Streamlined Efficient Synthesis and Antioxidant Activity of γ-[Glutamyl](n≥1)-tryptophan Peptides by Glutaminase from Bacillus amyloliquefaciens
Abstract
1. Introduction
2. Results and Discussion
2.1. Acceptor Amino Acid Screening
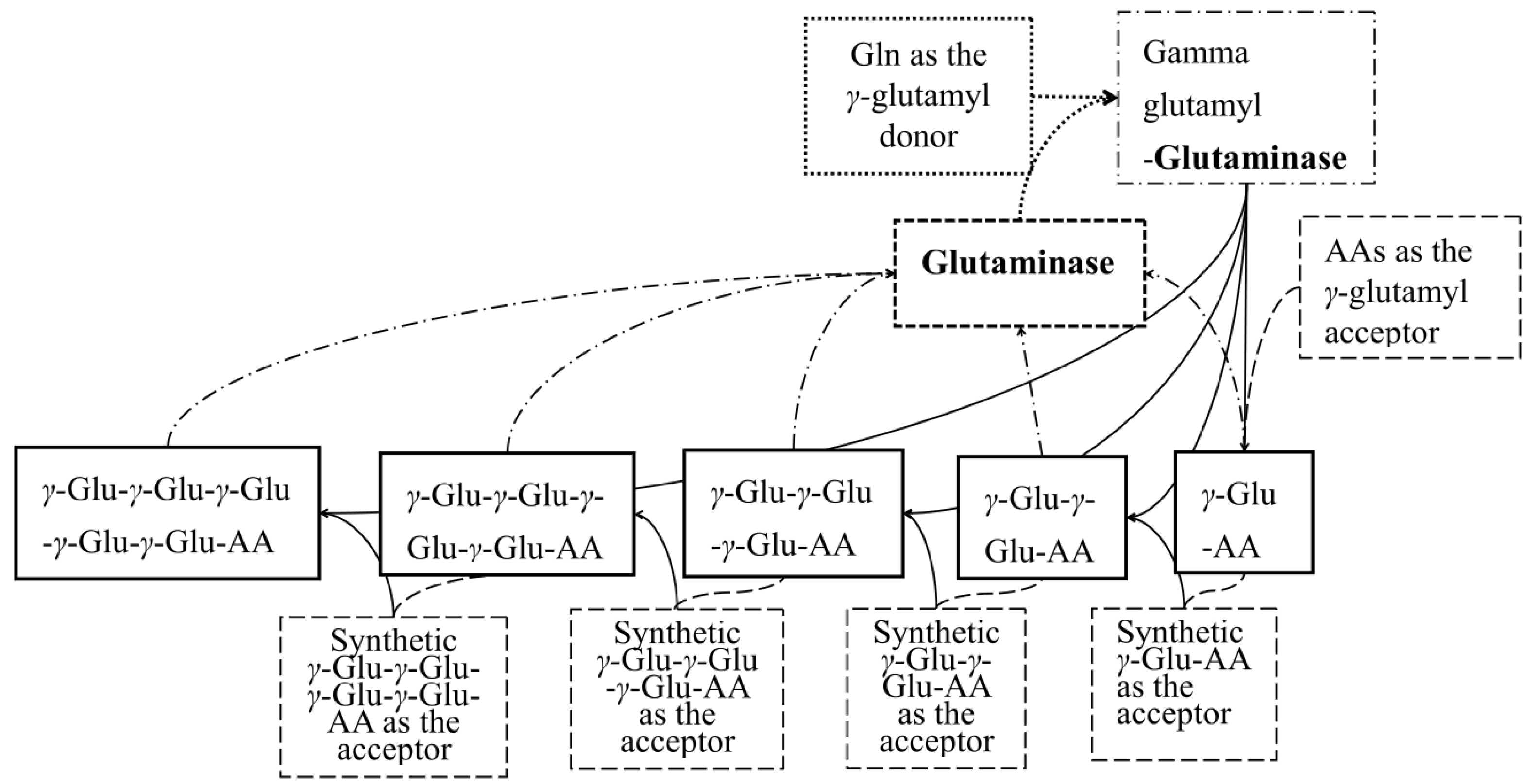
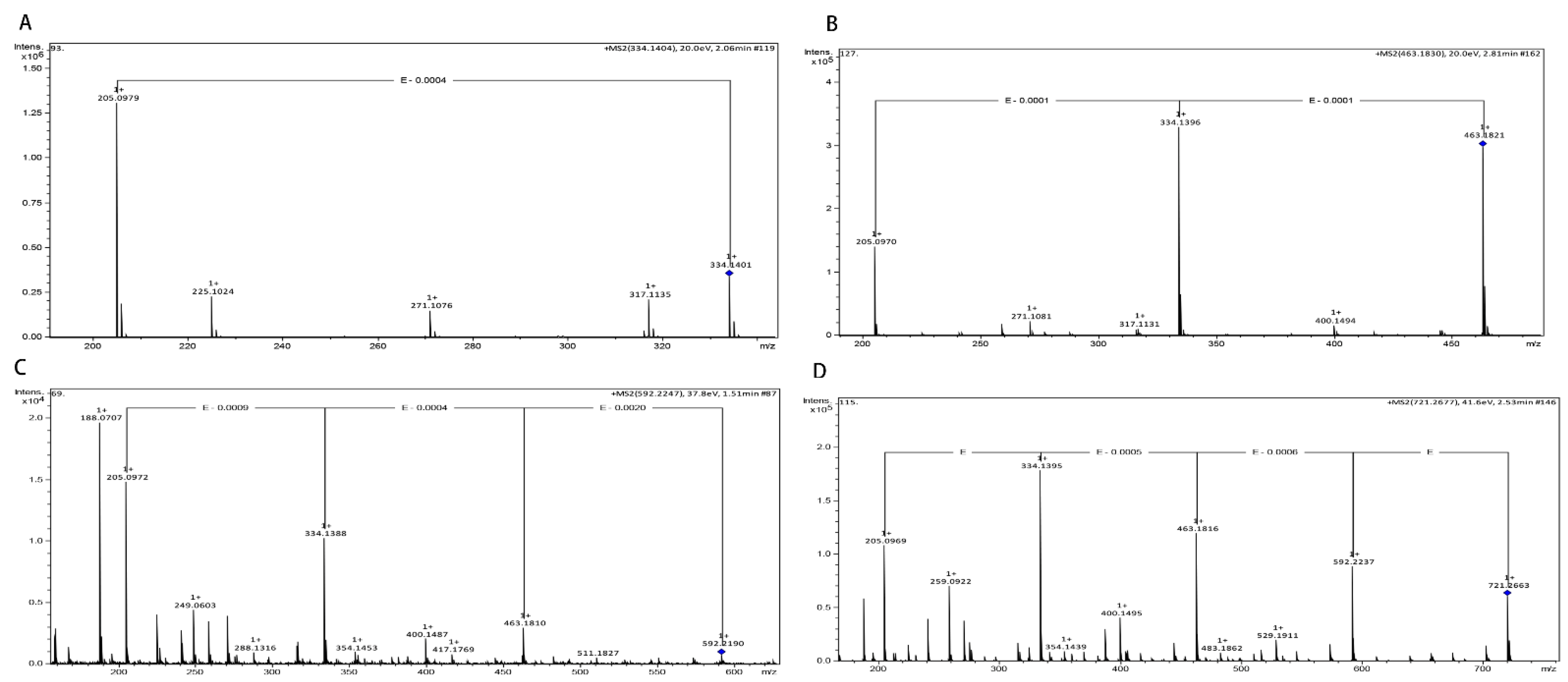
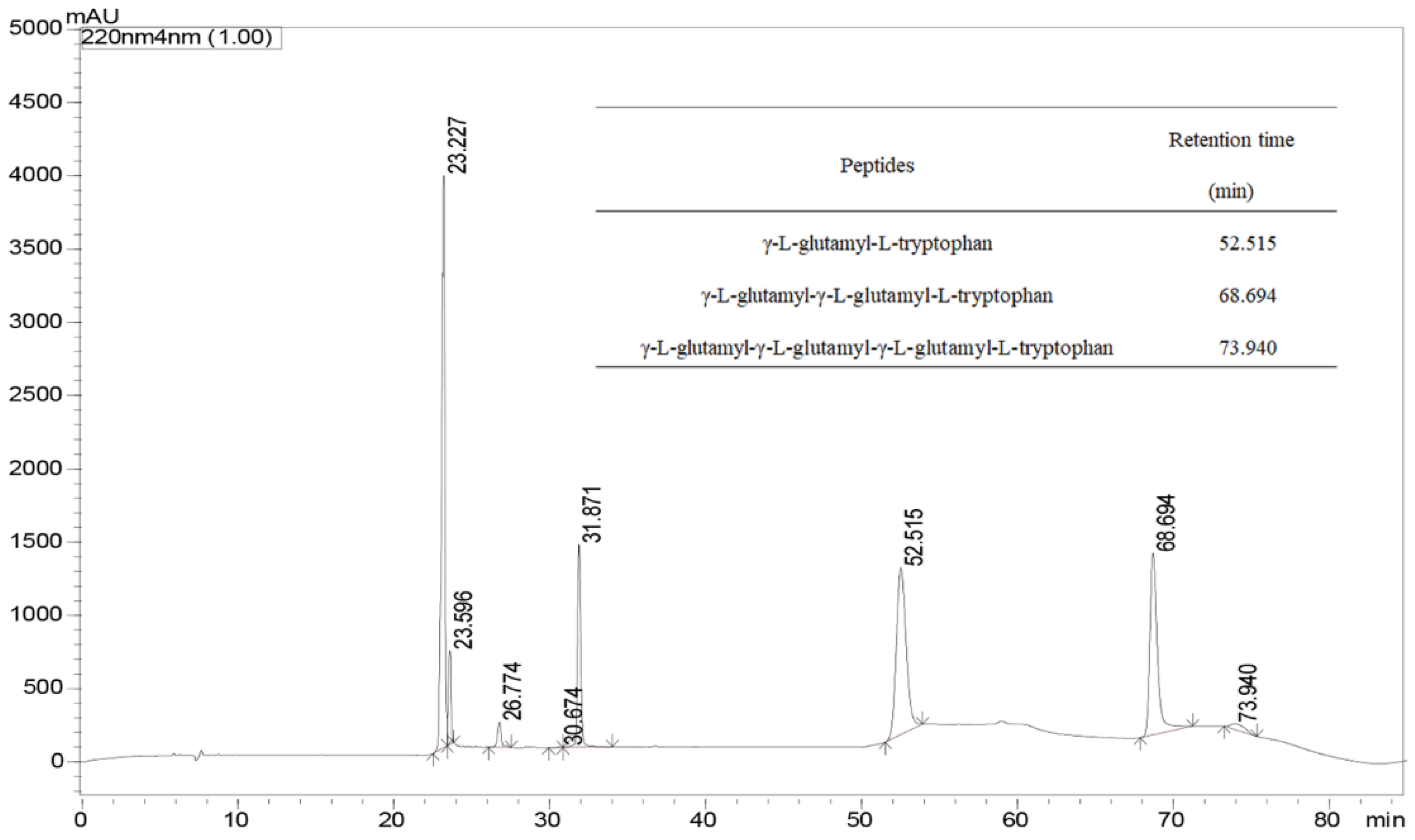
2.2. Identification of γ-[Glu]n-Trp Peptides
2.3. Parameter Optimization of γ-Glu-Peptides Synthesis
2.3.1. Effect of pH
2.3.2. Effect of Temperature
2.3.3. Effect of Enzyme Load
2.3.4. Effect of Synthesis Time
2.3.5. Effect of Substrate Concentration
2.3.6. Effects of Donor/Acceptor Ratio
2.4. Antioxidant Activity
2.4.1. DPPH• Scavenging Assay
2.4.2. ABTS•+ Radical-Scavenging Activity
2.4.3. Reducing Power
2.4.4. Ferrous Ion-Chelating Activity
2.4.5. Superoxide Radical Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. γ-Glutamyl Acceptor Amino Acid Screening
3.3. Identification of γ-[Glu](n=1, 2, 3, 4)-Trp Using UPLC-Q-TOF-MS/MS
3.4. Optimization of γ-Glutamyl Peptides Synthesis Conditions
3.5. Determination of Antioxidant Activity
3.5.1. DPPH• Scavenging Activity
3.5.2. ABTS Radical Scavenging Activity
3.5.3. Reducing Power
3.5.4. Fe2+-Chelating Ability
3.5.5. O2•− Scavenging Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lv, R.; Dong, Y.; Bao, Z.; Zhang, S.; Lin, S.; Sun, N. Advances in the activity evaluation and cellular regulation pathways of food-derived antioxidant peptides. Trends Food Sci. Technol. 2022, 122, 171–186. [Google Scholar] [CrossRef]
- Nishi, K.; Iwaihara, Y.; Tsunoda, T.; Doi, K.; Sakata, T.; Shirasawa, S.; Ishikura, S. ROS-induced cleavage of NHLRC2 by caspase-8 leads to apoptotic cell death in the HCT116 human colon cancer cell line. Cell Death Dis. 2017, 8, 3218. [Google Scholar] [CrossRef] [PubMed]
- Gracia, K.C.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, X.; Zheng, X.; Wang, X.; He, J. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y. Preparation, receptors, bioactivity and bioavailability of γ-glutamyl peptides: A comprehensive review. Trends Food Sci. Technol. 2021, 113, 301–314. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Piu, L.D.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Dunkel, A.; Koster, J.; Hofmann, T. Molecular and sensory characterization of gamma-glutamyl peptides as key contributors to the Kokumi taste of edible beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 6712–6719. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Comprehensive Review of gamma-Glutamyl Peptides (gamma-GPs) and Their Effect on Inflammation Concerning Cardiovascular Health. J. Agric. Food Chem. 2022, 70, 7851–7870. [Google Scholar] [CrossRef]
- Yang, J.; Bai, W.; Zeng, X.; Cui, C. Gamma glutamyl peptides: The food source, enzymatic synthesis, kokumi-active and the potential functional properties—A review. Trends Food Sci. Technol. 2019, 91, 339–346. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Y.Y.; Wang, W.; Zhao, J.; Chen, W.; Ganzle, M. gamma-Glutamyl Cysteine Ligase of Lactobacillus reuteri Synthesizes gamma-Glutamyl Dipeptides in Sourdough. J. Agric. Food Chem. 2018, 66, 12368–12375. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun-Waterhouse, D.; Cui, C.; Dong, K.; Wang, W. Synthesis and Sensory Characteristics of Kokumi gamma-[Glu](n)-Phe in the Presence of Glutamine and Phenylalanine: Glutaminase from Bacillus amyloliquefaciens or Aspergillus oryzae as the Catalyst. J. Agric. Food Chem. 2017, 65, 8696–8703. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun-Waterhouse, D.; Zhou, W.; Cui, C.; Wang, W. Glutaminase-catalyzed γ-glutamylation to produce CCK secretion-stimulatory γ-[Glu]n-Trp peptides superior to tryptophan. J. Funct. Foods 2019, 60, 103418. [Google Scholar] [CrossRef]
- Balagurunathan., R.; Radhakrishnan., M.; Somasundaram., S.T. L-glutaminase producing actinomycetes from marine sediments-selective isolation, semi quantitative assay and characterization of potential strain. Aust. J. Basic Appl. Sci. 2010, 4, 698–705. [Google Scholar]
- Lin, J.; Sun-Waterhouse, D.; Cui, C.; Lu, H. Increasing antioxidant activities of the glutamine-cysteine mixture by the glutaminase from Bacillus amyloliquefaciens. Food Chem. 2020, 308, 125701. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, T.; Jiang, B.; Hua, Y.; Mu, C.-F. An efficient method for the high-yield production of l-theanine using a newly isolated glutaminase-producing organism. Food Biosci. 2019, 28, 164–169. [Google Scholar] [CrossRef]
- Yang, J.; Sun-Waterhouse, D.; Cui, C.; Zhao, H.; Dong, K. Gamma-glutamylation of the white particulates of sufu and simultaneous synthesis of multiple acceptor amino acids-containing γ-glutamyl peptides: Favorable catalytic actions of glutaminase. LWT Food Sci. Technol. 2018, 96, 315–321. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Dong, H.; Huang, G.; Yu, L.; Bai, W.; Zeng, X. The application of L-glutaminase for the synthesis of the immunomodulatory γ-D-glutamyl-L-tryptophan and the kokumi-imparting γ-D-glutamyl peptides. Food Sci. Nutr. 2020, 8, 5841–5849. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, Q.; Sun-Waterhouse, D.; Li, W.; Liu, S.; Cui, C. gamma-[Glu]n-Trp ameliorates anxiety/depression-like behaviors and its anti-inflammatory effect in an animal model of anxiety/depression. Food Funct. 2019, 10, 5544–5554. [Google Scholar] [CrossRef]
- Yang, J.; Sun-Waterhouse, D.; Cui, C.; Dong, K.; Zhao, M. γ-Glu-Met synthesised using a bacterial glutaminase as a potential inhibitor of dipeptidyl peptidase IV. Int. J. Food Sci. Technol. 2018, 53, 1166–1175. [Google Scholar] [CrossRef]
- Yang, J.; Sun-Waterhouse, D.; Xie, J.; Wang, L.; Chen, H.-Z.; Cui, C.; Zhao, M. Comparison of kokumi gamma-[Glu](n>1)-Val and gamma-[Glu](n>1)-Met synthesized through transpeptidation catalyzed by glutaminase from Bacillus amyloliquefaciens. Food Chem. 2018, 247, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alterovitz, G.; Tuthill, C.; Rios, I.; Modelska, K.; Sonis, S. Personalized medicine for mucositis: Bayesian networks identify unique gene clusters which predict the response to gamma-D-glutamyl-L-tryptophan (SCV-07) for the attenuation of chemoradiation-induced oral mucositis. Oral Oncol. 2011, 47, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Bindal, S.; Gupta, R. Heterologous expression of γ-glutamyl transpeptidase from Bacillus atrophaeus GS-16 and its application in the synthesis of γ-D-glutamyl-L-tryptophan, a known immunomodulatory peptide. Enzym. Microb. Technol. 2017, 99, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Chun, J.; Thomas, D.G.; Schnieders, M.J.; Marucho, M.; Zhang, J.; Baker, N.A. Biomolecular electrostatics and solvation: A computational perspective. Q. Rev. Biophys. 2012, 45, 427–491. [Google Scholar] [CrossRef]
- Tang, R.; Sun-Waterhouse, D.; Xiong, J.; Cui, C.; Wang, W. Feasibility of synthesizing γ-[Glu] (n≥1)-Gln using high solid concentrations and glutaminase from Bacillus amyloliquefaciens as the catalyst. Food Chem. 2020, 310, 125920. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 2002, 40, 945–948. [Google Scholar] [CrossRef]
- Byun, H.-G.; Lee, J.K.; Park, H.G.; Jeon, J.-K.; Kim, S.-K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Zhao, H.; Wang, H.; Zhao, M.; Wang, W.; Dong, K. The effect of high solid concentrations on enzymatic hydrolysis of soya bean protein isolate and antioxidant activity of the resulting hydrolysates. Int. J. Food Sci. Technol. 2018, 53, 954–961. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Ganzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.-K. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G.; Leeuwenburgh, C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J. Agric. Food Chem. 2008, 56, 10359–10367. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Castilla, J.; Hernandez-Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Giron-Calle, J.; Vioque, J.; Davila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, X.; Gao, M.; Zhang, C.; Peng, B. Resource Utilization of Bovine Neck Ligament: Enzymatic Preparation of Elastin Peptide and Its Antioxidant Activity. Appl. Biochem. Biotechnol. 2022, 195, 33–50. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloglu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea desf ex DC), sage (Salvia triloba L.), and black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhang, W.G.; Zhou, G.H.; Xu, X.L.; Kang, Z.L.; Yin, Y. Isolation and Identification of Antioxidant Peptides from Jinhua Ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef]
- Abeynayake, R.; Zhang, S.; Yang, W.; Chen, L. Development of antioxidant peptides from brewers’ spent grain proteins. LWT Food Sci. Technol. 2022, 158, 113162. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A Comprehensive Review of Corn Protein-derived Bioactive Peptides: Production, Characterization, Bioactivities, and Transport Pathways. Compr. Rev. Food Sci. Food Saf. 2019, 18, 329–345. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.A.; Yu, X.; Owusu, J.; Ma, H.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Kerekes, J.P. Potential of Red Edge Spectral Bands in Future Landsat Satellites on Agroecosystem Canopy Green Leaf Area Index Retrieval. Remote Sens. 2018, 10, 1458. [Google Scholar] [CrossRef]
- Cui, Z.; Kerekes, J. Potential of Red Edge Spectral Bands in Future Landsat Satellites on Agroecosystem Canopy Chlorophyll Content Retrieval. In Proceedings of the IGARSS 2019—2019 IEEE International Geoscience and Remote Sensing Symposium, Yokohama, Japan, 28 July–2 August 2019; pp. 7168–7171. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation and characterisation of enzymatic hydrolysed peptides with antioxidant activities from green tender sorghum. LWT Food Sci. Technol. 2017, 84, 608–616. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, Y.; Huang, M.; Zhou, G. Identification and Characterization of Antioxidant Peptides from Enzymatic Hydrolysates of Duck Meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Huang, X.; Kelimu, A.; Li, W.; Cui, C. Streamlined Efficient Synthesis and Antioxidant Activity of γ-[Glutamyl](n≥1)-tryptophan Peptides by Glutaminase from Bacillus amyloliquefaciens. Molecules 2023, 28, 4944. https://doi.org/10.3390/molecules28134944
He W, Huang X, Kelimu A, Li W, Cui C. Streamlined Efficient Synthesis and Antioxidant Activity of γ-[Glutamyl](n≥1)-tryptophan Peptides by Glutaminase from Bacillus amyloliquefaciens. Molecules. 2023; 28(13):4944. https://doi.org/10.3390/molecules28134944
Chicago/Turabian StyleHe, Wenjiang, Xiaoling Huang, Abulimiti Kelimu, Wenzhi Li, and Chun Cui. 2023. "Streamlined Efficient Synthesis and Antioxidant Activity of γ-[Glutamyl](n≥1)-tryptophan Peptides by Glutaminase from Bacillus amyloliquefaciens" Molecules 28, no. 13: 4944. https://doi.org/10.3390/molecules28134944
APA StyleHe, W., Huang, X., Kelimu, A., Li, W., & Cui, C. (2023). Streamlined Efficient Synthesis and Antioxidant Activity of γ-[Glutamyl](n≥1)-tryptophan Peptides by Glutaminase from Bacillus amyloliquefaciens. Molecules, 28(13), 4944. https://doi.org/10.3390/molecules28134944




