Abstract
Connexin 43 (Cx43) is most widely distributed in mammals, especially in the cardiovascular and nervous systems. Its phosphorylation state has been found to be regulated by the action of more than ten kinases and phosphatases, including mitogen-activated protein kinase/extracellular signaling and regulating kinase signaling. In addition, the phosphorylation status of different phosphorylation sites affects its own synthesis and assembly and the function of the gap junctions (GJs) to varying degrees. The phosphorylation of Cx43 can affect the permeability, electrical conductivity, and gating properties of GJs, thereby having various effects on intercellular communication and affecting physiological or pathological processes in vitro and in vivo. Therefore, clarifying the relationship between Cx43 phosphorylation and specific disease processes will help us better understand the disease. Based on the above clinical and preclinical findings, we present in this review the functional significance of Cx43 phosphorylation in multiple diseases and discuss the potential of Cx43 as a drug target in Cx43-related disease pathophysiology, with an emphasis on the importance of connexin 43 as an emerging therapeutic target in cardiac and neuroprotection.
1. Introduction
Connexins are proteins that are broadly distributed throughout the body and are crucial for heart and brain health. At the plasma membrane, six connexin subunits come together to form a linker or hemichannel. Gap junction channels (GJCs) are created as a result of the two hemichannels interacting with one another in a head-to-head configuration [1]. Gap junctions supply the intracellular communications that are linked to prolonged physiological processes, cellular development, and stability [2,3,4]. These communications are also associated with the rapid exchange of cations, transmitters, and second messengers, which are fundamental atoms for electrophysiological excitability and its transmission. The regulation of intracellular Ca2+ mobilization and K+ buffering, in particular, helps the astroglial gap junction contribute to the cytoplasm-to-cytoplasm communication of biochemical or ionic mobilization between a cell and adjacent cells, resulting in the regulation of ionic and several other types of homeostasis [5]. Through the formation of functioning syncytial networks in the central nervous system (CNS), astrocytes serve crucial homeostatic roles. Compared to other connexins, such as Cx26, Cx30, and Cx32, connexin 43 (Cx43), which is expressed by astrocytes, is the most prevalent connexin in the brain and is one of the key components of gap junctions [6]. Similarly, Cx43 is also the most prominent connexin in the ventricles, which forms gap junctions and hemichannels and is also present in the mitochondria of the heart [7]. Thus, it plays an important role in the cardiovascular system and the nervous system.
Approximately 80% of astrocyte coupling in the hippocampus is mediated by Cx43, which has at least 21 phosphorylation sites on its C-terminus [8]. Protein kinase C (PKC) has been demonstrated to phosphorylate Cx43 at Ser368 and Ser372 in vitro, and tissue polypeptide antigen (TPA) has been shown to promote Ser262 and Ser368 phosphorylation [9]. Ser368 phosphorylation has also been demonstrated to be responsible for the TPA-induced decrease in intercellular communication. Purified recombinant Cx43 was phosphorylated by PKC, which eliminated sucrose and Lucifer yellow permeability and caused a conformational shift in Cx43. In cardiomyocytes, PKC was discovered to connect with Cx43. In lens epithelial cells, PKC binds with Cx43 [10]. Fibroblast growth factor-2 increased the colocalization of PKC with Cx43 via increasing Cx43 phosphorylation and decreasing cardiomyocyte gap junctional conductance [11]. It has been demonstrated that casein kinase 1 (CK1) interacts with and phosphorylates Cx43, especially the isoform [12]. There are other kinases that undoubtedly phosphorylate Cx43, and it indicates that different kinases can phosphorylate the same position. For instance, mitogen-activated protein kinase (MAPK) has been observed to phosphorylate Ser255 [13]. Therefore, a large body of evidence suggests that Cx43 is a highly phosphorylated and tightly controlled protein.
Cx43 phosphorylation regulates the opening and closing of gap junction channels and hemichannels. During a steady state, cultured astrocytes exhibit that gap junctional communication is active at a high level, while hemichannel activity is modest [14]. In contrast, depolarization, ischemia, specific cation mobilization, and connexin phosphorylation activate the hemichannel, resulting in the astroglial sustained release of excitatory L-glutamate, D-serine, adenosine triphosphate, kyonurenine metabolites, and eicosanoids [2,15,16]. At several stages of the connexin ‘life cycle’, phosphorylation plays a role in gap junctional cell-to-cell communication (GJIC), including trafficking, assembly/disassembly, and the gating of gap junction channels [17,18]. The phosphorylation of Cx43 is a fundamental regulation process during disease progression. Many growth factors, oncogenes, and tumor-promoting chemicals can phosphorylate Cxs and may be involved in the occurrence and development of depression, cerebral ischemia, arrhythmia, neurodegenerative diseases, and other diseases [19] (Figure 1). Additionally, it has been shown in various systems that a number of important serine phosphorylation sites are crucial for mediating both slow and quick changes in Cx43 expression or plaque stability. For instance, the ubiquitin protein ligase Nedd4 binds the gap junction-specific protein Cx43 in living organisms. The interaction between Nedd4 and Cx43 does not require the Ser279/Ser282 phosphorylation of Cx43. Instead, the binding of both proteins may be modulated by the Cx43 phosphorylation status. The interaction of the two proteins definitely affects both the internalization of gap junctions and the post-internalization degradation of the gap-junction proteins [20]. Thus, the molecular mechanism of Cx43 phosphorylation is of great importance.
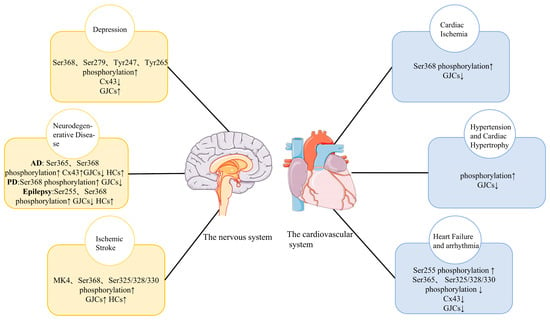
Figure 1.
Changes in Cx43 phosphorylation in major diseases of the cardiovascular system and the nervous system and its influence on Cx43 HCs and Cx43 GJIC. Cx43, Connexin 43; HC, hemichannel; GJIC, gap junction intercellular communication; ↑, Increase; ↓, Decrease.
2. Functional Significance of Phosphorylation of Cx43 in the Nervous System
2.1. Changes in Cx43 Phosphorylation Associated with Depression
Among many types of connexins, Cx43 is a major component of gap junctions in the brain, expressed primarily in astrocytes, and is required for astrocyte-mediated neuroprotection. Accumulating evidence suggests that astrocytes play an important role in the pathogenesis of major depression disorder (MDD). In particular, gap junction dysfunction in astrocytes is a potential target for MDD therapy. Several lines of evidence suggested the mechanism of stress-induced gap junction dysfunction in the prefrontal cortex and hippocampal astrocytes. Additionally, they experimentally demonstrated that corticosterone (CORT) disrupted the function of gap junctions due to the reduced distribution of Cx43 on the cell membrane and enhanced the phosphorylation of Cx43 at the Ser368 site [21]. When patients with depression undergo in vitro therapy, CORT simulates high levels of glucocorticoids. Previous data showed that Cx43 and Cx30 were significantly downregulated in patients with depression and in animal models of depression [22,23,24,25,26]. In addition, Cx43 mRNA has decreased in depressed patients [22,23,25,27]. In addition, several postmortem investigations showed that major depressive patients’ locus coeruleus, frontal cortex, mediodorsal thalamic nucleus, and caudate nucleus Cx43 expression was diminished in comparison to healthy people [22,23,24,25]. Moreover, it has been demonstrated that alterations in Cx43 expression in astrocytes are closely related to the development of various neuropathological diseases, including epilepsy, ischemia, Parkinson’s disease, and Huntington’s disease [28,29,30]. Furthermore, a recent study showed that interfering with cell communication by downregulating Cx43 gap junctions in the rat prefrontal cortex induces depression-like behaviors [26]. Studies have shown that Cx43 phosphorylation at Ser368 is elevated in both the corticosterone-exposed prefrontal cortex and hippocampal astrocytes. [31,32,33]. Another study found that the phosphorylation of Cx43 at Ser368 induced the internalization and degradation of gap junctions [34] (Figure 2). It has been demonstrated that the monomer ginsenoside Rg1 used in traditional Chinese medicine has antidepressant properties. By reducing the malfunctioning of gap junction channels (GJCs), Rg1 has antidepressant effects. It is possible that the improved function of GJCs may be due to Rg1 reversing the increased phosphorylation of Cx43 at Tyr265 and Ser279 sites induced by CORT [35]. Chronic unpredictable stress can increase C-Src expression. C-Src can directly phosphorylate Cx43 at Tyr247 and Tyr265 to cause the closure of the GJs channel [36]. Combined with these findings, targeting Cx43 phosphorylation to modulate gap junction function may be an effective approach for the treatment of MDD.
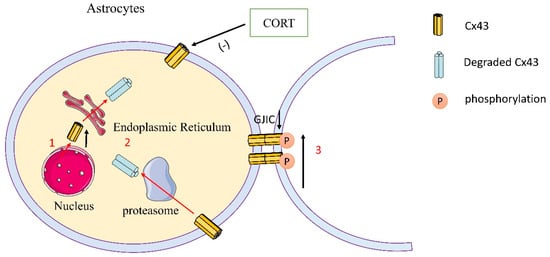
Figure 2.
Schematic representation of Cx43 expression and phosphorylation of astrocytes in depression. (1) CORT reduced the synthesis of Cx43 and the distribution of Cx43 in the membrane in astrocytes. (2) Cx43 was degraded by a proteasome. (3) CORT reduced the distribution of Cx43 in the membrane and in the meantime increased the phosphorylation of Cx43 at Ser368 in the prefrontal cortical astrocytes and hippocampal astrocytes, thereby inhibiting the communication mediated by gap junctions.
2.2. Changes in Cx43 Phosphorylation in Neurodegenerative Diseases
2.2.1. Alzheimer’s Disease
Cx43 has been reported to be involved in developing certain neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and epilepsy [37,38] (Figure 3). The prominent pathological feature of Alzheimer’s disease (AD) is the formation of amyloid plaques, which are deposits of different sizes of small peptides called β-amyloid (Aβ). These Aβ plaques are derived via sequential proteolytic cleavages of the Aβ precursor protein (APP) by the action of β- and γ-secretases. Among the different Aβ fragments, Aβ1–40 and Aβ1–42 are thought to be critical elements in AD pathogenesis due to their neuropil and vascular accumulation. Researchers pointed out that AD brains showed the upregulation of A2AR and Cx43, which form hemichannels in astrocytes that can mediate ATP release [39]. A2AR directly regulates hemichannel activity and inhibits the upregulation and phosphorylation of Cx43 in Aβ1–42-exposed astrocytes. While hemichannels can open under resting conditions to facilitate basal paracrine communication, they are generally believed to become more active under pathological conditions [14,40,41,42,43,44]. The activity of hemichannels is highly regulated by connexin phosphorylation. In particular, Cx43 can be phosphorylated by several kinases, such as (PKC), protein kinase A (PKA), cyclin-dependent kinase p34cdc2, and casein kinase 1 [45,46,47]. Notably, Cx43 levels were upregulated in astrocyte processes near Aβ plaques in both animal AD models and brain samples from human AD patients [48,49,50,51]. A2AR regulates Cx43 levels and phosphorylation in Aβ1–42-exposed astrocytes. Connexin phosphorylation at Ser 368 can modulate hemichannel activity in astrocyte cultures [6,52]. Furthermore, extracellular ATP-derived adenosine plays an important role in the maintenance of A2AR overactivation in the Aβ1–42-induced upregulation of Cx43 levels and phosphorylation status. The extracellular conversion of ATP to adenosine by CD73 maintains the A2AR-mediated increase in Cx43 hemichannel activity to support the further release of ATP and other astrocyte signals that contribute to neurodegeneration. Astrocyte Cx43 hemichannels are increasingly recognized as relevant mediators of memory impairment in AD patients. Previous research is the first to demonstrate that the A2AR-mediated control mechanism of Cx43 activity in astrocytes may be related to the phosphorylation of Cx43, a well-established mechanism for regulating Cx43 hemichannel activity [44,46,53,54]. In Aβ1–42-stimulated astrocytes, the phosphorylation of Cx43 Ser368 residues was enhanced, and a surge in hemichannel activity and ATP release was observed. Studies have shown that the phosphorylation of connexin 43 increases at week 42 of AD, which is consistent with the dysregulation of gap junctions and the activation of astrocytes [55]. The study identified two sites of increased phosphorylation on Cx43, Ser365, and Ser368. Increased phosphorylation at Ser368 is functionally associated with a decrease in gap junction permeability and is generally associated with decreased traumatic brain injury (TBI) regions and glutamate clearance [56]. Therefore, targeting astrocyte Cx43 hemichannels may be a promising therapeutic approach for AD.
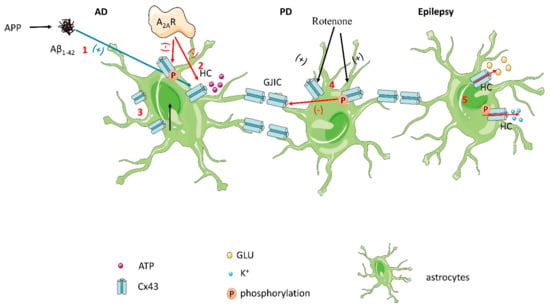
Figure 3.
(1) In Aβ1–42-stimulated astrocytes, phosphorylation of Cx43 Ser368 residues is enhanced, resulting in a surge in hemichannel activity and ATP release. (2) A2AR directly regulates hemichannel activity and inhibits the upregulation and phosphorylation of Cx43 in Aβ1–42-exposed astrocytes. (3) Cx43 expression is increased in AD patients. (4) In rotenone-treated cultured astrocytes, Cx43 protein levels and phosphorylation levels were enhanced, and phosphorylated Cx43 led to gap junction cell-to-cell communication dysfunction. (5) In epilepsy, phosphorylation of Cx43 results in the release of K+ and glutamate, which increases connection conductance.
2.2.2. Parkinson’s Disease
Recent evidence revealed that the major connexin in astrocytes, connexin 43, was enhanced in a rotenone-induced model of rat Parkinson’s disease (PD) [57]. In rotenone-treated cultured astrocytes, the enhancement of Cx43 protein levels paralleled the increase in gap junction cell-to-cell communication but was not accompanied by an increase in Cx43 mRNA levels. In addition, rotenone-induced increases in Cx43 protein levels were associated with increased levels of phosphorylated Cx43. In a rat model of PD, phosphorylated Cx43 was selectively enhanced in the basal ganglia region, which contains dopamine (DA) neurons or their terminal regions. Cx43 is primarily phosphorylated at Ser368 residues and exists in multiple isoforms after electrophoretic separation, non-phosphorylated (P0~41 kDa), or phosphorylated (P1~44 and P2~46 kDa) variants [58,59]. The phosphorylation levels of P0 and P1 were enhanced during the induction of Cx43 total protein by rotenone [58]. The findings suggest that the regulation of Cx43 protein phosphorylation in astrocytes, which leads to a reduction in gap junction cell communication, may play an important role in PD pathology.
2.2.3. Epilepsy
The role of Cx43 GJs in epilepsy is complex and obscure. According to the literature, there are two possible theories: GJIC is thought to be critical for maintaining tissue homeostasis and propagating electrical and metabolic signals in cell populations [60]. Consistent with this, GJs can suppress seizure activity by redistributing K+ and glutamate (Glu) [61,62,63,64,65]. Astrocytes are electrically and metabolically connected to each other through gap junctions mainly composed of Cx43 and Cx30, forming a functional network [66,67]. It was found that coupling was completely absent in hippocampal tissue excised from patients with mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS). In contrast, coupled astrocytes were abundantly present in the hippocampus of non-sclerotic MTLE patients [68]. Recent reports using phospho-specific antibodies suggest that the phosphorylation of Ser365 is necessary for conversion to the P1 isoform [69]. In Co2+-induced epileptiform activity in hippocampal slices, a marked increase in P1 and a slight increase in P2 and P0 Cx43 have been reported [70]. The phosphorylation of Cx43 at Ser255 and/or 368 was enhanced. Phosphorylation at Ser255 and Ser368 of the Cx43 c-terminal reduces junctional conductance. The phosphorylation of MAPK at Ser255 reduces the open probability of Cx43 channels, whereas Ser368 is targeted by PKC, resulting in an increased open probability [4,71]. Taken together, the redistribution of the Cx43 protein and/or the alteration of its C-terminal phosphorylation may contribute to the uncoupling of MTLE-HS. Importantly, it provides new insights into the molecular mechanisms underlying astrocyte dysfunction in epilepsy, which may be key to the development and progression of epilepsy disorders.
2.3. Variations in Cx43 Phosphorylation in Conditions Affecting Cerebral Blood Flow
2.3.1. Ischemic Stroke Induces the Occurrence of Cx43 Phosphorylation
The function of Connexin 43 is influenced by kinases that phosphorylate specific serine sites near its C-terminus. Stroke is a powerful inducer of kinase activity. Researchers studied wild-type (WT) and knock-in Cx43 at PKC site Cx43 Ser368, casein kinase 1 (CK1) site Cx43 Ser325/328/330, and MAPK site Cx43 Ser255/262/279/282 (MK4) in a permanent middle cerebral artery occlusion (pMCAO) stroke model [72]. The results of the research showed that astrocyte Cx43 gap junction channels (GJCs) affect neuronal survival under ischemic conditions [73,74,75]. The MAPK phosphorylation levels were more pronounced in WT and MK4 astrocytes when stressed in vitro for 3 h in ischemia buffer. Under normal circumstances, astrocyte Cx43 can exist in hemichannels or couple with other hemichannels on astrocytes, neurons, or oligodendrocytes to form glial syncytia, which are involved in the interaction of communication cells. The exchange of metabolites between them, thereby, maintains the nervous system environment. In ischemic stroke, the phosphorylation of Cx43 may lead to the degradation of gap junctions and the opening of hemichannels, thereby promoting the release of inflammatory mediators. The remaining gap junctions can facilitate the exchange of protective and harmful metabolites between healthy and damaged cells, protecting damaged cells to some extent. Various studies have shown that the phosphorylation state of the astrocyte Cx43 C-terminus is an important mediator of the regulation of gap junction channels and hemichannels after ischemic stroke, thereby affecting astrocyte and neuronal function [76,77]. It has been reported that the C-terminus of Cx43 in astrocytes after ischemic stroke can be phosphorylated by a variety of protein kinases, including protein kinase C, mitogen-activated protein kinase (MAPK), pp60Src kinase, and casein kinase 1δ, inducing Cx43 internalization and further promoting the uncoupling process of astrocytes [18,78,79,80,81,82]. Interestingly, other studies have found that hypoxia in vitro may lead to C-terminal dephosphorylation of astrocyte Cx43, accompanied by astrocyte uncoupling [52,82]. These studies suggest that the gap junction uncoupling process in astrocytes is intermediate between the phosphorylation and dephosphorylation of Cx43 and may be the result of Cx43 phosphorylation and the cause of hemichannel Cx43 dephosphorylation. Furthermore, hemichannel opening induced by Cx43 dephosphorylation in astrocytes promotes the release of inflammatory mediators and increases neuroinflammation after ischemic stroke [65,83]. In ischemic stroke, the phosphorylation of astrocyte Cx43 may lead to the uncoupling of gap junctions between astrocytes and other parenchymal cells, thereby reducing the direct communication between these cells. The subsequent dephosphorylation of Cx43 on hemichannels activates hemichannel opening, promoting the release of various proinflammatory mediators and toxic molecules, such as ATP and Glu.
2.3.2. Phosphorylation of Cx43 in Cerebral Ischemia
The process of cerebral ischemia/reperfusion (I/R) injury is often accompanied by the enhancement of gap junction (GJ) function and the increase in gap junction protein expression. It has been reported that in the event of I/R injury, the increased expression of Cx43 increases the release of Glu, ultimately leading to the injury of astrocytes [84]. There is a growing body of literature that reveals that Cx43 expression is abnormally elevated when the brain is damaged by ischemia, and the inhibition of Cx43 expression can improve brain I/R injury [85]. During cerebral ischemia-reperfusion, normal cells provide energy support and signal transmission to neighboring cells through gap junctions to reduce cell damage. In the ischemic brain, the expression of Cx43 in activated astrocytes is drastically decreased, followed by rapid dephosphorylation and internalization of superficial protein epitopes, leading to reduced neuroprotective effects of gap junctions [86]. Studies have shown that Cx43 localization and phosphorylation are significantly regulated during ischemia and injury [87,88]. In addition, increased Cx43 and elevated levels of Cx43 phosphorylation have been detected after ischemia induction [89]. These results not only confirm the formation of Cx43 heteromeric channels but also suggest that Cx43 and phosphorylated-Cx43 heteromeric channels are involved in brain injury during ischemia. Previous research has established that astrocytes mainly contain phosphorylated Cx43 and that these undergo dephosphorylation after hypoxia [80]. In a recent study, researchers found that the phosphorylation of Cx43 was increased in the brain after ischemia. The membrane Cx40/Cx43 and Cx40/phospho-Cx43 complexes have been shown to be increased during ischemia [90]. Heteromeric channels are reported to be inconsistent with homo-polymeric channels. Heteromeric channels display strongly asymmetric voltage-dependent gating responses. Therefore, increased Cx40/Cx43 and Cx40/phospho-Cx43 heteromeric phosphor-Cx43 membrane may contribute to the changes in gap junctions after ischemia induction that can lead to brain damage. Furthermore, the degradation of Cx43 during cerebral ischemia is caused by selective autophagy, thereby affecting neuroinflammation and apoptosis. It has been found that Cx43 is phosphorylated at Ser368, Tyr247, and Tyr265 during cerebral ischemia [91]. The phosphorylation of Cx43 is a mechanistic initiator leading to its autophagic degradation during cerebral ischemia. In HS (vascular dementia due to cerebral ischemia, hypoperfusion) with hippocampal sclerosis, Cx43 is characterized by enhanced C-terminal phosphorylation affecting channel permeability and enhanced phosphorylation at Ser255 and/or 368. Phosphorylation at positions Ser255 and Ser368 of the Cx43 C-terminal reduces junctional conductance.
2.3.3. Phosphorylation of Cx43 in Cerebral Vasospasm
Cerebrovascular disease is the second leading cause of death among Chinese citizens, among which cerebral vasospasm caused by spontaneous subarachnoid hemorrhage is the main factor regarding the high disability and mortality rates. Recent evidence suggested that the phosphorylation of Cx43 might play an important role in cerebral vasospasm after subarachnoid hemorrhage [92,93]. The authors found that in a rabbit model of secondary subarachnoid hemorrhage, the phosphorylation level of Cx43 in the cerebral basilar artery was significantly increased compared with the normal group, and it showed a chronological change, whereas the dephosphorylation was the opposite. At the same time, the basilar artery angiography in rabbits revealed that its contraction was positively correlated with the phosphorylation level of Cx43, which further indicated that the phosphorylation of Cx43 was related to cerebral vasospasm. In addition, PKC activity was noticeably increased in the membrane fraction of vasospastic cerebral arteries, and PKC controls GJIC spatially and dynamically by phosphorylating Cx43 at Ser368 [94].
2.4. Cx43 Phosphorylation and Its Associated GJs Play Important Roles in Cancer
All the available data suggest that Cx43 and its associated GJs play an important role in cancer. The expression level of Cx43 showed a downward trend and increased levels in malignant tumors, especially in astrocytomas. GJ intercellular communication activity in glioma cells can be regulated by Cx43 phosphorylation and by combining Cx43 and its related proteins [95]. Cx43 can enhance the motility and invasiveness of astrocytoma cells. It also affects the separation of glioma cells from the tumor core into the peritumoral neocortex [96]. Decreased GJIC in tumor cells is often associated with decreased Cx43 expression [97]. Cx43 can be the potential target of therapy for malignant gliomas. In addition, the epidermal growth factor receptor (EGF receptor) signal transduction pathways are intricately involved in various processes that contribute to the development of malignancies, such as cell cycle progression, the inhibition of apoptosis, angiogenesis, tumor cell motility, and metastasis [98]. EGF has been shown to induce Cx43 serine phosphorylation and the inhibition of gap junctional intercellular communication in cell lines derived from multiple tissue types [99,100].
3. Functional Significance of Cx43 Phosphorylation in the Cardiovascular System
Cx43 is the major ventricular gap junction protein, but it is also expressed in atrial and endothelial cells. Numerous studies have shown that post-translational phosphorylation of Cx43 severely affects intercellular coupling through gap junction remodeling under pathological conditions. While many kinases have been shown to phosphorylate Cx43, only Akt, MAPK, and PKC have been shown to modulate the activity of the Cx43 hemichannel, potentially affecting cardiac excitability. For example, the phosphorylation of AKT, also known as protein kinase B (PKB), on Ser373 increases hemichannel function by increasing the levels of surface Cx43, while PKC inhibits hemichannel activity through the phosphorylation of Ser368 [101,102,103,104,105,106,107]. Src is a tyrosine kinase known to both phosphorylate Cx43 (residues Tyr247 and Tyr265) and affect gap junction intercellular communication. In diseased cardiac tissue, in which Src is active, an increase in intercalated disc and intracellular localized Cx43 phos-Tyr313 has been observed [108].
3.1. Cx43 Phosphorylation and Dephosphorylation Are Associated with Cardiac Ischemia/Reperfusion Injury
Myocardial ischemia leads to the dephosphorylation of Cx43 and the redistribution of gap junction components away from intercalated discs. In this way, the Cx43 protein loses its supportive role in coordinating contractile activation and triggers arrhythmias in the ischemic heart [109]. Furthermore, the repositioned Cx43 can operate as an open hemichannel. By blocking these channels to prevent the loss of key metabolites, the store ion imbalance, hindered cell swelling, and myocardial ischemia/reperfusion injury can be prevented [110,111]. Prolonged ischemia/hypoxia (>15 min) has been reported to induce sarcolemmal redistribution of Cx43 in isolated rat and rabbit hearts [112,113]. The dephosphorylation of Ser365 occurred rapidly (5 min) after ischemia, followed by increased phosphorylation of Ser368. This phenomenon is consistent with the ‘gatekeeper’ concept that Ser365 phosphorylation prevents Ser368 phosphorylation, resulting in an inverse relationship in vivo [114,115]. Therefore, Cx43 phosphorylation is essential in regulating hypoxia-induced cardiac injury.
3.2. Cx43 Phosphorylation in Hypertension and Cardiac Hypertrophy
The conclusions of studies related to Cx43 phosphorylation in hypertension are controversial. For instance, a recent study found that Cx43 expression was upregulated and Cx43 phosphorylation levels were increased [116,117,118]. However, Cx43 expression decreased in some studies [119,120]. It has also been suggested that some of the observed differences may be related to the degree of left ventricular hypertrophy associated with hypertension because in the human heart, mild hypertrophy increases, while extensive hypertrophy decreases left ventricular Cx43 expression [121]. Increased Cx43 protein levels and enhanced Cx43 phosphorylation in early cardiac hypertrophy have been observed. The prevention or attenuation of maladaptive myocardial Cx43 remodeling and dysfunction caused by hypertension may reduce the risk of arrhythmic death and heart failure [84]. Furthermore, all the studies on diabetes and hypercholesterolemia showed increased Cx43 phosphorylation [122,123].
3.3. Cx43 Phosphorylation in Heart Failure and Arrhythmia
In some heart failure studies, Cx43 was dephosphorylated and the phosphorylation level of the Ser255 residue of Cx43 was increased [124,125]. Decreased phosphorylation of Cx43 at Ser365 (PKA-dependent site) or Ser325/328/330 (CK1δ-dependent site) results in slowed cardiac conduction and enhanced arrhythmia [126,127,128]. In addition, studies have shown that chronic heart disease and arrhythmias, including cardiac hypertrophy and heart failure, are associated with decreased total Cx43 but increased dephosphorylated Cx43 [129]. Restricted cell-to-cell communication, hampered action potential propagation, and the emergence of cardiac arrhythmia are all a result of Cx43 being phosphorylated or dephosphorylated by p38. Additionally, p38 inhibition enhances cell-to-cell communication and diminishes arrhythmia occurrence [130]. Studies in wild-type and transgenic mice show that enhanced CK1 phosphorylation by Cx43 protects against arrhythmias [83].
4. Functional Significance of Cx43 Phosphorylation in Other Tissues
4.1. Cx43 Phosphorylation in Endothelial Tissue
Connexins expressed in the vasculature include Cx37, 40, 43, and 45 [131]. Cx43 phosphorylation has also been shown to change during atherogenesis, in which minimally modified low-density lipoprotein accumulates in the vessel wall to form plaques [132]. In a mouse model of atherosclerosis, the total Cx43 expression was decreased in the carotid arteries, whereas the phosphorylation levels of Ser368 and Ser279/282 were significantly increased [133].
4.2. Cx43 Is Expressed in Many Epithelial Tissues
The phosphorylation of Cx43 at Ser368 has been detected in the intestinal epithelial cells of MDR1α-deficient mice [134]. In normal adult mammary glands, Cx43 phosphorylated at Ser279/282 was detected in myoepithelial cells [135]. Cx43 plays a role in the repair of damaged organs in the epidermis by being phosphorylated by various kinases. It was also observed that the phosphorylation of Cx43 at Ser368 was specifically elevated in the basal cell compartment proximal to the wound edge. The increase in phosphorylation levels reached a maximum level at 24 h post-injury and returned to baseline levels by 72 h [136].
4.3. Phosphorylation of Cx43 as a Role in Proliferative Retinopathy
Cx43 plays a critical role in astrocyte apoptosis and the resulting preretinal neovascularization in a mouse model of hypoxia-induced retinopathy. The increased coupling in response to hypoxia is due to the phosphorylation of Cx43 by CK1δ. The inhibition of this phosphorylation with a CK1δ inhibitor or in site-specific phosphorylation-deficient mice similarly protected astrocytes from hypoxic injury [137]. This study suggests that targeting Cx43 phosphorylation in astrocytes is a potential treatment for proliferative retinopathy. The inhibition of Cx43 Ser325/328/330 phosphorylation enhances retinal revascularization and improves pathological neovascularization.
4.4. Phosphorylation of Cx43 and Chronic Pain
Chronic pain, including inflammatory, neuropathic pain, is a serious clinical issue. Cx43 phosphorylation has been demonstrated in chronic pain models. After sciatic nerve chronic constriction injury (CCI), increased phosphorylation at Ser368 of spinal astrocytic Cx43 was observed during the maintenance phase (10–20 days after CCI), and this response was induced by the downregulation of K-ATP channels [138]. Furthermore, increased phosphorylation at Ser368 of spinal astrocytic Cx43 has been reported in the bone cancer pain model. Although it was also reported that phosphorylated Cx43 is involved in mechanical allodynia through the production of chemokine CXCL12, the mechanism mediating phosphorylated Cx43-induced CXCL12 production was not elaborated [139,140].
5. Conclusions and Perspectives
The most abundant connexin in the brain and heart is Cx43. Cx43 phosphorylation can affect changes in Cx43-interacting protein binding, subsequently affecting the underlying signaling pathways of gap junction communication, hemichannel function, kinase activity, and cell biological function. The phosphorylation status of Cx43 at various serine, threonine, and tyrosine sites can significantly affect the expression and function of Cx43 in various pathological conditions, such as depression, neurodegenerative disease, cerebral ischemia, and myocardial ischemia (Table 1). In the future, the phosphorylation of Cx43 can be used as an emerging target to study its regulation on the nervous system and the cardiovascular system and its significance for the occurrence and development of diseases.

Table 1.
Summary table of the expression and phosphorylation of Cx43 in multiple diseases.
Author Contributions
Writing—original draft preparation, M.Z.; formal analysis, Z.-Z.W.; conceptualization, funding acquisition, and writing—review and editing, Z.-Z.W. and N.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82130109, 81773924, 81573636).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Cx43 | Connexin 43 |
| CNS | Central nervous system |
| Ser | Serine |
| Tyr | Tyrosine |
| GJ | Gap junctions |
| GJIC | Gap junctional cell-to-cell communication |
| MDD | Major depression disorder |
| CORT | Corticosterone |
| AD | Alzheimer’s disease |
| Aβ | β-amyloid |
| APP | Aβ precursor protein |
| PKC | Protein kinase C |
| PKA | Protein kinase A |
| TBI | Traumatic brain injury |
| TPA | Tissue polypeptide antigen |
| PD | Parkinson’s disease |
| DA | Dopamine |
| MTLE-HS | Mesial temporal lobe epilepsy with hippocampal sclerosis |
| pMCAO | Permanent middle cerebral artery occlusion |
| CK1 | Casein kinase 1 |
| PKB | Protein kinase B |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
References
- Pun, R.; Kim, M.H.; North, B.J. Role of Connexin 43 phosphorylation on Serine-368 by PKC in cardiac function and disease. Front. Cardiovasc. Med. 2022, 9, 1080131. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Fukuyama, K.; Shiroyama, T.; Murata, M. A Working Hypothesis Regarding Identical Pathomechanisms between Clinical Efficacy and Adverse Reaction of Clozapine via the Activation of Connexin43. Int. J. Mol. Sci. 2020, 21, 7019. [Google Scholar] [CrossRef] [PubMed]
- Lapato, A.S.; Tiwari-Woodruff, S.K. Connexins and pannexins: At the junction of neuro-glial homeostasis & disease. J. Neurosci. Res. 2018, 96, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Q.Q.; Jia, J.N.; Liu, Z.Q.; Zhou, H.H.; Mao, X.Y. Targeting gap junction in epilepsy: Perspectives and challenges. Biomed. Pharmacother. 2019, 109, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of connexin 43 in different forms of intercellular communication—gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, L.; Ma, D.; Yin, P.; Wang, X.; Hou, S.; Hao, Y.; Zhang, J.; Xin, M.; Feng, J. Roles of astrocytic connexin-43, hemichannels, and gap junctions in oxygen-glucose deprivation/reperfusion injury induced neuroinflammation and the possible regulatory mechanisms of salvianolic acid B and carbenoxolone. J. Neuroinflamm. 2018, 15, 97. [Google Scholar] [CrossRef]
- Boengler, K.; Rohrbach, S.; Weissmann, N.; Schulz, R. Importance of Cx43 for Right Ventricular Function. Int. J. Mol. Sci. 2021, 22, 987. [Google Scholar] [CrossRef]
- Gosejacob, D.; Dublin, P.; Bedner, P.; Hüttmann, K.; Zhang, J.; Tress, O.; Willecke, K.; Pfrieger, F.; Steinhäuser, C.; Theis, M. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia 2011, 59, 511–519. [Google Scholar] [CrossRef]
- Akoyev, V.; Takemoto, D.J. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signal. 2007, 19, 958–967. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, J.; Zelenka, P.S.; Takemoto, D.J. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5259–5268. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Rodríguez-Sinovas, A. Connexin 43 phosphorylation in subsarcolemmal mitochondria: A general cardioprotective signal targeted by fibroblast growth factor-2? Cardiovasc. Res. 2014, 103, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rosado, L.; Solan, J.L.; Dunn, C.A.; Norris, R.P.; Lampe, P.D. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim. Biophys. Acta 2012, 1818, 1985–1992. [Google Scholar] [CrossRef]
- Nimlamool, W.; Andrews, R.M.; Falk, M.M. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol. Biol. Cell 2015, 26, 2755–2768. [Google Scholar] [CrossRef]
- Chever, O.; Lee, C.Y.; Rouach, N. Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J. Neurosci. 2014, 34, 11228–11232. [Google Scholar] [CrossRef]
- Fukuyama, K.; Ueda, Y.; Okada, M. Effects of Carbamazepine, Lacosamide and Zonisamide on Gliotransmitter Release Associated with Activated Astroglial Hemichannels. Pharmaceuticals 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okubo, R.; Murata, M.; Shiroyama, T.; Okada, M. Activation of Astroglial Connexin is Involved in Concentration-Dependent Double-Edged Sword Clinical Action of Clozapine. Cells 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, L.N.; Calloe, K.; Holstein-Rathlou, N.H.; Nielsen, M.S. Managing the complexity of communication: Regulation of gap junctions by post-translational modification. Front. Pharmacol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef]
- Morioka, N.; Suekama, K.; Zhang, F.F.; Kajitani, N.; Hisaoka-Nakashima, K.; Takebayashi, M.; Nakata, Y. Amitriptyline up-regulates connexin43-gap junction in rat cultured cortical astrocytes via activation of the p38 and c-Fos/AP-1 signalling pathway. Br. J. Pharmacol. 2014, 171, 2854–2867. [Google Scholar] [CrossRef]
- Leykauf, K.; Salek, M.; Bomke, J.; Frech, M.; Lehmann, W.D.; Dürst, M.; Alonso, A. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J. Cell Sci. 2006, 119, 3634–3642. [Google Scholar] [CrossRef]
- Xia, C.Y.; Wang, Z.Z.; Zhang, Z.; Chen, J.; Wang, Y.Y.; Lou, Y.X.; Gao, Y.; Luo, P.; Ren, Q.; Du, G.H.; et al. Corticosterone impairs gap junctions in the prefrontal cortical and hippocampal astrocytes via different mechanisms. Neuropharmacology 2018, 131, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Kerman, I.A.; Thompson, R.C.; Jones, E.G.; Bunney, W.E.; Barchas, J.D.; Schatzberg, A.F.; Myers, R.M.; Akil, H.; Watson, S.J. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol. Psychiatry 2011, 16, 634–646. [Google Scholar] [CrossRef]
- Ernst, C.; Nagy, C.; Kim, S.; Yang, J.P.; Deng, X.; Hellstrom, I.C.; Choi, K.H.; Gershenfeld, H.; Meaney, M.J.; Turecki, G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol. Psychiatry 2011, 70, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J.; Wilson, B.A.; Hussain, S.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J. Psychiatr. Res. 2014, 55, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Torres-Platas, S.G.; Mechawar, N.; Turecki, G. Repression of Astrocytic Connexins in Cortical and Subcortical Brain Regions and Prefrontal Enrichment of H3K9me3 in Depression and Suicide. Int. J. Neuropsychopharmacol. 2017, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.D.; Liu, Y.; Yuan, Y.H.; Li, J.; Chen, N.H. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- Nakase, T.; Yoshida, Y.; Nagata, K. Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia 2006, 54, 369–375. [Google Scholar] [CrossRef]
- Fonseca, L.; Mayer, L.; Orange, D.; Driscoll, N. The high-frequency backscattering angular response of gassy sediments: Model/data comparison from the Eel River Margin, California. J. Acoust. Soc. Am. 2002, 111, 2621–2631. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Rothstein, J.D. Mechanisms of Disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006, 2, 679–689. [Google Scholar] [CrossRef]
- Pahujaa, M.; Anikin, M.; Goldberg, G.S. Phosphorylation of connexin43 induced by Src: Regulation of gap junctional communication between transformed cells. Exp. Cell Res. 2007, 313, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014, 588, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Nuriya, M.; Morita, A.; Shinotsuka, T.; Yamada, T.; Yasui, M. Norepinephrine induces rapid and long-lasting phosphorylation and redistribution of connexin 43 in cortical astrocytes. Biochem. Biophys. Res. Commun. 2018, 504, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cone, A.C.; Cavin, G.; Ambrosi, C.; Hakozaki, H.; Wu-Zhang, A.X.; Kunkel, M.T.; Newton, A.C.; Sosinsky, G.E. Protein kinase Cδ-mediated phosphorylation of Connexin43 gap junction channels causes movement within gap junctions followed by vesicle internalization and protein degradation. J. Biol. Chem. 2014, 289, 8781–8798. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, H.; Wang, H.; Wang, Y.; Peng, Y.; Liu, Y.; Xia, C.; Yan, X.; Chu, S.; Zhang, Y.; et al. Novel Antidepressant Mechanism of Ginsenoside Rg1 in Regulating the Dysfunction of the Glutamatergic System in Astrocytes. Int. J. Mol. Sci. 2022, 24, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Hou, X.; Lu, K.; He, Y.; Yang, B.; Qin, Y. Xiaoyao powder alleviates the hippocampal neuron damage in chronic unpredictable mild stress-induced depression model rats in hippocampus via connexin 43Cx43/glucocorticoid receptor/brain-derived neurotrophic factor signaling pathway. Bioengineered 2022, 13, 383–394. [Google Scholar] [CrossRef]
- Freitas-Andrade, M.; Naus, C.C. Astrocytes in neuroprotection and neurodegeneration: The role of connexin43 and pannexin1. Neuroscience 2016, 323, 207–221. [Google Scholar] [CrossRef]
- Garbelli, R.; Frassoni, C.; Condorelli, D.F.; Trovato Salinaro, A.; Musso, N.; Medici, V.; Tassi, L.; Bentivoglio, M.; Spreafico, R. Expression of connexin 43 in the human epileptic and drug-resistant cerebral cortex. Neurology 2011, 76, 895–902. [Google Scholar] [CrossRef]
- Madeira, D.; Dias, L.; Santos, P.; Cunha, R.A.; Canas, P.M.; Agostinho, P. Association Between Adenosine A(2A) Receptors and Connexin 43 Regulates Hemichannels Activity and ATP Release in Astrocytes Exposed to Amyloid-β Peptides. Mol. Neurobiol. 2021, 58, 6232–6248. [Google Scholar] [CrossRef]
- Abudara, V.; Bechberger, J.; Freitas-Andrade, M.; De Bock, M.; Wang, N.; Bultynck, G.; Naus, C.C.; Leybaert, L.; Giaume, C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front. Cell. Neurosci. 2014, 8, 306. [Google Scholar] [CrossRef]
- Stehberg, J.; Moraga-Amaro, R.; Salazar, C.; Becerra, A.; Echeverría, C.; Orellana, J.A.; Bultynck, G.; Ponsaerts, R.; Leybaert, L.; Simon, F.; et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012, 26, 3649–3657. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Leybaert, L.; Naus, C.C.; Sáez, J.C. Connexin and pannexin hemichannels in brain glial cells: Properties, pharmacology, and roles. Front. Pharmacol. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Pannasch, U.; Ezan, P.; Rouach, N. Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130596. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Contreras, J.E.; Bukauskas, F.F.; Retamal, M.A.; Bennett, M.V. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol. Scand. 2003, 179, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Lampe, P.D.; Lau, A.F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000, 384, 205–215. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17 (Suppl. S1), 11. [Google Scholar] [CrossRef]
- De Smet, M.A.; Lissoni, A.; Nezlobinsky, T.; Wang, N.; Dries, E.; Pérez-Hernández, M.; Lin, X.; Amoni, M.; Vervliet, T.; Witschas, K.; et al. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Mei, X.; Ezan, P.; Giaume, C.; Koulakoff, A. Astroglial connexin immunoreactivity is specifically altered at β-amyloid plaques in β-amyloid precursor protein/presenilin1 mice. Neuroscience 2010, 171, 92–105. [Google Scholar] [CrossRef]
- Nagy, J.I.; Li, W.; Hertzberg, E.L.; Marotta, C.A. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res. 1996, 717, 173–178. [Google Scholar] [CrossRef]
- Angeli, S.; Kousiappa, I.; Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Papacostas, S.S.; Kleopa, K.A. Altered Expression of Glial Gap Junction Proteins Cx43, Cx30, and Cx47 in the 5XFAD Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 582934. [Google Scholar] [CrossRef]
- Huang, X.; Su, Y.; Wang, N.; Li, H.; Li, Z.; Yin, G.; Chen, H.; Niu, J.; Yi, C. Astroglial Connexins in Neurodegenerative Diseases. Front. Mol. Neurosci. 2021, 14, 657514. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Grißmer, A.; Wolf, S.; Recktenwald, J.; Meier, C. Oxygen-Glucose Deprivation in Mouse Astrocytes is Associated with Ultrastructural Changes in Connexin 43 Gap Junctions. Neuroscience 2019, 397, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta 2005, 1711, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta 2005, 1711, 172–182. [Google Scholar] [CrossRef]
- Hoos, M.D.; Richardson, B.M.; Foster, M.W.; Everhart, A.; Thompson, J.W.; Moseley, M.A.; Colton, C.A. Longitudinal study of differential protein expression in an Alzheimer’s mouse model lacking inducible nitric oxide synthase. J. Proteome Res. 2013, 12, 4462–4477. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.H.; Denisova, J.V.; Park, W.M.; Fontes, J.D.; Belousov, A.B. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J. Neurosci. 2012, 32, 713–725. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Samiei, M.; Maleki Dizaj, S.; Vinken, M. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell Signal. 2019, 58, 111–118. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 2007, 217, 35–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, Y.; Li, Q.; Quan, H.; Liu, D.; Zhou, H. Connexin43 inhibition attenuated dopaminergic neuronal loss in the lipopolysaccharide-induced mice model of Parkinson’s disease. Neurosci. Lett. 2022, 771, 136471. [Google Scholar] [CrossRef]
- Delvaeye, T.; Vandenabeele, P.; Bultynck, G.; Leybaert, L.; Krysko, D.V. Therapeutic Targeting of Connexin Channels: New Views and Challenges. Trends Mol. Med. 2018, 24, 1036–1053. [Google Scholar] [CrossRef]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef]
- Cheung, G.; Sibille, J.; Zapata, J.; Rouach, N. Activity-Dependent Plasticity of Astroglial Potassium and Glutamate Clearance. Neural Plast. 2015, 2015, 109106. [Google Scholar] [CrossRef]
- Hansson, E.; Muyderman, H.; Leonova, J.; Allansson, L.; Sinclair, J.; Blomstrand, F.; Thorlin, T.; Nilsson, M.; Rönnbäck, L. Astroglia and glutamate in physiology and pathology: Aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem. Int. 2000, 37, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Onodera, M.; Meyer, J.; Furukawa, K.; Hiraoka, Y.; Aida, T.; Tanaka, K.; Tanaka, K.F.; Rose, C.R.; Matsui, K. Exacerbation of Epilepsy by Astrocyte Alkalization and Gap Junction Uncoupling. J. Neurosci. 2021, 41, 2106–2118. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Wang, Z.Z.; Chen, N.H. Connexin 43: An Interface Connecting Neuroinflammation to Depression. Molecules 2023, 28, 1820. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nature reviews. Neuroscience 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Rash, J.E. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Rev. 2000, 32, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.; Li, T.; Herde, M.K.; Becker, A.; Vatter, H.; Schwarz, M.K.; Henneberger, C.; Steinhäuser, C.; Bedner, P. Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 2017, 65, 1809–1820. [Google Scholar] [CrossRef]
- Walrave, L.; Vinken, M.; Leybaert, L.; Smolders, I. Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment. Biomolecules 2020, 10, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, S.; Zhang, L.; Wu, C.; Zhang, Z.J.; Samoilova, M.; Eubanks, J.; Carlen, P.L.; Poulter, M.O. Hippocampal seizures alter the expression of the pannexin and connexin transcriptome. J. Neurochem. 2010, 112, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, A.F.; Kowal, T.J.; Fong, J.T.; Kells, R.M.; Fisher, C.G.; Falk, M.M. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology 2013, 28, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Andrade, M.; Wang, N.; Bechberger, J.F.; De Bock, M.; Lampe, P.D.; Leybaert, L.; Naus, C.C. Targeting MAPK phosphorylation of Connexin43 provides neuroprotection in stroke. J. Exp. Med. 2019, 216, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Siushansian, R.; Bechberger, J.F.; Cechetto, D.F.; Hachinski, V.C.; Naus, C.C. Connexin43 null mutation increases infarct size after stroke. J. Comp. Neurol. 2001, 440, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Söhl, G.; Theis, M.; Willecke, K.; Naus, C.C. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am. J. Pathol. 2004, 164, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Kozoriz, M.G.; Bechberger, J.F.; Bechberger, G.R.; Suen, M.W.; Moreno, A.P.; Maass, K.; Willecke, K.; Naus, C.C. The connexin43 C-terminal region mediates neuroprotection during stroke. J. Neuropathol. Exp. Neurol. 2010, 69, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 2005, 1711, 99–125. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, X.; Hao, Y.; Qiu, L.; Lou, Y.; Zhang, Y.; Ma, D.; Feng, J. The Multifaceted Role of Astrocyte Connexin 43 in Ischemic Stroke Through Forming Hemichannels and Gap Junctions. Front. Neurol. 2020, 11, 703. [Google Scholar] [CrossRef]
- Lampe, P.D.; TenBroek, E.M.; Burt, J.M.; Kurata, W.E.; Johnson, R.G.; Lau, A.F. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 2000, 149, 1503–1512. [Google Scholar] [CrossRef]
- Warn-Cramer, B.J.; Lampe, P.D.; Kurata, W.E.; Kanemitsu, M.Y.; Loo, L.W.; Eckhart, W.; Lau, A.F. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J. Biol. Chem. 1996, 271, 3779–3786. [Google Scholar] [CrossRef]
- Loo, L.W.; Berestecky, J.M.; Kanemitsu, M.Y.; Lau, A.F. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 1995, 270, 12751–12761. [Google Scholar] [CrossRef]
- Cooper, C.D.; Lampe, P.D. Casein kinase 1 regulates connexin-43 gap junction assembly. J. Biol. Chem. 2002, 277, 44962–44968. [Google Scholar] [CrossRef] [PubMed]
- Li, W.E.; Nagy, J.I. Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. Eur. J. Neurosci. 2000, 12, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Ek-Vitorín, J.F.; Pontifex, T.K.; Burt, J.M. Cx43 Channel Gating and Permeation: Multiple Phosphorylation-Dependent Roles of the Carboxyl Terminus. Int. J. Mol. Sci. 2018, 19, 1659. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Tan, X.; Kostrzewa, R.M.; Du, G.; Chen, Y.; Zhu, J.; Miao, Z.; Yu, H.; Kong, J.; et al. Inhibition of connexin43 improves functional recovery after ischemic brain injury in neonatal rats. Glia 2015, 63, 1553–1567. [Google Scholar] [CrossRef]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Zhao, M.; Hou, S.; Feng, L.; Shen, P.; Nan, D.; Zhang, Y.; Wang, F.; Ma, D.; Feng, J. Vinpocetine Protects Against Cerebral Ischemia-Reperfusion Injury by Targeting Astrocytic Connexin43 via the PI3K/AKT Signaling Pathway. Front. Neurosci. 2020, 14, 223. [Google Scholar] [CrossRef]
- Axelsen, L.N.; Stahlhut, M.; Mohammed, S.; Larsen, B.D.; Nielsen, M.S.; Holstein-Rathlou, N.H.; Andersen, S.; Jensen, O.N.; Hennan, J.K.; Kjølbye, A.L. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123). J. Mol. Cell. Cardiol. 2006, 40, 790–798. [Google Scholar] [CrossRef]
- Turner, M.S.; Haywood, G.A.; Andreka, P.; You, L.; Martin, P.E.; Evans, W.H.; Webster, K.A.; Bishopric, N.H. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ. Res. 2004, 95, 726–733. [Google Scholar] [CrossRef]
- Meier, C.; Rosenkranz, K. Cx43 expression and function in the nervous system-implications for stem cell mediated regeneration. Front. Physiol. 2014, 5, 106. [Google Scholar] [CrossRef]
- Chen, W.; Feng, J.; Tong, W. Phosphorylation of astrocytic connexin43 by ERK1/2 impairs blood-brain barrier in acute cerebral ischemia. Cell Biosci. 2017, 7, 43. [Google Scholar] [CrossRef]
- Wang, X.; Feng, L.; Xin, M.; Hao, Y.; Wang, X.; Shang, P.; Zhao, M.; Hou, S.; Zhang, Y.; Xiao, Y.; et al. Mechanisms underlying astrocytic connexin-43 autophagy degradation during cerebral ischemia injury and the effect on neuroinflammation and cell apoptosis. Biomed. Pharmacother. 2020, 127, 110125. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Wang, H.; Wang, Y.; Wang, H. Effects of gap junctional blockers on cerebral vasospasm after subarachnoid hemorrhage in rabbits. Neurol. Res. 2009, 31, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Ruan, Y.; Cai, C.; He, B.; Zhao, D. Role of P38 mitogen-activated protein kinase on Cx43 phosphorylation in cerebral vasospasm after subarachnoid hemorrhage. Int. J. Neurosci. 2019, 129, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, J.; Zhang, J.A.; Zhou, X.H.; Fang, C.; Zeng, E.M.; Tang, B.; Duan, J.; Lu, G.H.; Hong, T. The important role of connexin 43 in subarachnoid hemorrhage-induced cerebral vasospasm. J. Transl. Med. 2019, 17, 433. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, X.W.; Wang, X.; Yang, Y.; Luo, J.W.; Liu, Y.H.; Mao, Q. Complex role of connexin 43 in astrocytic tumors and possible promotion of glioma-associated epileptic discharge (Review). Mol. Med. Rep. 2017, 16, 7890–7900. [Google Scholar] [CrossRef]
- Ye, X.Y.; Jiang, Q.H.; Hong, T.; Zhang, Z.Y.; Yang, R.J.; Huang, J.Q.; Hu, K.; Peng, Y.P. Altered expression of connexin43 and phosphorylation connexin43 in glioma tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 4296–4306. [Google Scholar]
- Mesnil, M.; Crespin, S.; Avanzo, J.L.; Zaidan-Dagli, M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nature reviews. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Fong, J.T.; Nimlamool, W.; Falk, M.M. EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Lett. 2014, 588, 836–844. [Google Scholar] [CrossRef]
- Sirnes, S.; Kjenseth, A.; Leithe, E.; Rivedal, E. Interplay between PKC and the MAP kinase pathway in Connexin43 phosphorylation and inhibition of gap junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 41–45. [Google Scholar] [CrossRef]
- Batra, N.; Riquelme, M.A.; Burra, S.; Kar, R.; Gu, S.; Jiang, J.X. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J. Biol. Chem. 2014, 289, 10582–10591. [Google Scholar] [CrossRef] [PubMed]
- Salas, D.; Puebla, C.; Lampe, P.D.; Lavandero, S.; Sáez, J.C. Role of Akt and Ca2+ on cell permeabilization via connexin43 hemichannels induced by metabolic inhibition. Biochim. Biophys. Acta 2015, 1852, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Altenberg, G.A.; Reuss, L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am. J. Physiol. Cell Physiol. 2004, 286, C647–C654. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Reuss, L.; Altenberg, G.A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004, 279, 20058–20066. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lee, S.C.; Reuss, L.; Altenberg, G.A. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc. Natl. Acad. Sci. USA 2007, 104, 4919–4924. [Google Scholar] [CrossRef] [PubMed]
- Hawat, G.; Baroudi, G. Differential modulation of unapposed connexin 43 hemichannel electrical conductance by protein kinase C isoforms. Pflug. Arch. Eur. J. Physiol. 2008, 456, 519–527. [Google Scholar] [CrossRef]
- D’Hondt, C.; Ponsaerts, R.; Srinivas, S.P.; Vereecke, J.; Himpens, B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Investig. Ophthalmol. Vis. Sci. 2007, 48, 120–133. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Cannon, A.; Trease, A.J.; Spagnol, G.; Zheng, H.; Radio, S.; Patel, K.; Batra, S.; Sorgen, P.L. Phosphorylation of Cx43 residue Y313 by Src contributes to blocking the interaction with Drebrin and disassembling gap junctions. J. Mol. Cell. Cardiol. 2019, 126, 36–49. [Google Scholar] [CrossRef]
- Ma, D.; Feng, L.; Cheng, Y.; Xin, M.; You, J.; Yin, X.; Hao, Y.; Cui, L.; Feng, J. Astrocytic gap junction inhibition by carbenoxolone enhances the protective effects of ischemic preconditioning following cerebral ischemia. J. Neuroinflamm. 2018, 15, 198. [Google Scholar] [CrossRef]
- Hou, S.; Shen, P.P.; Zhao, M.M.; Liu, X.P.; Xie, H.Y.; Deng, F.; Feng, J.C. Mechanism of Mitochondrial Connexin43’s Protection of the Neurovascular Unit under Acute Cerebral Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2016, 17, 679. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Tansey, E.E.; Kwaku, K.F.; Hammer, P.E.; Cowan, D.B.; Federman, M.; Levitsky, S.; McCully, J.D. Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann. Thorac. Surg. 2006, 82, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Beardslee, M.A.; Lerner, D.L.; Tadros, P.N.; Laing, J.G.; Beyer, E.C.; Yamada, K.A.; Kléber, A.G.; Schuessler, R.B.; Saffitz, J.E. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 2000, 87, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Solan, J.L.; Gaietta, G.M.; Ngan, L.; Lee, G.J.; Mackey, M.R.; Lampe, P.D. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem. J. 2007, 408, 375–385. [Google Scholar] [CrossRef]
- Solan, J.L.; Marquez-Rosado, L.; Sorgen, P.L.; Thornton, P.J.; Gafken, P.R.; Lampe, P.D. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J. Cell Biol. 2007, 179, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Bacova, B.; Radosinska, J.; Knezl, V.; Kolenova, L.; Weismann, P.; Navarova, J.; Barancik, M.; Mitasikova, M.; Tribulova, N. Omega-3 fatty acids and atorvastatin suppress ventricular fibrillation inducibility in hypertriglyceridemic rat hearts: Implication of intracellular coupling protein, connexin-43. J. Physiol. Pharmacol. 2010, 61, 717–723. [Google Scholar]
- Bačová, B.; Radošinská, J.; Viczenczová, C.; Knezl, V.; Dosenko, V.; Beňova, T.; Navarová, J.; Gonçalvesová, E.; van Rooyen, J.; Weismann, P.; et al. Up-regulation of myocardial connexin-43 in spontaneously hypertensive rats fed red palm oil is most likely implicated in its anti-arrhythmic effects. Can. J. Physiol. Pharmacol. 2012, 90, 1235–1245. [Google Scholar] [CrossRef]
- Radosinska, J.; Bacova, B.; Bernatova, I.; Navarova, J.; Zhukovska, A.; Shysh, A.; Okruhlicova, L.; Tribulova, N. Myocardial NOS activity and connexin-43 expression in untreated and omega-3 fatty acids-treated spontaneously hypertensive and hereditary hypertriglyceridemic rats. Mol. Cell. Biochem. 2011, 347, 163–173. [Google Scholar] [CrossRef]
- Bacharova, L.; Plandorova, J.; Klimas, J.; Krenek, P.; Kyselovic, J. Discrepancy between increased left ventricular mass and “normal” QRS voltage is associated with decreased connexin 43 expression in early stage of left ventricular hypertrophy in spontaneously hypertensive rats. J. Electrocardiol. 2008, 41, 730–734. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, G.; Hu, X.; Wang, M.; Li, H.; Ye, Y.; Du, Q.; Yao, J.; Bao, Z.; Hong, W.; et al. Aliskiren-attenuated myocardium apoptosis via regulation of autophagy and connexin-43 in aged spontaneously hypertensive rats. J. Cell. Mol. Med. 2014, 18, 1247–1256. [Google Scholar] [CrossRef]
- Kostin, S.; Dammer, S.; Hein, S.; Klovekorn, W.P.; Bauer, E.P.; Schaper, J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc. Res. 2004, 62, 426–436. [Google Scholar] [CrossRef]
- Anna, Z.; Angela, S.; Barbara, B.; Jana, R.; Tamara, B.; Csilla, V.; Victor, D.; Oleksiy, M.; Narcisa, T. Heart-protective effect of n-3 PUFA demonstrated in a rat model of diabetic cardiomyopathy. Mol. Cell. Biochem. 2014, 389, 219–227. [Google Scholar] [CrossRef]
- Howarth, F.C.; Chandler, N.J.; Kharche, S.; Tellez, J.O.; Greener, I.D.; Yamanushi, T.T.; Billeter, R.; Boyett, M.R.; Zhang, H.; Dobrzynski, H. Effects of streptozotocin-induced diabetes on connexin43 mRNA and protein expression in ventricular muscle. Mol. Cell. Biochem. 2008, 319, 105–114. [Google Scholar] [CrossRef]
- Ai, X.; Jiang, A.; Ke, Y.; Solaro, R.J.; Pogwizd, S.M. Enhanced activation of p21-activated kinase 1 in heart failure contributes to dephosphorylation of connexin 43. Cardiovasc. Res. 2011, 92, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ohkusa, T.; Honjo, H.; Suzuki, S.; Yoshida, M.A.; Ishiguro, Y.S.; Nakagawa, H.; Yamazaki, M.; Yano, M.; Kodama, I.; et al. Altered expression of connexin43 contributes to the arrhythmogenic substrate during the development of heart failure in cardiomyopathic hamster. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1164–H1173. [Google Scholar] [CrossRef] [PubMed]
- Remo, B.F.; Giovannone, S.; Fishman, G.I. Connexin43 cardiac gap junction remodeling: Lessons from genetically engineered murine models. J. Membr. Biol. 2012, 245, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kalcheva, N.; Qu, J.; Sandeep, N.; Garcia, L.; Zhang, J.; Wang, Z.; Lampe, P.D.; Suadicani, S.O.; Spray, D.C.; Fishman, G.I. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc. Natl. Acad. Sci. USA 2007, 104, 20512–20516. [Google Scholar] [CrossRef]
- Himelman, E.; Lillo, M.A.; Nouet, J.; Gonzalez, J.P.; Zhao, Q.; Xie, L.H.; Li, H.; Liu, T.; Wehrens, X.H.; Lampe, P.D.; et al. Prevention of connexin-43 remodeling protects against Duchenne muscular dystrophy cardiomyopathy. J. Clin. Investig. 2020, 130, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Severs, N.J.; Bruce, A.F.; Dupont, E.; Rothery, S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 2008, 80, 9–19. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef]
- Harris, A.; Locke, D. Connexins: A Guide; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Furnkranz, A.; Schober, A.; Bochkov, V.N.; Bashtrykov, P.; Kronke, G.; Kadl, A.; Binder, B.R.; Weber, C.; Leitinger, N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.R.; Ross, J.; Rizzo, M.J.; Straub, A.C.; Lampe, P.D.; Leitinger, N.; Isakson, B.E. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am. J. Pathol. 2009, 175, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Ey, B.; Eyking, A.; Gerken, G.; Podolsky, D.K.; Cario, E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J. Biol. Chem. 2009, 284, 22332–22343. [Google Scholar] [CrossRef] [PubMed]
- Gould, V.E.; Mosquera, J.M.; Leykauf, K.; Gattuso, P.; Dürst, M.; Alonso, A. The phosphorylated form of connexin43 is up-regulated in breast hyperplasias and carcinomas and in their neoformed capillaries. Hum. Pathol. 2005, 36, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.S.; Dunn, C.A.; Carter, W.G.; Usui, M.L.; Olerud, J.E.; Lampe, P.D. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J. Cell Biol. 2004, 167, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Slavi, N.; Toychiev, A.H.; Kosmidis, S.; Ackert, J.; Bloomfield, S.A.; Wulff, H.; Viswanathan, S.; Lampe, P.D.; Srinivas, M. Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy. Proc. Natl. Acad. Sci. USA 2018, 115, E5934–E5943. [Google Scholar] [CrossRef]
- Wu, X.F.; Liu, W.T.; Liu, Y.P.; Huang, Z.J.; Zhang, Y.K.; Song, X.J. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain 2011, 152, 2605–2615. [Google Scholar] [CrossRef]
- Hang, L.H.; Li, S.N.; Luo, H.; Shu, W.W.; Mao, Z.M.; Chen, Y.F.; Shi, L.L.; Shao, D.H. Connexin 43 Mediates CXCL12 Production from Spinal Dorsal Horn to Maintain Bone Cancer Pain in Rats. Neurochem. Res. 2016, 41, 1200–1208. [Google Scholar] [CrossRef]
- Morioka, N.; Nakamura, Y.; Zhang, F.F.; Hisaoka-Nakashima, K.; Nakata, Y. Role of Connexins in Chronic Pain and Their Potential as Therapeutic Targets for Next-Generation Analgesics. Biol. Pharm. Bull. 2019, 42, 857–866. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).