Wisden, W.; Yu, X.; Franks, N.P. GABA Receptors and the Pharmacology of Sleep. Handb. Exp. Pharmacol. 2019, 253, 279–304. [Google Scholar]
- Flanagan, R.J.; Ruprah, M.; Meredith, T.J.; Ramsey, J.D. An introduction to the clinical toxicology of volatile substances. Drug Safety 1990, 5, 359–383. [Google Scholar] [CrossRef]
- Cocchi, V.; Gasperini, S.; Hrelia, P.; Tirri, M.; Marti, M.; Lenzi, M. Novel Psychoactive Phenethylamines: Impact on Genetic Material. Int. J. Mol. Sci. 2020, 21, 9616. [Google Scholar] [CrossRef]
- Heal, D.J.; Smith, S.L.; Gosden, J.; Nutt, D.J. Amphetamine, past and present—A pharmacological and clinical perspective. J. Psychopharmacol. 2013, 27, 479–496. [Google Scholar] [CrossRef]
- Müller, F.; Brändle, R.; Liechti, M.E.; Borgwardt, S. Neuroimaging of chronic MDMA (“ecstasy”) effects: A meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.; Margolis, S.; de Wit, H.; Lyubomirsky, S. Does ±3,4-methylenedioxymethamphetamine (ecstasy) induce subjective feelings of social connection in humans? A multilevel meta-analysis. PLoS ONE 2021, 16, e0258849. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Rosca, P.; Fattore, L.; London, E.D. Synthetic Cathinone and Cannabinoid Designer Drugs Pose a Major Risk for Public Health. Front. Psychiatry 2017, 8, 156. [Google Scholar] [CrossRef]
- Gebrie, A.; Alebel, A.; Zegeye, A.; Tesfaye, B. Prevalence and predictors of khat chewing among Ethiopian university students: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195718. [Google Scholar] [CrossRef] [PubMed]
- Roque Bravo, R.; Faria, A.C.; Brito-da-Costa, A.M.; Carmo, H.; Mladěnka, P.; Dias da Silva, D.; Remião, F.; on behalf of the Oemonom Researchers. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- Wee, S.; Orio, L.; Ghirmai, S.; Cashman, J.R.; Koob, G.F. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology 2009, 205, 565–575. [Google Scholar] [CrossRef]
- De Sa Nogueira, D.; Bourdy, R.; Filliol, D.; Romieu, P.; Befort, K. Hippocampal mu opioid receptors are modulated following cocaine self-administration in rat. Eur. J. Neurosci. 2021, 53, 3341–3349. [Google Scholar] [CrossRef]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Gress, K.; Charipova, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Hasoon, J.; Kassem, H.; Kaye, A.D.; Viswanath, O. Cannabis Use and its Association with Psychological Disorders. Psychopharmac. Bull. 2020, 50, 56–67. [Google Scholar]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol. Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- Howlett, A.C.; Johnson, M.R.; Melvin, L.S.; Milne, G.M. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: Development of a cannabinoid receptor model. Mol. Pharmacol. 1988, 33, 297–302. [Google Scholar] [PubMed]
- Gunderson, E.W.; Haughey, H.M.; Ait-Daoud, N.; Joshi, A.S.; Hart, C.L. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am. J. Addict. 2012, 21, 320–326. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic serotonergic psychedelics for mood and depressive symptoms: A meta-analysis of mood disorder patients and healthy participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Therap. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E. Modern Clinical Research on LSD. Neuropsychopharmacology 2017, 42, 2114–2127. [Google Scholar] [CrossRef]
- Miliano, C.; Serpelloni, G.; Rimondo, C.; Mereu, M.; Marti, M.; De Luca, M.A. Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 2016, 10, 153. [Google Scholar] [CrossRef]
- Florou, D.; Boumba, V.A. Hair analysis for New Psychoactive Substances (NPS): Still far from becoming the tool to study NPS spread in the community? Toxicol. Rep. 2021, 8, 1699–1720. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
- Baconi, D.; Bălălău, D.; Abraham, P. Abuzul și Toxicodependența. Mecanisme, Manifestări, Tratament, Legislație; Editura Medicală: Bucharest, Romania, 2008. [Google Scholar]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11 (Suppl. 2), S133–S153. [Google Scholar] [CrossRef] [PubMed]
- Krantz, M.J.; Palmer, R.B.; Haigney, M. Cardiovascular Complications of Opioid Use: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 205–223. [Google Scholar] [CrossRef]
- Chikritzhs, T.; Livingston, M. Alcohol and the Risk of Injury. Nutrients 2021, 13, 2777. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N. Baclofen for alcohol withdrawal. Cochrane Database Syst. Rev. 2019, 2019, CD008502. [Google Scholar] [CrossRef]
- Petursson, H.; Lader, M.H. Withdrawal from long-term benzodiazepine treatment. BMJ 1981, 283, 643–645. [Google Scholar] [CrossRef]
- Nelson, J.; Chouinard, G. Guidelines for the clinical use of benzodiazepines: Pharmacokinetics, dependency, rebound and withdrawal. Canadian Society for Clinical Pharmacology. Can. J. Physiol. Pharmacol. 1999, 6, 69–83. [Google Scholar]
- Howard, P.; Twycross, R.; Shuster, J.; Mihalyo, M.; Wilcock, A. Benzodiazepines. J. Pain Symptom Manage. 2014, 47, 955–964. [Google Scholar] [CrossRef]
- Andrade, A.L.M.; De Micheli, D.; da Silva, E.A.; Lopes, F.M.; de Oliveira Pinheiro, B.; Reichert, R.A. (Eds.) Psychology of Substance Abuse: Psychotherapy, Clinical Management and Social Intervention; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Wiener, R.C.; Waters, C.; Bhandari, R.; Shockey, A. Epidemiology and Characteristics of People with Injury Due to Volatile Substance Use to Induce Euphoria. Subst. Use Misuse 2021, 56, 169. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Świt, P.; Malek, K. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOME): A Harmful Hallucinogen Review. J. Anal. Toxicol. 2021, 44, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamines: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine, and D-amphetamine. J. Biol. Chem. 2011, 392, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Harro, J. Neuropsychiatric Adverse Effects of Amphetamine and Methamphetamine. Int. Rev. Neurobiol. 2015, 120, 179–204. [Google Scholar] [PubMed]
- Bickel, W.K.; Quisenberry, A.J.; Snider, S.E. Does impulsivity change rate dependently following stimulant administration? A translational selective review and re-analysis. Psychopharmacology 2016, 233, 1–18. [Google Scholar] [CrossRef]
- Studerus, E.; Vizeli, P.; Harder, S.; Ley, L.; Liechti, M.E. Prediction of MDMA response in healthy humans: A pooled analysis of placebo-controlled studies. J. Psychopharmacol. 2021, 35, 556–565. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef] [PubMed]
- Penders, T.M.; Gestring, R.E.; Vilensky, D.A. Excited delirium following use of synthetic cathinones (bath salts). Gen. Hosp. Psychiatry 2012, 34, 647–650. [Google Scholar] [CrossRef]
- John, M.E.; Thomas-Rozea, C.; Hahn, D. Bath Salts Abuse Leading to New-Onset Psychosis and Potential for Violence. Clin. Schizophr. Relat. Psychoses 2017, 11, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ciucă Anghel, D.-M.; Anghel, E.-E.; Stan, M.; Tudor, G.; Dumitriu, A.S.; Paunica, S.; Baconi, D.L. Psychological and psychiatric characterization of various groups of drugs users. J. Mind Med. Sci. 2022, 9, 8. [Google Scholar] [CrossRef]
- Edwards, B.; Atkins, N. Exploring the association between khat use and psychiatric symptoms: A systematic review. BMJ Open 2022, 12, e061865. [Google Scholar] [CrossRef]
- Ganesh, S.; Cortes-Briones, J.; Ranganathan, M.; Radhakrishnan, R.; Skosnik, P.D.; D’Souza, D.C. Psychosis-Relevant Effects of Intravenous Delta-9-Tetrahydrocannabinol: A Mega Analysis of Individual Participant-Data from Human Laboratory Studies. Int. J. Neuropsychopharmacol. 2020, 23, 559–570. [Google Scholar] [CrossRef]
- Patel, J.; Marwaha, R. Cannabis Use Disorder. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Iyalomhe, G.B. Cannabis abuse and addiction: A contemporary literature review. Niger. J. Med. 2009, 18, 128–133. [Google Scholar] [CrossRef]
- Hindley, G.; Beck, K.; Borgan, F.; Ginestet, C.E.; McCutcheon, R.; Kleinloog, D.; Ganesh, S.; Radhakrishnan, R.; D’Souza, D.C.; Howes, O.D. Psychiatric symptoms caused by cannabis constituents: A systematic review and meta-analysis. Lancet Psychiatry 2020, 7, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.; Di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef]
- Jett, J.; Stone, E.; Warren, G.; Cummings, K.M. Cannabis Use, Lung Cancer, and Related Issues. J. Thorac. Oncol. 2018, 13, 480–487. [Google Scholar] [CrossRef]
- Adamowicz, P.; Gieroń, J. Acute intoxication of four individuals following use of the synthetic cannabinoid MAB-CHMINACA. Clin. Toxicol. 2016, 54, 650–654. [Google Scholar] [CrossRef]
- Katz, K.D.; Leonetti, A.L.; Bailey, B.C.; Surmaitis, R.M.; Eustice, E.R.; Kacinko, S.; Wheatley, S.M. Case Series of Synthetic Cannabinoid Intoxication from One Toxicology Center. West. J. Emerg. Med. 2016, 17, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Armenian, P.; Darracq, M.; Gevorkyan, J.; Clark, S.; Kaye, B.; Brandehoff, N.P. Intoxication from the novel synthetic cannabinoids AB-PINACA and ADB-PINACA: A case series and review of the literature. Neuropharmacology 2018, 134 Pt A, 82–91. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Müller, D.; Kithinji, J.; Angerer, V.; Franz, F.; Eyer, F.; Neurath, H.; Liebetrau, G.; Auwärter, V. Acute side effects after consumption of the new synthetic cannabinoids AB-CHMINACA and MDMB-CHMICA. Clin. Toxicol. 2018, 56, 404–411. [Google Scholar] [CrossRef]
- Lessin, A.W.; Long, R.F.; Parkes, M.W. Central stimulant actions of alpha-alkyl substituted tryptamines in mice. Br. J. Pharmacol. Chemother. 1965, 24, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Shechet, B.; Nicholas, C.R.; Ng, C.W.; Deole, G.; Chen, Z.; Raison, C.L. Post-acute psychological effects of classical serotonergic psychedelics: A systematic review and meta-analysis. Psychol. Med. 2020, 50, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Dini, G.; Toletone, A.; Rahmani, A.; Montecucco, A.; Massa, E.; Manca, A.; Guglielmi, O.; Garbarino, S.; Debarbieri, N.; et al. Patterns of Harmful Alcohol Consumption among Truck Drivers: Implications for Occupational Health and Work Safety from a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1121. [Google Scholar] [CrossRef]
- Rehm, J.; Rovira, P.; Llamosas-Falcón, L.; Shield, K.D. Dose-Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients 2021, 13, 2652. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef]
- Roozen, S.; Peters, G.Y.; Kok, G.; Townend, D.; Nijhuis, J.; Koek, G.; Curfs, L. Systematic literature review on which maternal alcohol behaviours are related to fetal alcohol spectrum disorders (FASD). BMJ Open 2018, 8, e022578. [Google Scholar] [CrossRef]
- Baandrup, L.; Ebdrup, B.H.; Rasmussen, J.Ø.; Lindschou, J.; Gluud, C.; Glenthøj, B.Y. Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users. Cochrane Database Syst. Rev. 2018, 3, CD011481. [Google Scholar] [CrossRef] [PubMed]
- Hennissen, L.; Bakker, M.J.; Banaschewski, T.; Carucci, S.; Coghill, D.; Danckaerts, M.; Dittmann, R.W.; Hollis, C.; Kovshoff, H.; McCarthy, S.; et al. Cardiovascular Effects of Stimulant and Non-Stimulant Medication for Children and Adolescents with ADHD: A Systematic Review and Meta-Analysis of Trials of Methylphenidate, Amphetamines and Atomoxetine. CNS Drugs 2017, 31, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.; Theunissen, E.L.; van Wel, J.H.; de Sousa Fernandes Perna, E.B.; Linssen, A.; Sambeth, A.; Schultz, B.G.; Ramaekers, J.G. Verbal Memory Impairment in Polydrug Ecstasy Users: A Clinical Perspective. PLoS ONE 2016, 11, e0149438. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.; de la Torre, R.; Farre, M.; Pujadas, M.; Ramaekers, J.G. Inhibition of MDMA-induced increase in cortisol does not prevent acute impairment of verbal memory. Br. J. Pharmacol. 2013, 168, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.G.; Wagner, M.; Jessen, F.; Kühn, K.U.; Joe, A.; Seifritz, E.; Maier, W.; Biersack, H.J.; Quednow, B.B. Verbal memory deficits are correlated with prefrontal hypometabolism in (18)FDG PET of recreational MDMA users. PLoS ONE 2013, 8, e61234. [Google Scholar] [CrossRef]
- Keboa, M.T.; Enriquez, N.; Martel, M.; Nicolau, B.; Macdonald, M.E. Oral Health Implications of Cannabis Smoking: A Rapid Evidence Review. J. Can. Dent. Assoc. 2020, 86, k2. [Google Scholar]
- Kozak, K.; Smith, P.H.; Lowe, D.J.E.; Weinberger, A.H.; Cooper, Z.D.; Rabin, R.A.; George, T.P. A systematic review and meta-analysis of sex differences in cannabis use disorder amongst people with comorbid mental illness. Am. J. Drug Alcohol Abuse 2021, 47, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.; Cotter, J.; Firth, J.; Bradshaw, T.; Yung, A.R. Cannabis use and symptom severity in individuals at ultra high risk for psychosis: A meta-analysis. Acta Psychiatr. Scand. 2017, 136, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.; Winsper, C.; Marwaha, S.; Gilbert, E.; Broome, M.; Singh, S.P. Cannabis use and mania symptoms: A systematic review and meta-analysis. J. Affect. Disord. 2015, 171, 39–47. [Google Scholar] [CrossRef]
- Sabe, M.; Zhao, N.; Kaiser, S. Cannabis, nicotine and the negative symptoms of schizophrenia: Systematic review and meta-analysis of observational studies. Neurosci. Biobehav. Rev. 2020, 116, 415–425. [Google Scholar] [CrossRef]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, K.K.; Laeber, L.T. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population—A meta-analysis of 31 studies. BMC Psychiatry 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.; Shaw, C.; Stanley, J.; Signal, V.; Sarfati, D. Cannabis exposure and risk of testicular cancer: A systematic review and meta-analysis. BMC Cancer 2015, 15, 897. [Google Scholar] [CrossRef]
- Belladelli, F.; Del Giudice, F.; Kasman, A.; Kold Jensen, T.; Jørgensen, N.; Salonia, A.; Eisenberg, M.L. The association between cannabis use and testicular function in men: A systematic review and meta-analysis. Andrology 2021, 9, 503–510. [Google Scholar] [CrossRef]
- Pizzol, D.; Demurtas, J.; Stubbs, B.; Soysal, P.; Mason, C.; Isik, A.T.; Solmi, M.; Smith, L.; Veronese, N. Relationship Between Cannabis Use and Erectile Dysfunction: A Systematic Review and Meta-Analysis. Am. J. Men’s Health 2019, 13, 1557988319892464. [Google Scholar] [CrossRef]
- Zeraatkar, D.; Cooper, M.A.; Agarwal, A.; Vernooij, R.W.M.; Leung, G.; Loniewski, K.; Dookie, J.E.; Ahmed, M.M.; Hong, B.Y.; Hong, C.; et al. Long-term and serious harms of medical cannabis and cannabinoids for chronic pain: A systematic review of non-randomised studies. BMJ Open 2022, 12, e054282. [Google Scholar] [CrossRef]
- Gunn, J.K.; Rosales, C.B.; Center, K.E.; Nuñez, A.; Gibson, S.J.; Christ, C.; Ehiri, J.E. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016, 6, e009986. [Google Scholar] [CrossRef]
- Skipina, T.M.; Upadhya, B.; Soliman, E.Z. Cannabis Use and Electrocardiographic Myocardial Injury. Am. J. Cardiol. 2021, 151, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ciucă Anghel, D.-M.; Vlăsceanu, A.-M.; Tudor, G.; Ciobanu, A.-M.; Stan, M.; Nițescu, G.V.; Florou, D.; Tsatsakis, A.M.; Baconi, D.L. Characterization Of Various Groups Of Drugs Users. Highlights On Legal Highs Users. Farmacia 2023, 71, 18–28. [Google Scholar] [CrossRef]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of Synthetic Cathinones. Handb. Exp. Pharmacol. 2018, 252, 113–142. [Google Scholar]
- Aydin Sunbul, E.; Sunbul, M.; Terzi, A.; Calli, S.; Koca, E.; Bilici, R.; Citak, S. The Effect of Synthetic Cannabinoids on P-Wave Dispersion: An Observational Study. Med. Princ. Pract. 2016, 25, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, H.M.; Erdogan, M.; Alsancak, Y.; Yarlioglues, M.; Duran, M.; Boztas, M.H.; Murat, S.N.; Ozturk, S. Electrocardiographic alterations in patients consuming synthetic cannabinoids. J. Psychopharmacol. 2018, 32, 296–301. [Google Scholar] [CrossRef]
- McKeever, R.G.; Vearrier, D.; Jacobs, D.; LaSala, G.; Okaneku, J.; Greenberg, M.I. K2—Not the spice of life; synthetic cannabinoids and ST elevation myocardial infarction: A case report. J. Med. Toxicol. 2015, 11, 129–131. [Google Scholar] [CrossRef]
- Guimarães, F.; Camões, J.; Pereira, M.; Araujo, R. Cannabinoids: A Cause of Severe Bradycardia. Cureus 2021, 13, e16560. [Google Scholar] [CrossRef]
- Sivagnanam, K.; Chaudari, D.; Lopez, P.; Sutherland, M.E.; Ramu, V.K. “Bath salts” induced severe reversible cardiomyopathy. Am. J. Case Rep. 2013, 14, 288–291. [Google Scholar] [PubMed]
- Efe, T.H.; Felekoglu, M.A.; Çimen, T.; Doğan, M. Atrial fibrillation following synthetic cannabinoid abuse. Turk Kardiyol Dern Ars 2017, 45, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. Heroin dependence. WMJ 2004, 103, 20–26. [Google Scholar]
- Hosztafi, S. A heroin addikció [Heroin addiction]. Acta Pharm. Hung. 2011, 81, 173–183. [Google Scholar] [PubMed]
- Leung, J.; Santo, T.; Colledge-Frisby, S.; Mekonen, T.; Thomson, K.; Degenhardt, L.; Connor, J.P.; Hall, W.; Stjepanović, D. Mood and Anxiety Symptoms in Persons Taking Prescription Opioids: A Systematic Review with Meta-Analyses of Longitudinal Studies. Pain Med. 2022, 23, 1442–1456. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, M.; Schwan, R.; Loiseaux-Meunier, M.N.; Albuisson, E.; Deteix, P. Patients admitted to emergency services for drunkenness: Moderate alcohol users or harmful drinkers? Am. J. Psychiatry 2001, 158, 96–99. [Google Scholar] [CrossRef]
- Martel, M.L.; Klein, L.R.; Lichtenheld, A.J.; Kerandi, A.M.; Driver, B.E.; Cole, J.B. Etiologies of altered mental status in patients with presumed ethanol intoxication. Am. J. Emerg. Med. 2018, 36, 1057–1059. [Google Scholar] [CrossRef]
- Wood, E.; Albarqouni, L.; Tkachuk, S.; Green, C.J.; Ahamad, K.; Nolan, S.; McLean, M.; Klimas, J. Will This Hospitalized Patient Develop Severe Alcohol Withdrawal Syndrome?: The Rational Clinical Examination Systematic Review. JAMA 2018, 320, 825–833. [Google Scholar] [CrossRef]
- Wøien, V.A.; Horwitz, H.; Høgberg, L.C.; Askaa, B.; Jürgens, G. Cannabismisbrug og dets konsekvenser [Cannabis–abuse and consequences]. Ugeskr. Laeg. 2015, 177, V04140228. [Google Scholar]
- Mashhoon, Y.; Sagar, K.A.; Gruber, S.A. Cannabis Use and Consequences. Pediatr. Clin. N. Am. 2019, 66, 1075–1086. [Google Scholar] [CrossRef]
- Bahji, A.; Stephenson, C.; Tyo, R.; Hawken, E.R.; Seitz, D.P. Prevalence of Cannabis Withdrawal Symptoms Among People With Regular or Dependent Use of Cannabinoids: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e202370. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; Daminello-Raimundo, R.; de Abreu, L.C. Main artifacts in electrocardiography. Ann. Noninvasive Electrocardiol. 2018, 23, e12494. [Google Scholar] [CrossRef]
- Harkness, W.T.; Hicks, M. Right Bundle Branch Block. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2021. [Google Scholar]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; de Rezende Barbosa, M.; Daminello-Raimundo, R.; de Abreu, L.C.; Nikus, K. Left bundle branch block: Epidemiology, etiology, anatomic features, electrovectorcardiography, and classification proposal. Ann. Noninvasive Electrocardiol. 2019, 24, e12572. [Google Scholar] [CrossRef] [PubMed]
- Scherbak, D.; Hicks, G.J. Left Bundle Branch Block. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2021. [Google Scholar]
- Schamroth, L.; Chesler, E. Phasic aberrant ventricular conduction. Br. Heart J. 1963, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kulbertus, H.E.; de Laval-Rutten, F.; Casters, P. Vectorcardiographic study of aberrant conduction anterior displacement of QRS: Another form of intraventricular block. Br. Heart J. 1976, 38, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, Y.; Grossman, S.A. Sinus Bradycardia. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2021. [Google Scholar]
- Bramwell, C. Tachycardia. BMJ 1953, 1, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Ziccardi, M.; Goyal, A.; Maani, C.V. Atrial Flutter. In StatPearls; StatPearls Publishing: Orlando, FL, USA, 2021. [Google Scholar]
- Wallner, C.; Stöllberger, C.; Hlavin, A.; Finsterer, J.; Hager, I.; Hermann, P. Electrocardiographic abnormalities in opiate addicts. Addiction 2008, 103, 1987–1993. [Google Scholar] [CrossRef]



| Receptor/System | Abuse Substance (Types of Receptors) |
|---|---|
| Opioidergic | Heroin (µ, κ, δ), alcohol (µ), cocaine (µ, κ, Σ); |
| GABAminergic | Alcohol (GABAA, GABAC), benzodiazepines (GABA2α, GABA1α); |
| Serotoninergic | Synthetic Cathinones (serotonin transporters 5-HT), LSD, amphetamines (5-HT1A, 5-HT2A, 5-HT2C), alcohol (5-HT3); |
| Dopaminergic | Synthetic Cathinones (dopamine transporters DAT), LSD (D2, D3), amphetamines, alcohol; |
| Adrenergic | Synthetic Cathinones (norepinephrine transporters NET), LSD (α2), amphetamines, cocaine; |
| Cannabimimetic | Synthetic cannabinoids (CB1, CB2), THC (CB1, CB2), alcohol. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciucă Anghel, D.-M.; Nițescu, G.V.; Tiron, A.-T.; Guțu, C.M.; Baconi, D.L. Understanding the Mechanisms of Action and Effects of Drugs of Abuse. Molecules 2023, 28, 4969. https://doi.org/10.3390/molecules28134969
Ciucă Anghel D-M, Nițescu GV, Tiron A-T, Guțu CM, Baconi DL. Understanding the Mechanisms of Action and Effects of Drugs of Abuse. Molecules. 2023; 28(13):4969. https://doi.org/10.3390/molecules28134969
Chicago/Turabian StyleCiucă Anghel, Daniela-Mădălina, Gabriela Viorela Nițescu, Andreea-Taisia Tiron, Claudia Maria Guțu, and Daniela Luiza Baconi. 2023. "Understanding the Mechanisms of Action and Effects of Drugs of Abuse" Molecules 28, no. 13: 4969. https://doi.org/10.3390/molecules28134969
APA StyleCiucă Anghel, D.-M., Nițescu, G. V., Tiron, A.-T., Guțu, C. M., & Baconi, D. L. (2023). Understanding the Mechanisms of Action and Effects of Drugs of Abuse. Molecules, 28(13), 4969. https://doi.org/10.3390/molecules28134969







