Improvement of Luminescence Properties of Eulytite Single-Phase White Emitting Ca3Bi (PO4)3: Ce3+/Dy3+ Phosphor
Abstract
:1. Introduction
2. Results and Discussion
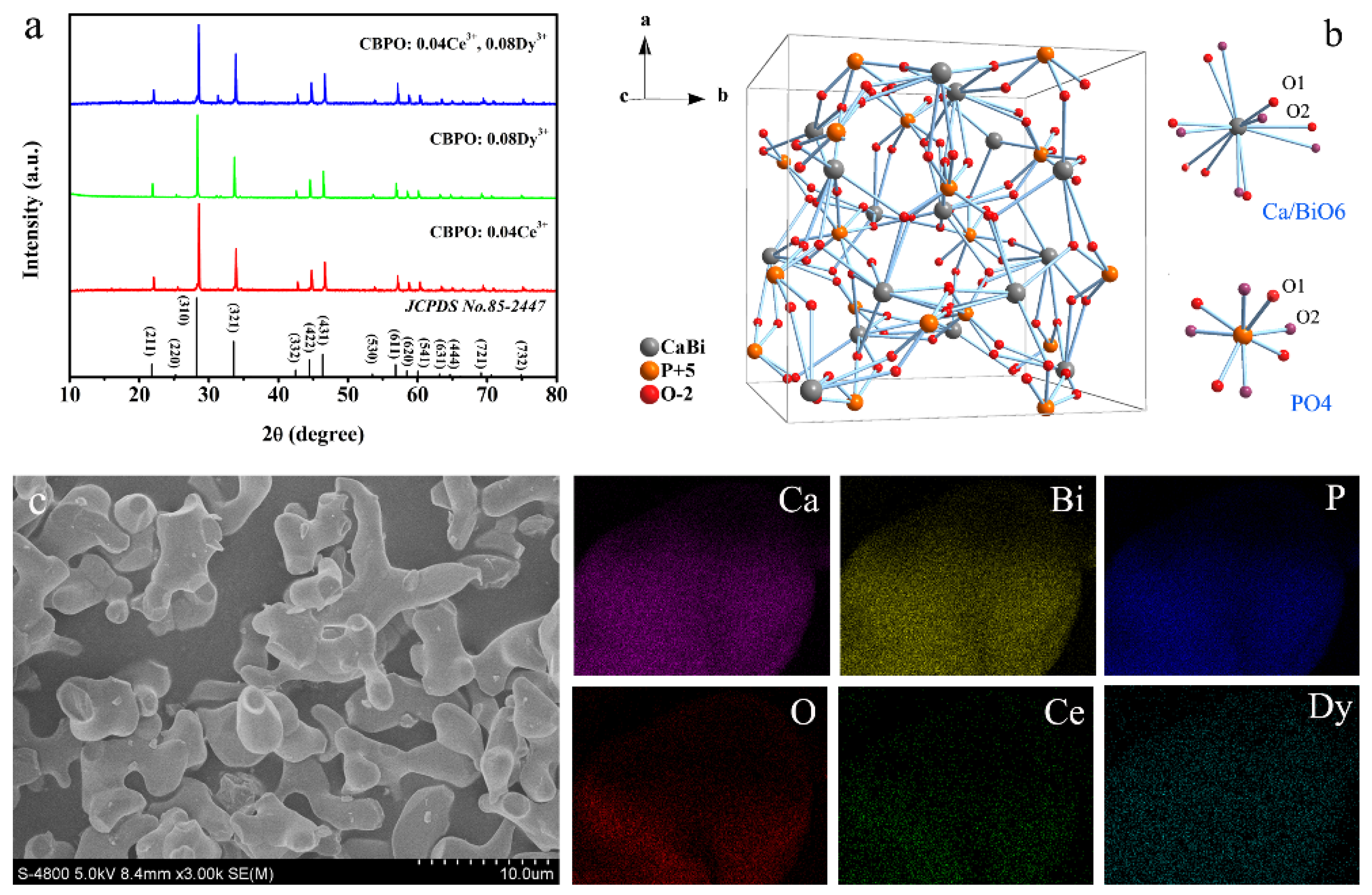
2.1. Phase, Structure, and Morphology Analysis
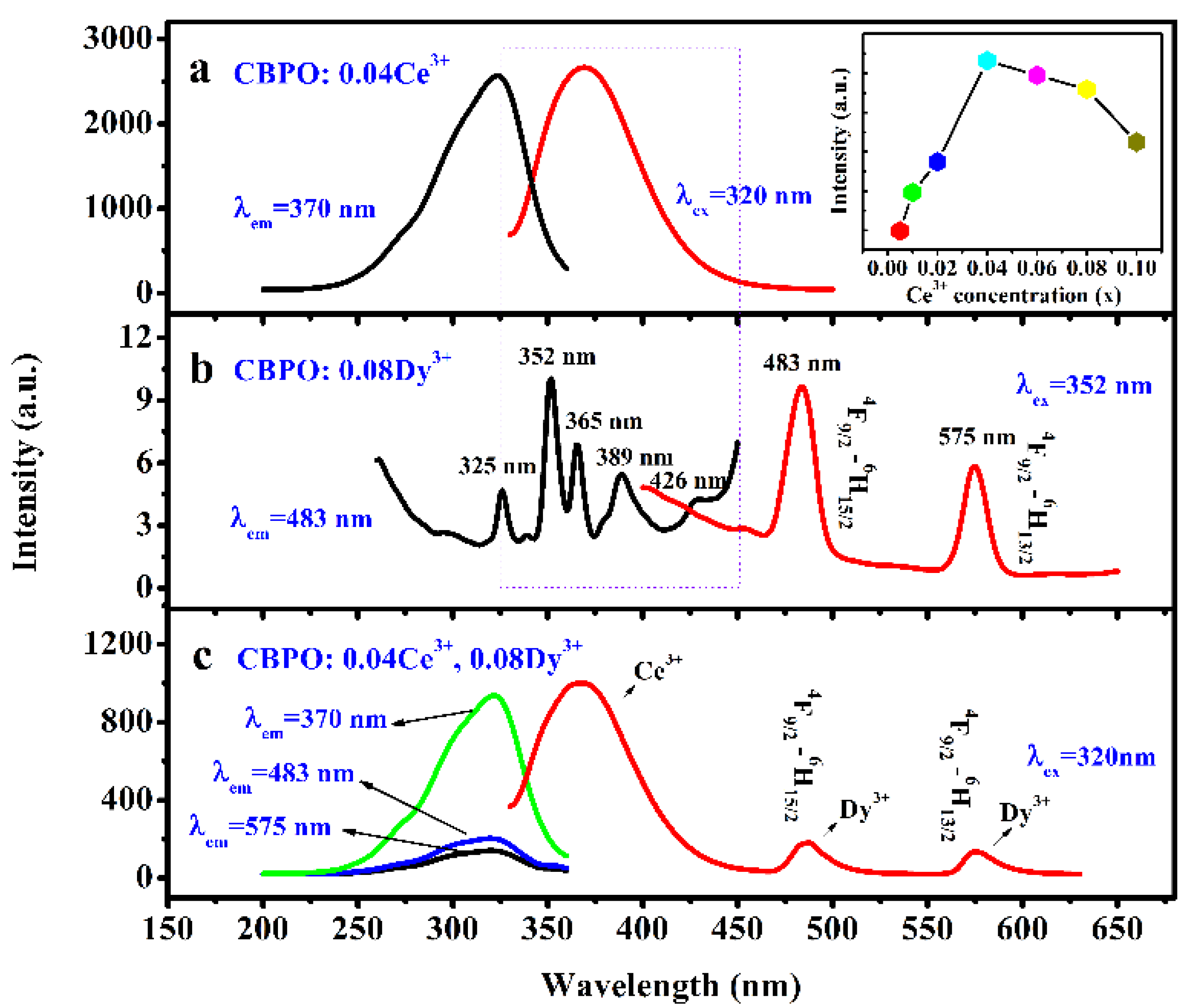
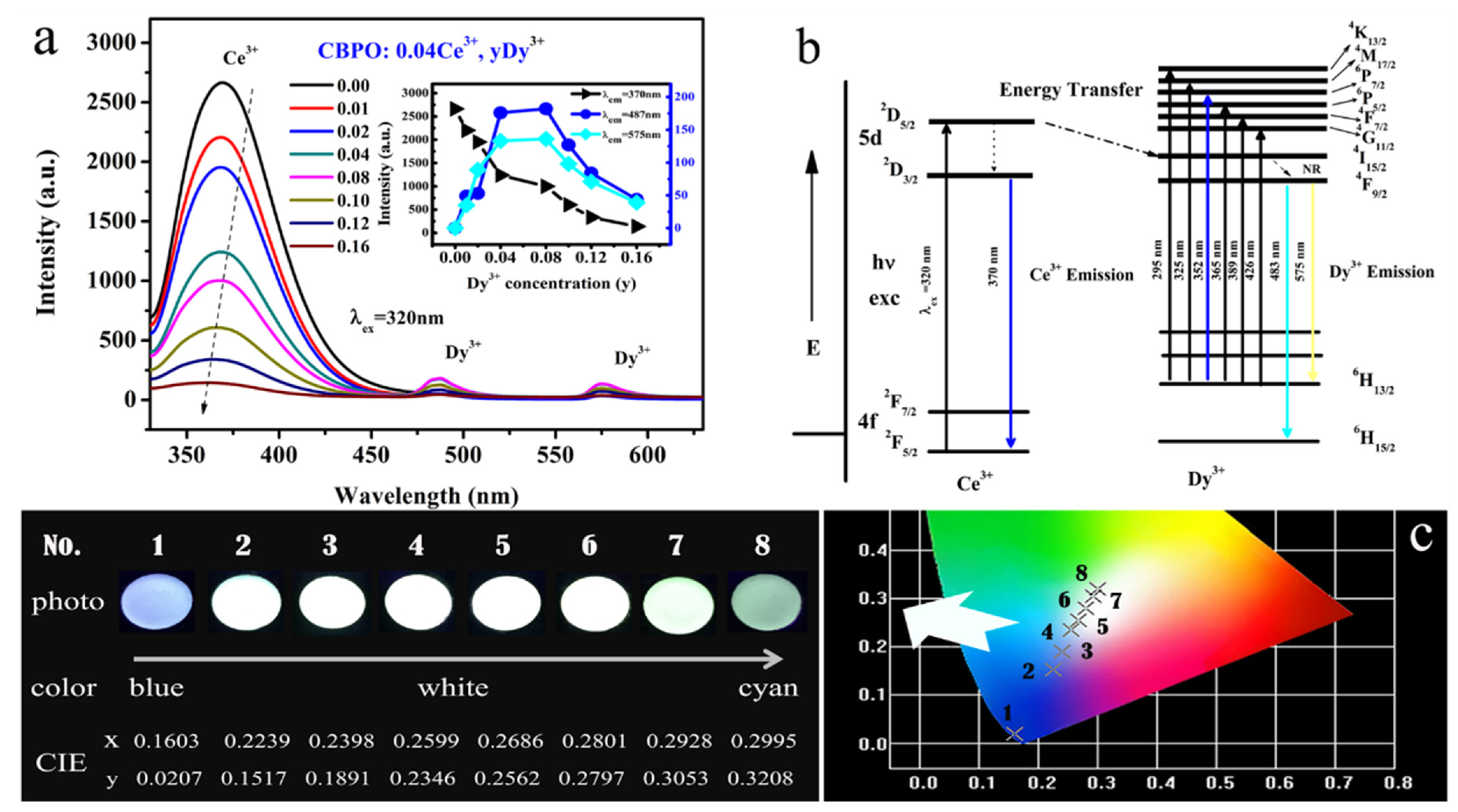
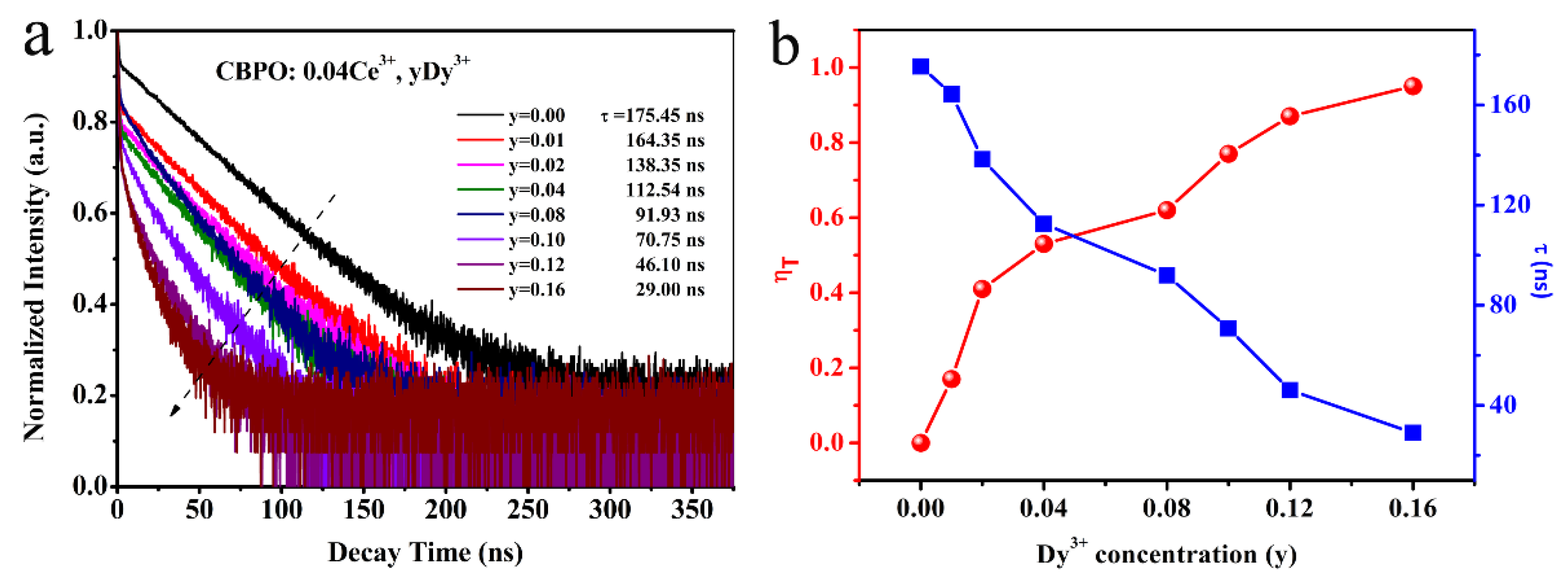
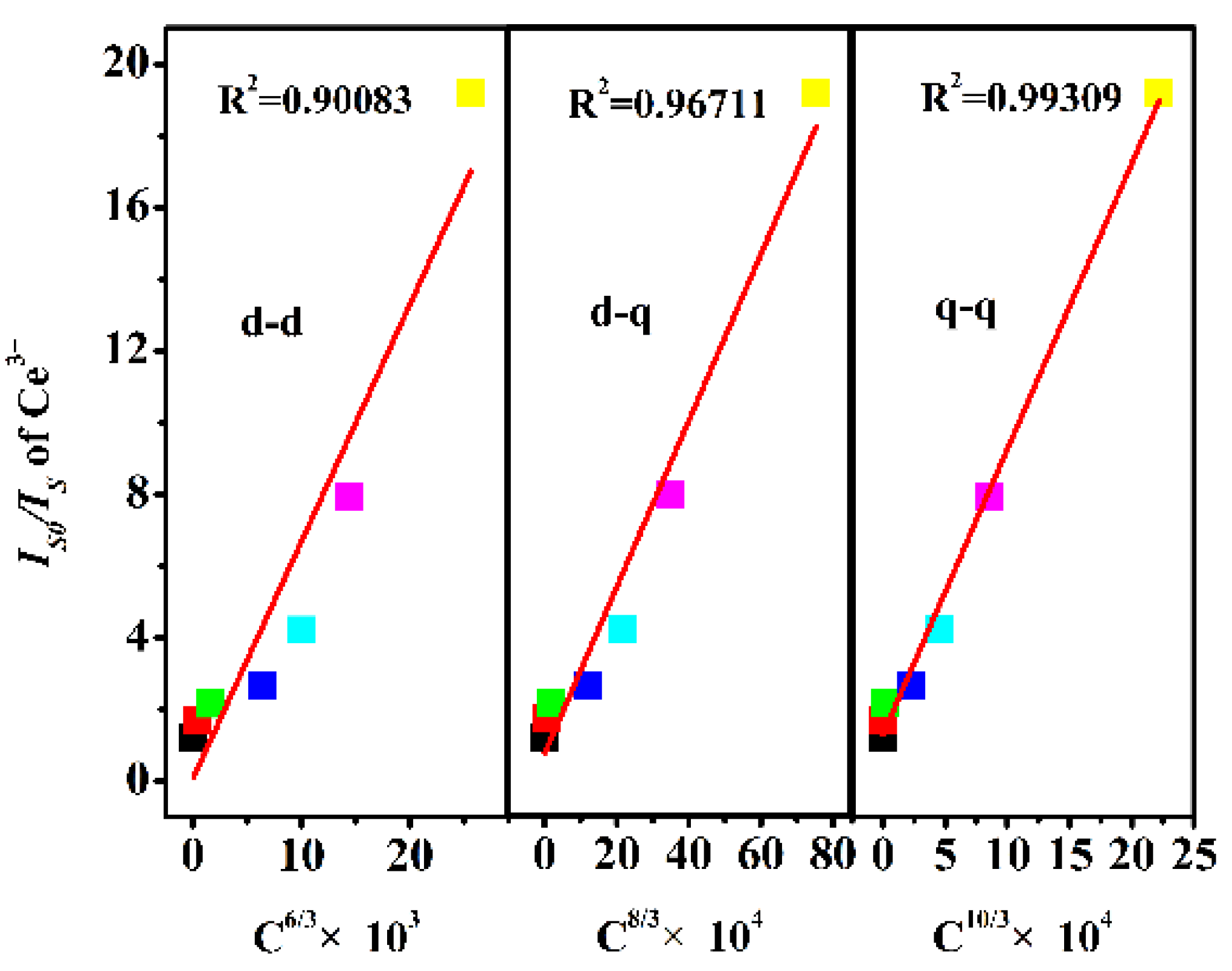
2.2. Ce3+/Dy3+ Photoluminescence Properties Analysis
2.3. Thermal Stability Analysis
3. Materials and Methods
3.1. Preparation Process
3.2. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lin, C.C.; Liu, R.S. Advances in phosphors for light-emitting diodes. J. Phys. Chem. Lett. 2011, 2, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.G.; Xu, Z.; Chen, M.Y.; Liu, Q.L. Recent developments in the new inorganic solid-state LED phosphors. Dalton Trans. 2016, 45, 11214–11232. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, S.; Bhagwan, S.; Singh, D. Rare earth (RE) doped phosphors and their emerging applications: A review. Ceram. Int. 2021, 47, 19282–19303. [Google Scholar] [CrossRef]
- Jayachandiran, M.; Kennedy, S.M.M. Synthesis and optical properties of Ba3Bi2(PO4)4: Dy3+ phosphors for white light emitting diodes. J. Alloys Compd. 2019, 775, 353–359. [Google Scholar] [CrossRef]
- Han, L.L.; Zhu, D.C.; Wang, Y.J.; Jiang, F.; Yang, X.Y.; Wang, S.; Zhao, J.; Ci, Z.P.; Wang, C.W. Luminescence and thermal stability enhancement by matrix luminescence center dispersion in Sc(V, P)O4: Dy3+ nano/submicron phosphors. CrystEngComm 2018, 20, 7526–7535. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Zhu, D.C.; Pu, Y. A zero-thermal-quenching phosphor Sr3La(AlO)3(BO3)4: Dy3+ for near ultraviolet excitation white-LEDs. J. Lumin. 2022, 243, 118610. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xu, J.; Ju, Z.H.; Zhao, S.S.; Pei, P.X.; Ma, X.Y.; Liu, W.S. Novel white-emitting afterglow phosphor Na2CaSn2Ge3O12: Dy3+: Preparation, photoluminescence, and phosphorescence properties. J. Alloys Compd. 2021, 856, 157230. [Google Scholar] [CrossRef]
- Alajlani, Y.; Can, N. Novel Dy and Sm activated BaSi2O5 phosphors: Insights into the structure, intrinsic and extrinsic luminescence, and influence of doping concentration. J. Lumin. 2021, 230, 117718. [Google Scholar] [CrossRef]
- Yu, H.; Su, L.B.; Qian, X.B.; Jiang, D.P.; Wu, Q.H.; Tang, F.; Wang, J.Y.; Xu, J. Influence of Gd3+ on the optical properties of Dy3+-activated CaF2 single crystal for white LED application. J. Electron. Mater. 2019, 48, 2910–2915. [Google Scholar] [CrossRef]
- Li, K.; Shang, M.M.; Lian, H.Z.; Lin, J. Recent development in phosphors with different emitting colors via energy transfer. J. Mater. Chem. C 2016, 4, 5507–5530. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, C.F.; Chen, Z.T.; Shi, M.L.; Meng, X.G. Ce3+ and Dy3+ doped Ca3(P1−xBxO4)2 phosphors for white light-emitting applications. J. Alloys Compd. 2019, 775, 1044–1051. [Google Scholar] [CrossRef]
- Nair, G.B.; Dhoble, S.J. White light emission through efficient energy transfer from Ce3+ to Dy3+ ions in Ca3Mg3(PO4)4 matrix aided by Li+ charge compensator. J. Lumin. 2017, 192, 1157–1166. [Google Scholar] [CrossRef]
- Dev, K.; Selot, A.; Nair, G.B.; Barai, V.L.; Haque, F.Z.; Aynyas, M.; Dhoble, S.J. Energy transfer from Ce3+ to Dy3+ ions for white light emission in Sr2MgAl22O36: Ce3+, Dy3+ phosphor. J. Lumin. 2019, 206, 380–385. [Google Scholar] [CrossRef]
- Huo, J.S.; Dong, L.P.; Lü, W.; Shao, B.Q.; You, H.P. Novel tunable green-red-emitting oxynitride phosphors co-activated with Ce3+, Tb3+, and Eu3+: Photoluminescence and energy transfer. Phys. Chem. Chem. Phys. 2017, 19, 17314–17323. [Google Scholar] [CrossRef]
- Sun, L.L.; Devakumar, B.; Liang, J.; Wang, S.Y.; Sun, Q.; Huang, X.Y. Highly efficient Ce3+→Tb3+ energy transfer induced bright narrowband green emissions from garnet-type Ca2YZr2(AlO4)3: Ce3+, Tb3+ phosphors for white LEDs with high color rendering index. J. Mater. Chem. C 2019, 7, 10471–10480. [Google Scholar] [CrossRef]
- Shang, M.M.; Liang, S.S.; Lian, H.Z.; Lin, J. Luminescence properties of Ca19Ce(PO4)14: A (A= Eu3+/Tb3+/Mn2+) phosphors with abundant colors: Abnormal coexistence of Ce4+/3+- Eu3+ and energy transfer of Ce3+→Tb3+/Mn2+ and Tb3+- Mn2+. Inorg. Chem. 2017, 56, 6131–6140. [Google Scholar] [CrossRef]
- Zou, X.; He, L.H.; Li, R.; Zheng, Q.J.; Liu, Y.F.; Xu, C.G.; Lin, D.M. Structure and luminescent properties of Ca3Bi(PO4)3: Sm3+ orange phosphor. J. Mater. Sci. Mater. Electron. 2017, 28, 826–2832. [Google Scholar] [CrossRef]
- Li, K.; Liang, S.S.; Shang, M.M.; Lian, H.Z.; Lin, J. Photoluminescence and energy transfer properties with Y+SiO4 substituting Ba+PO4 in Ba3Y(PO4)3: Ce3+/Tb3+, Tb3+/Eu3+ phosphors for w-LEDs. Inorg. Chem. 2016, 55, 7593–7604. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Wang, Y.; Geng, L. A new eulytite-type Pb3Bi(PO4)3: Eu3+ red-emitting phosphor: Synthesis, structure and photoluminescence characteristics. J. Lumin. 2020, 220, 116978. [Google Scholar] [CrossRef]
- Jiang, D.C.; Geng, L.; Zhou, S.S.; Wang, Y.J. Photoluminescence properties and energy transfer in the Sm3+ and Eu3+ co-doped Ca3Bi(PO4)3 red phosphor. Inorg. Chem. Commun. 2022, 142, 109668. [Google Scholar] [CrossRef]
- Sahu, M.K.; Jayasimhadri, M.; Haranath, D. Temperature-dependent photoluminescence and optical thermometry performance in Ca3Bi(PO4)3: Er3+ phosphors. Solid State Sci. 2022, 131, 106956. [Google Scholar] [CrossRef]
- Cao, R.P.; Chen, C.P.; Cheng, F.R.; Chen, T.; Lan, B.; Li, L.; Liu, R.; Wang, J. Synthesis and luminescence properties of Eu3+, Dy3+ co-doped Ca3Bi(PO4)3 single-phase phosphor. J. Lumin. 2023, 257, 119731. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Li, K.; Van Deun, R. Photoluminescence and energy transfer properties of a novel molybdate KBaY(MoO4)3: Ln3+ (Ln3+ = Tb3+, Eu3+, Sm3+, Tb3+/Eu3+, Tb3+/Sm3+) as a multi-color emitting phosphor for UV w-LEDs. Dalton Trans. 2018, 47, 6995–7004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Single-component and warm-white-emitting phosphor NaGd(WO4)2: Tm3+, Dy3+, Eu3+: Synthesis, luminescence, energy transfer, and tunable color. Inorg. Chem. 2014, 53, 11457–11466. [Google Scholar] [CrossRef]
- Chen, C.T.; Lin, T.J.; Molokeev, M.S.; Liu, W.R. Synthesis, luminescent properties and theoretical calculations of novel orange-red-emitting Ca2Y8(SiO4)6O2: Sm3+ phosphors for white light-emitting diodes. Dyes Pigm. 2018, 150, 121–129. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, W.; Du, F.; Wen, J.; Zhuang, W.; Liu, R.; Duan, C.K.; Wang, L.; Lin, K. Identifying the emission centers and probing the mechanism for highly efficient and thermally stable luminescence in the La3Si6N11: Ce3+ phosphor. J. Phys. Chem. C 2018, 122, 7849–7858. [Google Scholar] [CrossRef]
- Wang, J.; Shang, M.M.; Cui, M.; Dang, P.; Liu, D.; Huang, D.; Lian, H.; Lin, J. Realizing an impressive red-emitting Ca9MnNa(PO4)7 phosphor through a dual function based on disturbing structural confinement and energy transfer. J. Mater. Chem. C 2020, 8, 285–295. [Google Scholar] [CrossRef]
- Xia, M.; Wu, X.; Zhong, Y.; Zhou, Z.; Wong, W.-Y. A novel Na3La(PO4)2/LaPO4: Eu blue-red dual-emitting phosphor with high thermal stability for plant growth lighting. J. Mater. Chem. C 2019, 7, 2385–2393. [Google Scholar] [CrossRef]
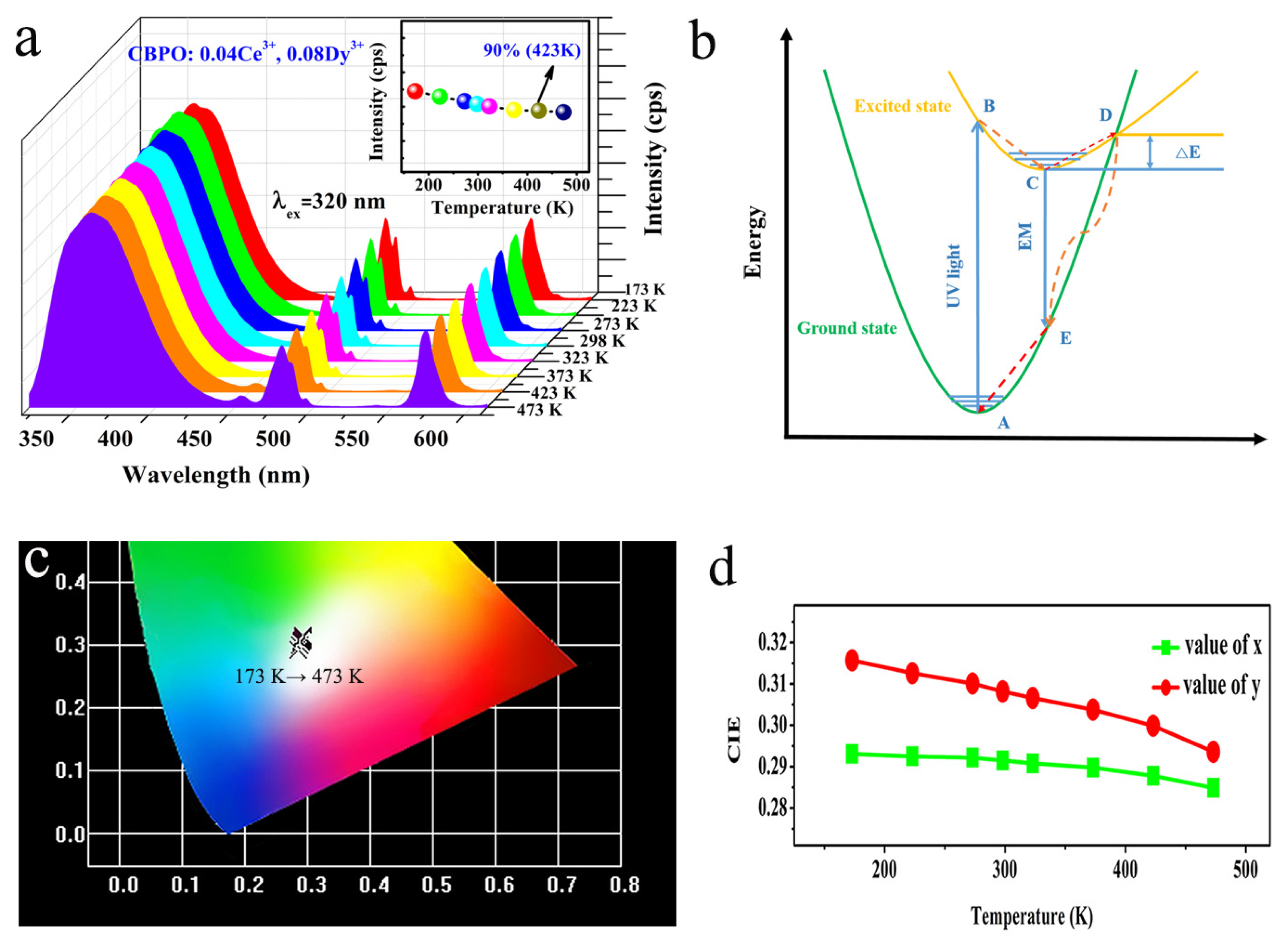
| T (K) | CIE (x) | CIE (y) |
|---|---|---|
| 173 | 0.2931 | 0.3157 |
| 223 | 0.2925 | 0.3126 |
| 273 | 0.2922 | 0.3101 |
| 298 | 0.2915 | 0.3082 |
| 323 | 0.2908 | 0.3066 |
| 373 | 0.2898 | 0.3038 |
| 423 | 0.2878 | 0.2999 |
| 473 | 0.2849 | 0.2936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liang, J.; Wang, L.; Guo, N.; Ai, L. Improvement of Luminescence Properties of Eulytite Single-Phase White Emitting Ca3Bi (PO4)3: Ce3+/Dy3+ Phosphor. Molecules 2023, 28, 4967. https://doi.org/10.3390/molecules28134967
Xu M, Liang J, Wang L, Guo N, Ai L. Improvement of Luminescence Properties of Eulytite Single-Phase White Emitting Ca3Bi (PO4)3: Ce3+/Dy3+ Phosphor. Molecules. 2023; 28(13):4967. https://doi.org/10.3390/molecules28134967
Chicago/Turabian StyleXu, Mengjiao, Jiamin Liang, Luxiang Wang, Nannan Guo, and Lili Ai. 2023. "Improvement of Luminescence Properties of Eulytite Single-Phase White Emitting Ca3Bi (PO4)3: Ce3+/Dy3+ Phosphor" Molecules 28, no. 13: 4967. https://doi.org/10.3390/molecules28134967
APA StyleXu, M., Liang, J., Wang, L., Guo, N., & Ai, L. (2023). Improvement of Luminescence Properties of Eulytite Single-Phase White Emitting Ca3Bi (PO4)3: Ce3+/Dy3+ Phosphor. Molecules, 28(13), 4967. https://doi.org/10.3390/molecules28134967





