Research on Electric Field—Induced Catalysis Using Single—Molecule Electrical Measurement
Abstract
1. Introduction
2. Electric Field Catalytic Reactions
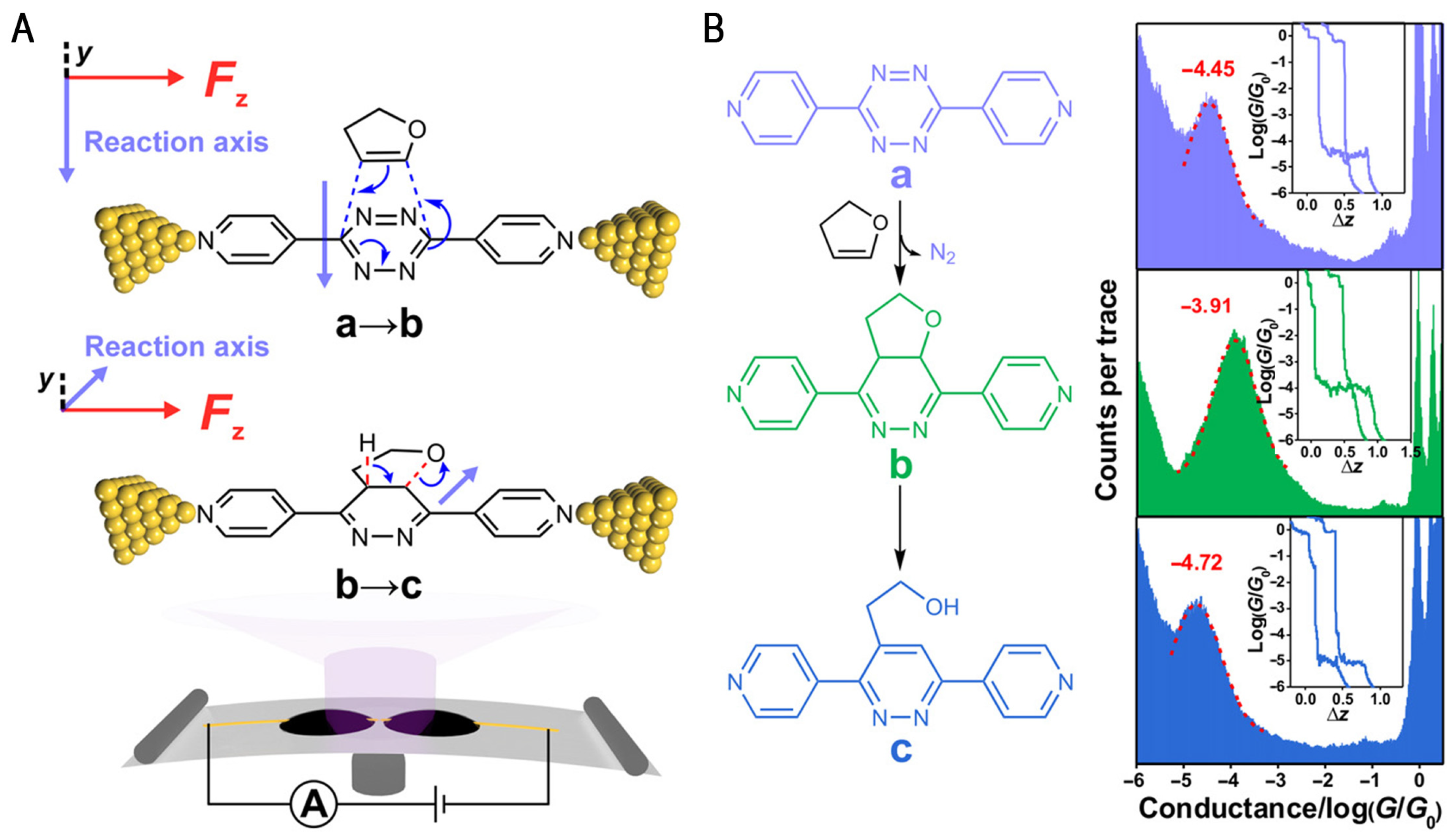
2.1. Diels–Alder Reactions
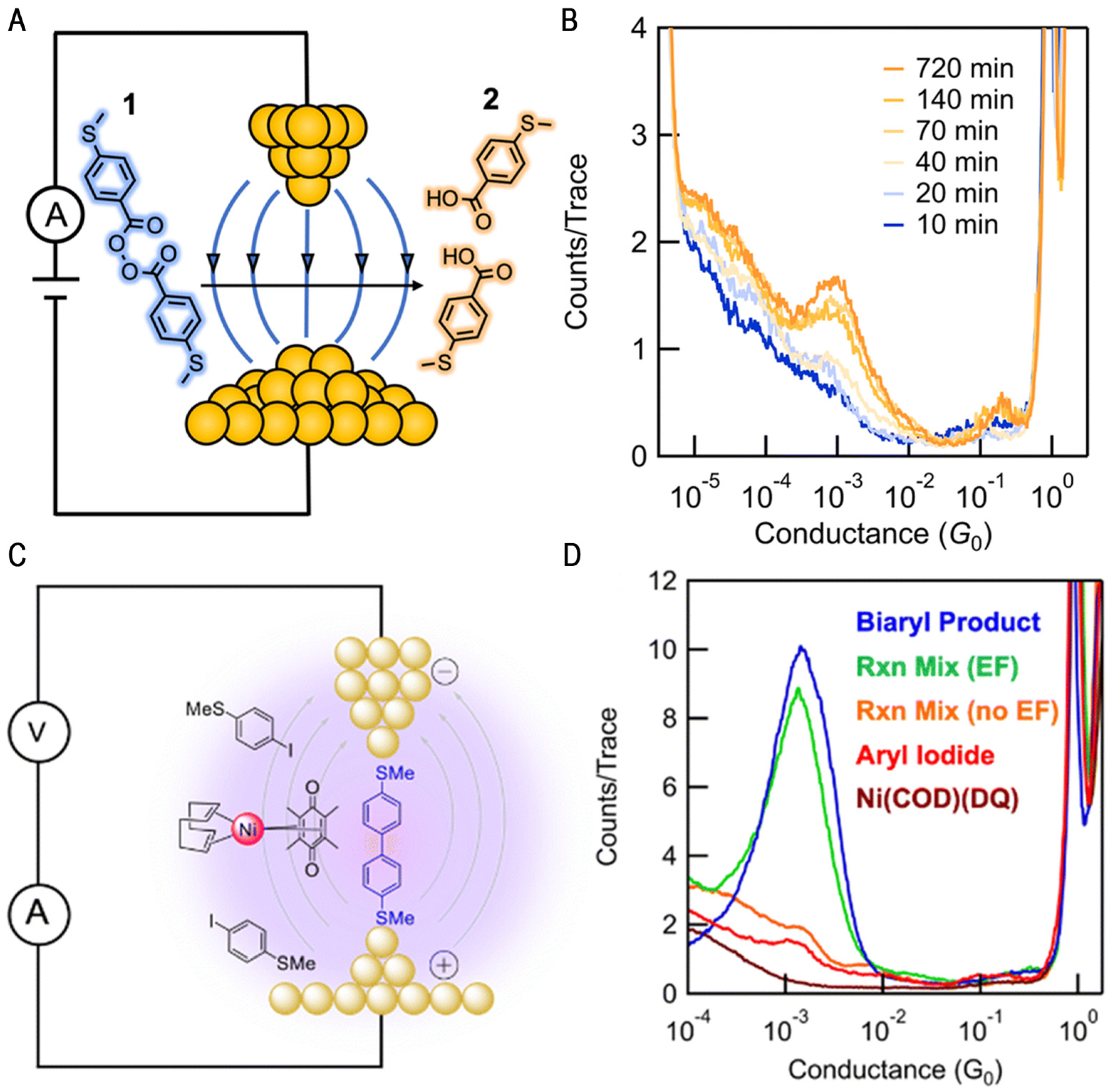
2.2. Cleavage Reactions
2.3. Coupling Reaction of Transition Metal Complexes
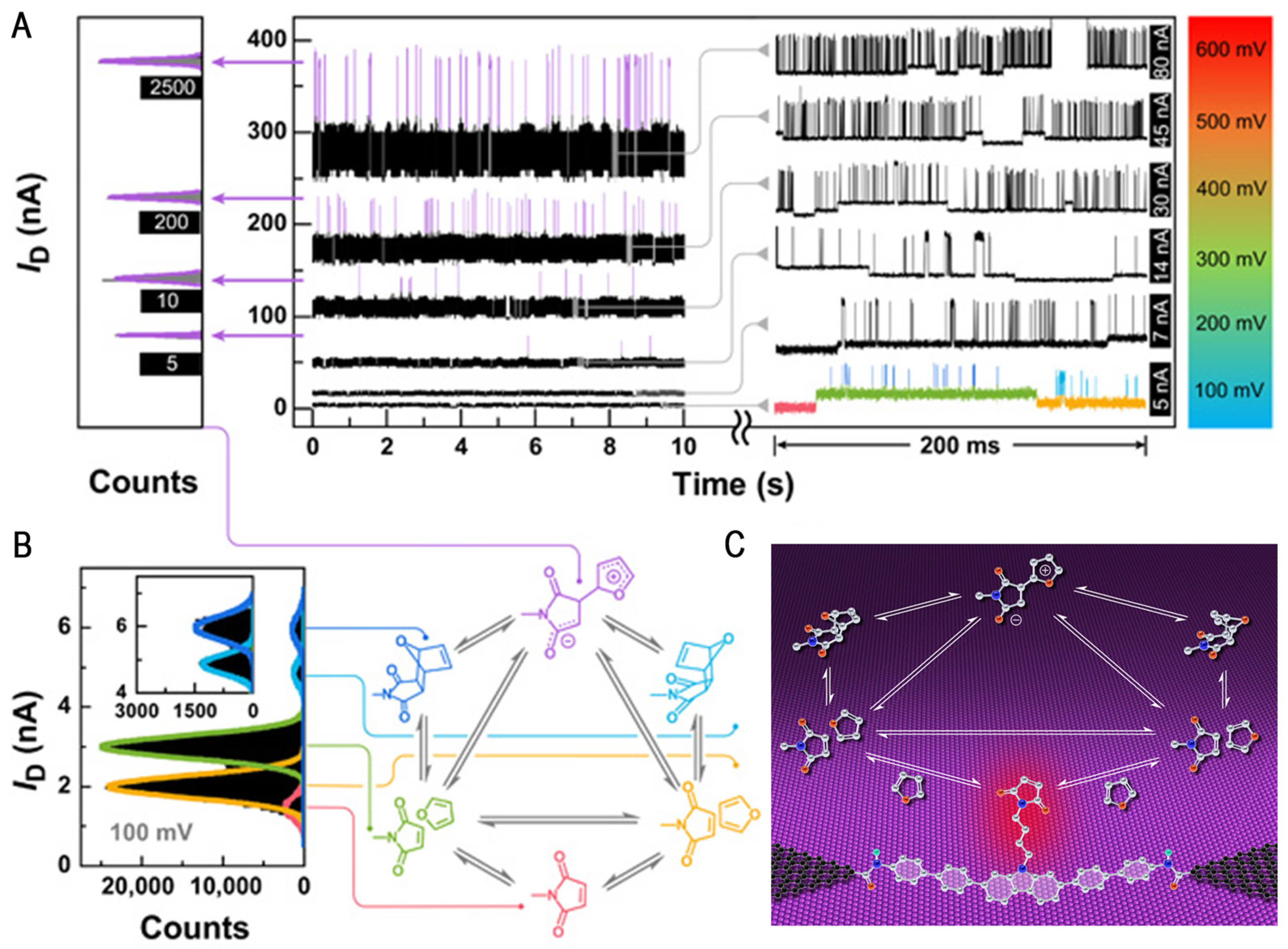
3. Electronic Catalysis
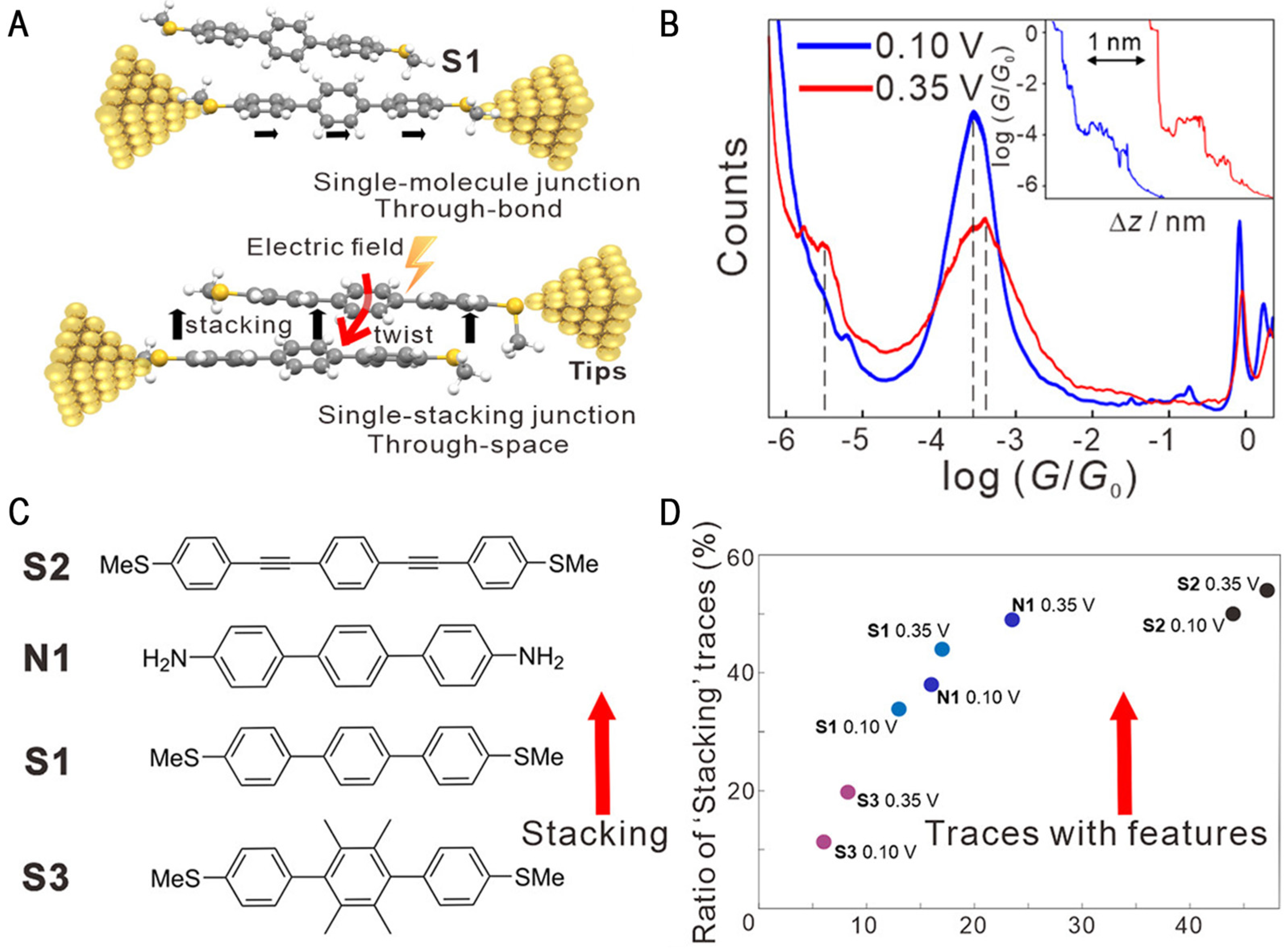
4. Electric Field Catalytic Assembly
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E.J. Controlled fluoroalkylation reactions by visible-light photoredox catalysis. Acc. Chem. Res. 2016, 49, 2284–2294. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis enabled by plasma activation of strong chemical bonds: A review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Cortes, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, G.; Demetriadou, A.; Narang, P. Challenges in plasmonic catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, X.B.; Zhang, G. TiO2-based catalysts for photothermal catalysis: Mechanisms, materials and applications. J. Clean. Prod. 2022, 381, 135156–135174. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Cui, X.Y.; Liu, H.L.; Guo, Z.G.; Dang, Y.F.; Fan, Z.X.; Zhang, Z.C.; Hu, W.P. Tandem catalysis in electrochemical CO2 reduction reaction. Nano Res. 2021, 14, 4471–4486. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.D.; Nunna, B.B.; Lee, E.S. Nitrogen-doped graphene nanomaterials for electrochemical catalysis/reactions: A review on chemical structures and stability. Carbon 2021, 185, 198–214. [Google Scholar] [CrossRef]
- Tang, B.Y.; Bisbey, R.P.; Lodaya, K.M.; Toh, W.L.; Surendranath, Y. Reaction environment impacts charge transfer but not chemical reaction steps in hydrogen evolution catalysis. Nat. Catal. 2023, 6, 339–350. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Fokas, A.S.; Grigoriou, D. Catalysis and autocatalysis of chemical synthesis and of hadronization. Appl. Catal. B Environ. 2017, 203, 582–590. [Google Scholar] [CrossRef]
- Ciampi, S.; Darwish, N.; Aitken, H.M.; Diez-Perez, I.; Coote, M.L. Harnessing electrostatic catalysis in single molecule, electrochemical and chemical systems: A rapidly growing experimental tool box. Chem. Soc. Rev. 2018, 47, 5146–5164. [Google Scholar] [CrossRef]
- Ramanan, R.; Danovich, D.; Mandal, D.; Shaik, S. Catalysis of methyl transfer reactions by oriented external electric fields: Are gold-thiolate linkers innocent? J. Am. Chem. Soc. 2018, 140, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.B.; Kong, Y.; Yue, Q.Y.; Liu, J.S.; Xu, X.; Kong, W.J.; Gao, Y.; Gao, B.Y. Magnetic field-enhanced radical intensity for accelerating norfloxacin degradation under FeCu/rGO photo-Fenton catalysis. Chem. Eng. J. 2021, 420, 127634. [Google Scholar] [CrossRef]
- Besalu-Sala, P.; Sola, M.; Luis, J.M.; Torrent-Sucarrat, M. Fast and simple evaluation of the catalysis and selectivity induced by external electric fields. ACS Catal. 2021, 11, 14467–14479. [Google Scholar] [CrossRef]
- Dulic, D.; van der Molen, S.J.; Kudernac, T.; Jonkman, H.T.; de Jong, J.J.D.; Bowden, T.N.; van Esch, J.; Feringa, B.L.; van Wees, B.J. One-way optoelectronic switching of photochromic molecules on gold. Phys. Rev. Lett. 2003, 91, 207402–207406. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Liu, B.; Li, X.L.; Yang, Z.L.; Ren, B.; Wu, S.T.; Tao, N.J.; Tian, Z.Q. Study of molecular junctions with a combined surface-enhanced raman and mechanically controllable break junction method. J. Am. Chem. Soc. 2006, 128, 14748–14749. [Google Scholar] [CrossRef]
- Venkataraman, L.; Klare, J.E.; Nuckolls, C.; Hybertsen, M.S.; Steigerwald, M.L. Dependence of single-molecule junction conductance on molecular conformation. Nature 2006, 442, 904–907. [Google Scholar] [CrossRef]
- Aragones, A.C.; Haworth, N.L.; Darwish, N.; Ciampi, S.; Bloomfield, N.J.; Wallace, G.G.; Diez-Perez, I.; Coote, M.L. Electrostatic catalysis of a Diels-Alder reaction. Nature 2016, 531, 88–91. [Google Scholar] [CrossRef]
- Guan, J.X.; Jia, C.C.; Li, Y.W.; Liu, Z.T.; Wang, J.Y.; Yang, Z.Y.; Gu, C.H.; Su, D.K.; Houk, K.N.; Zhang, D.Q.; et al. Direct single-molecule dynamic detection of chemical reactions. Sci. Adv. 2018, 4, eaar2177. [Google Scholar] [CrossRef]
- Ramsay, W.J.; Bell, N.A.W.; Qing, Y.J.; Bayley, H. Single-molecule observation of the intermediates in a catalytic cycle. J. Am. Chem. Soc. 2018, 140, 17538–17546. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Lu, C.X.; Zhou, S.Y.; Li, X.X.; Li, Y.W.; Yang, Y.; Li, Y.; Liu, Z.R.; Yang, J.L.; et al. Unveiling the full reaction path of the Suzuki-Miyaura cross-coupling in a single-molecule junction. Nat. Nanotechnol. 2021, 16, 1214–1223. [Google Scholar] [CrossRef]
- Huang, C.C.; Jevric, M.; Borges, A.; Olsen, S.T.; Hamill, J.M.; Zheng, J.T.; Yang, Y.; Rudnev, A.; Baghernejad, M.; Broekmann, P.; et al. Single-molecule detection of dihydroazulene photo-thermal reaction using break junction technique. Nat. Commun. 2017, 8, 15436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.C.; Xu, W.L.; Liu, G.K.; Panda, D.; Chen, P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Ge, X.; Cao, J.; Xiao, Y.; Wang, Y.; Zhang, W.; Song, P.; Xu, W.L. Revealing the catalytic kinetics and dynamics of individual Pt atoms at the single-molecule level. Proc. Natl. Acad. Sci. USA 2022, 119, e2114639103. [Google Scholar] [CrossRef]
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chen, L.C.; Zhang, Z.Q.; Cao, J.J.; Tang, C.; Liu, J.Y.; Duan, L.L.; Huo, Y.; Shao, X.F.; Hong, W.J.; et al. Distinguishing diketopyrrolopyrrole isomers in single-molecule junctions via reversible stimuli-responsive quantum interference. J. Am. Chem. Soc. 2018, 140, 6531–6535. [Google Scholar] [CrossRef]
- Frisenda, R.; Stefani, D.; van der Zant, H.S.J. Quantum transport through a single conjugated rigid molecule, a mechanical break junction study. Acc. Chem. Res. 2018, 51, 1359–1367. [Google Scholar] [CrossRef]
- Haiss, W.; Wang, C.S.; Grace, I.; Batsanov, A.S.; Schiffrin, D.J.; Higgins, S.J.; Bryce, M.R.; Lambert, C.J.; Nichols, R.J. Precision control of single-molecule electrical junctions. Nat. Mater. 2006, 5, 995–1002. [Google Scholar] [CrossRef]
- Mishchenko, A.; Zotti, L.A.; Vonlanthen, D.; Burkle, M.; Pauly, F.; Cuevas, J.C.; Mayor, M.; Wandlowski, T. Single-molecule junctions based on Nitrile-terminated biphenyls: A promising new anchoring group. J. Am. Chem. Soc. 2011, 133, 184–187. [Google Scholar] [CrossRef]
- Cui, L.J.; Hur, S.; Akbar, Z.A.; Klockner, J.C.; Jeong, W.; Pauly, F.; Jang, S.Y.; Reddy, P.; Meyhofer, E. Thermal conductance of single-molecule junctions. Nature 2019, 572, 628–633. [Google Scholar] [CrossRef]
- Feng, A.N.; Zhou, Y.; Al-Shebami, M.A.Y.; Chen, L.C.; Pan, Z.C.; Xu, W.; Zhao, S.Q.; Zeng, B.F.; Xiao, Z.Y.; Yang, Y.; et al. σ-σ stacked supramolecular junctions. Nat. Chem. 2022, 14, 1158–1164. [Google Scholar] [CrossRef]
- Xu, B.; Tao, N. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, X.X.; Gong, Z.L.; Jia, C.C.; Lin, Y.W.; Gu, C.H.; He, G.; Zhong, Y.W.; Yang, J.L.; Guo, X.F. Direct observation of single-molecule hydrogen-bond dynamics with single-bond resolution. Nat. Commun. 2018, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Su, D.K.; Zhou, S.Y.; Masai, H.; Liu, Z.H.; Zhou, C.; Yang, C.; Li, Z.Z.; Tsuda, S.; Liu, Z.R.; Terao, J.; et al. Stochastic binding dynamics of a photoswitchable single supramolecular complex. Adv. Sci. 2022, 9, 202200022. [Google Scholar] [CrossRef]
- Jia, C.C.; Migliore, A.; Xin, N.; Huang, S.Y.; Wang, J.Y.; Yang, Q.; Wang, S.P.; Chen, H.L.; Wang, D.M.; Feng, B.Y.; et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 2016, 352, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.K.; Hopson, T.J.; Rawlett, A.M.; Nagahara, L.A.; Primak, A.; Lindsay, S.M. A bond-fluctuation mechanism for stochastic switching in wired molecules. Science 2003, 300, 1413–1416. [Google Scholar] [CrossRef]
- Chiechi, R.C.; Weiss, E.A.; Dickey, M.D.; Whitesides, G.M. Eutectic Gallium-Indium (EGaIn): A moldable liquid metal for electrical characterization of self-assembled monolayers. Angew. Chem. Int. Ed. 2008, 47, 142–144. [Google Scholar] [CrossRef]
- Soh, E.J.H.; Astier, H.P.A.G.; Daniel, D.; Chua, J.Q.I.; Miserez, A.; Jia, Z.; Li, L.; O’Shea, S.J.; Bhaskaran, H.; Tomczak, N.; et al. AFM manipulation of EGaln microdroplets to generate controlled, on-demand contacts on molecular self-assembled monolayers. ACS Nano 2022, 16, 14370–14378. [Google Scholar] [CrossRef]
- Nerngchamnong, N.; Yuan, L.; Qi, D.C.; Li, J.; Thompson, D.; Nijhuis, C.A. The role of van der Waals forces in the performance of molecular diodes. Nat. Nanotechnol. 2013, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Rudnev, A.V.; Hong, W.J.; Wandlowski, T. Break junction under electrochemical gating: Testbed for single-molecule electronics. Chem. Soc. Rev. 2015, 44, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, S.J.; Yao, Y.R.; Zhang, C.Y.; Wu, Q.Q.; Wang, H.C.; Zhang, H.W.; Liu, X.Y.; Tang, C.; Wei, M.X.; et al. Room-temperature logic-in-memory operations in single-metallofullerene devices. Nat. Mater. 2022, 21, 917–923. [Google Scholar] [CrossRef]
- Zhang, L.; Laborda, E.; Darwish, N.; Noble, B.B.; Tyrell, J.H.; Pluczyk, S.; Le Brun, A.P.; Wallace, G.G.; Gonzalez, J.; Coote, M.L.; et al. Electrochemical and electrostatic cleavage of alkoxyamines. J. Am. Chem. Soc. 2018, 140, 766–774. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Schaack, C.; Prindle, C.R.; Vo, E.A.; Aziz, M.; Steigerwald, M.L.; Berkelbach, T.C.; Nuckolls, C.; Venkataraman, L. Electric fields drive bond homolysis. Chem. Sci. 2023, 14, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Orchanian, N.M.; Guizzo, S.; Steigerwald, M.L.; Nuckolls, C.; Venkataraman, L. Electric-field-induced coupling of aryl iodides with a nickel(0) complex. Chem. Commun. 2022, 58, 12556–12559. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Cao, J.X.; Hu, Y. In-Situ label-free single-molecule dynamic detection of thermal-reversible reactions. Chem. Eng. J. 2023, 451, 138779–138786. [Google Scholar] [CrossRef]
- Huang, X.Y.; Tang, C.; Li, J.Q.; Chen, L.C.; Zheng, J.T.; Zhang, P.; Le, J.B.; Li, R.H.; Li, X.H.; Liu, J.Y.; et al. Electric field-induced selective catalysis of single-molecule reaction. Sci. Adv. 2019, 5, eaaw3072. [Google Scholar] [CrossRef]
- Chen, H.L.; Jiang, F.; Hu, C.; Jiao, Y.; Chen, S.; Qiu, Y.Y.; Zhou, P.; Zhang, L.; Cai, K.; Song, B.; et al. Electron-catalyzed dehydrogenation in a single-molecule junction. J. Am. Chem. Soc. 2021, 143, 8476–8487. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhou, Y.; Zhou, D.H.; Chen, Y.R.; Xiao, Z.Y.; Shi, J.; Liu, J.Y.; Hong, W.J. Electric field-induced assembly in single-stacking terphenyl junctions. J. Am. Chem. Soc. 2020, 142, 19101–19109. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.; Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Corey, E.J. Catalytic enantioselective Diels-Alder reactions: Methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. 2002, 41, 1650–1667. [Google Scholar] [CrossRef]
- Warshel, A.; Sharma, P.K.; Kato, M.; Xiang, Y.; Liu, H.B.; Olsson, M.H.M. Electrostatic basis for enzyme catalysis. Chem. Rev. 2006, 106, 3210–3235. [Google Scholar] [CrossRef]
- Lai, W.; Chen, H.; Cho, K.B.; Shaik, S. External electric field can control the catalytic cycle of cytochrome P450cam: A QM/MM study. J. Phys. Chem. Lett. 2010, 1, 2082–2087. [Google Scholar] [CrossRef]
- Fried, S.D.; Bagchi, S.; Boxer, S.G. Extreme electric fields power catalysis in the active site of ketosteroid isomerase. Science 2014, 346, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Meir, R.; Chen, H.; Lai, W.Z.; Shaik, S. Oriented electric fields accelerate Diels-Alder reactions and control the endo/exo selectivity. Chemphyschem 2010, 11, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Z.T.; Li, Y.W.; Zhou, S.Y.; Lu, C.X.; Guo, Y.L.; Ramirez, M.; Zhang, Q.Z.; Li, Y.; Liu, Z.R.; et al. Electric field-catalyzed single-molecule Diels-Alder reaction dynamics. Sci. Adv. 2021, 7, eabf0689. [Google Scholar] [CrossRef]
- Mejia, L.; Garay-Ruiz, D.; Franco, I. Diels-Alder reaction in a molecular junction. J. Phys. Chem. C 2021, 125, 14599–14606. [Google Scholar] [CrossRef]
- Su, T.A.; Neupane, M.; Steigerwald, M.L.; Venkataraman, L.; Nuckolls, C. Chemical principles of single-molecule electronics. Nat. Rev. Mater. 2016, 1, 16002. [Google Scholar] [CrossRef]
- Chen, W.B.; Li, H.X.; Widawsky, J.R.; Appayee, C.; Venkataraman, L.; Breslow, R. Aromaticity decreases single-molecule junction conductance. J. Am. Chem. Soc. 2014, 136, 918–920. [Google Scholar] [CrossRef]
- Fujii, S.; Marques-Gonzalez, S.; Shin, J.Y.; Shinokubo, H.; Masuda, T.; Nishino, T.; Arasu, N.P.; Vazquez, H.; Kiguchi, M. Highly-conducting molecular circuits based on antiaromaticity. Nat. Commun. 2017, 8, 15984. [Google Scholar] [CrossRef]
- Pocker, Y.; Buchholz, R.F. Electrostatic catalysis by ionic aggregates. I. The ionization and dissociation of trityl chloride and hydrogen chloride in lithium perchlorate-diethyl ether solutions. J. Am. Chem. Soc. 1970, 92, 2075–2084. [Google Scholar] [CrossRef]
- Shaik, S.S. What happens to molecules as they react? A valence bond approach to reactivity. J. Am. Chem. Soc. 1981, 103, 3692–3701. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Marshall, D.L.; Blanksby, S.J.; Coote, M.L. Switching radical stability by pH-induced orbital conversion. Nat. Chem. 2013, 5, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Klinska, M.; Smith, L.M.; Gryn’ova, G.; Banwell, M.G.; Coote, M.L. Experimental demonstration of pH-dependent electrostatic catalysis of radical reactions. Chem. Sci. 2015, 6, 5623–5627. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Stuyver, T.; Shaik, S. Oriented external electric fields and ionic additives elicit catalysis and mechanistic crossover in oxidative addition reactions. J. Am. Chem. Soc. 2020, 142, 3836–3850. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Brasiliense, V.; Mo, J.S.; Zhang, L.; Jiao, Y.; Chen, Z.; Jones, L.O.; He, G.; Guo, Q.H.; Chen, X.Y.; et al. Single-molecule charge transport through positively charged electrostatic anchors. J. Am. Chem. Soc. 2021, 143, 2886–2895. [Google Scholar] [CrossRef]
- Brixner, T.; Gerber, G. Quantum control of gas-phase and liquid-phase femtochemistry. Chemphyschem 2003, 4, 418–438. [Google Scholar] [CrossRef]
- Bohler, E.; Warneke, J.; Swiderek, P. Control of chemical reactions and synthesis by low-energy electrons. Chem. Soc. Rev. 2013, 42, 9219–9231. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Curran, D.P. The Electron is a Catalyst. Nat. Chem. 2014, 6, 765–773. [Google Scholar] [CrossRef]
- Luca, O.R.; Gustafson, J.L.; Maddox, S.M.; Fenwick, A.Q.; Smith, D.C. Catalysis by electrons and holes: Formal potential scales and preparative organic electrochemistry. Org. Chem. Front. 2015, 2, 823–848. [Google Scholar] [CrossRef]
- Francke, R.; Little, R.D. Electrons and Holes as Catalysts in Organic Electrosynthesis. Chemelectrochem 2019, 6, 4373–4382. [Google Scholar] [CrossRef]
- Che, F.; Gray, J.T.; Ha, S.; Kruse, N.; Scott, S.L.; McEwen, J.-S. Elucidating the roles of electric fields in catalysis: A perspective. ACS Catal. 2018, 8, 5153–5174. [Google Scholar] [CrossRef]
- Stone, I.; Starr, R.L.; Zang, Y.P.; Nuckolls, C.; Steigerwald, M.L.; Lambert, T.H.; Roy, X.; Venkataraman, L. A single-molecule blueprint for synthesis. Nat. Rev. Chem. 2021, 5, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.; Vali, S.R.; Reddy, B.V.S. Electric-field-induced organic transformations-recent advances. Eur. J. Org. Chem. 2023, 26, e202300103. [Google Scholar] [CrossRef]
- Mattioli, E.J.; Bottoni, A.; Zerbetto, F.; Calvaresi, M. Oriented external electric fields affect rate and stereoselectivity of electrocyclic reactions. J. Phys. Chem. C 2019, 123, 26370–26378. [Google Scholar] [CrossRef]
- Stuyver, T.; Danovich, D.; De Proft, F.; Shaik, S. Electrophilic aromatic substitution reactions: Mechanistic landscape, electrostatic and electric-field control of reaction rates, and mechanistic crossovers. J. Am. Chem. Soc. 2019, 141, 9719–9730. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Silva, C.; Bertran, J.; Branchadell, V.; Oliva, A. Kemp elimination reaction catalyzed by electric fields. Chemphyschem 2020, 21, 295–306. [Google Scholar] [CrossRef]
- Westlake, B.C.; Brennaman, M.K.; Concepcion, J.J.; Paul, J.J.; Bettis, S.E.; Hampton, S.D.; Miller, S.A.; Lebedeva, N.V.; Forbes, M.D.E.; Moran, A.M.; et al. Concerted electron-proton transfer in the optical excitation of hydrogen-bonded dyes. Proc. Natl. Acad. Sci. USA 2011, 108, 8554–8558. [Google Scholar] [CrossRef]
- Wu, K.L.; Zhang, T.; Wang, Z.A.; Wang, L.; Zhan, L.S.; Gong, S.L.; Zhong, C.; Lu, Z.H.; Zhang, S.; Yang, C.L. De Novo design of excited-state intramolecular proton transfer emitters via a thermally activated delayed fluorescence channel. J. Am. Chem. Soc. 2018, 140, 8877–8886. [Google Scholar] [CrossRef]
- Grundmann, S.; Trabert, D.; Fehre, K.; Strenger, N.; Pier, A.; Kaiser, L.; Kircher, M.; Weller, M.; Eckart, S.; Schmidt, L.P.H.; et al. Zeptosecond birth time delay in molecular photoionization. Science 2020, 370, 339–341. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Sun, R.; Yang, Q.; Gan, P.; Yu, S.; Tan, Z. Research on Electric Field—Induced Catalysis Using Single—Molecule Electrical Measurement. Molecules 2023, 28, 4968. https://doi.org/10.3390/molecules28134968
Lv J, Sun R, Yang Q, Gan P, Yu S, Tan Z. Research on Electric Field—Induced Catalysis Using Single—Molecule Electrical Measurement. Molecules. 2023; 28(13):4968. https://doi.org/10.3390/molecules28134968
Chicago/Turabian StyleLv, Jieyao, Ruiqin Sun, Qifan Yang, Pengfei Gan, Shiyong Yu, and Zhibing Tan. 2023. "Research on Electric Field—Induced Catalysis Using Single—Molecule Electrical Measurement" Molecules 28, no. 13: 4968. https://doi.org/10.3390/molecules28134968
APA StyleLv, J., Sun, R., Yang, Q., Gan, P., Yu, S., & Tan, Z. (2023). Research on Electric Field—Induced Catalysis Using Single—Molecule Electrical Measurement. Molecules, 28(13), 4968. https://doi.org/10.3390/molecules28134968



