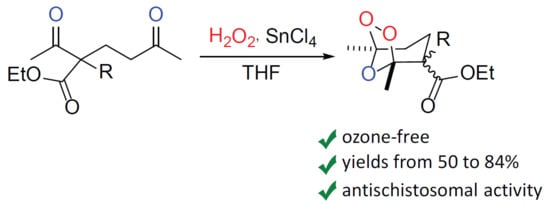
Bridged 1,2,4-Trioxolanes: SnCl4—Catalyzed Synthesis and an In Vitro Study against S. mansoni
Abstract
1. Introduction
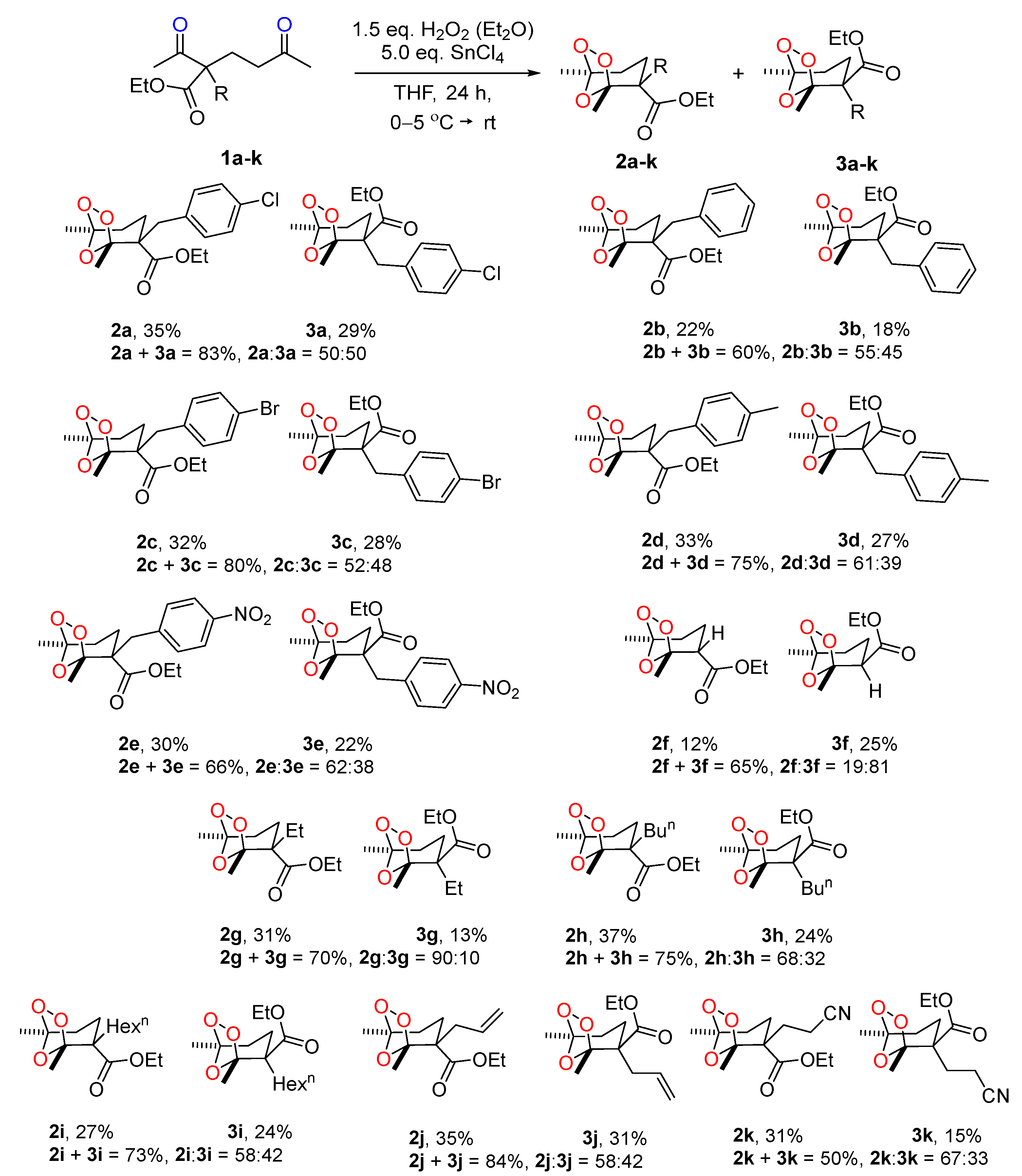
2. Results and Discussion
In Vitro Drug Assay on Newly Transformed Schistosomula (NTS) and Adult Schistosomes
3. Materials and Methods
3.1. General Materials and Methods
3.2. Synthesis of Starting Compounds
3.3. Procedure for the Synthesis of Ozonides 2a and 3a from 1,5-Diketone 1a, for Table 1
3.4. General Procedure for the Synthesis of Ozonides 2a–k and 3a–k from 1,5-Diketones 1a–k, for Scheme 1
3.4.1. Ethyl (1R*,2R*,5S*)-2-(4-chlorobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2a)
3.4.2. Ethyl (1R*,2S*,5S*)-2-(4-chlorobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3a)
3.4.3. Ethyl (1R*,2R*,5S*)-2-benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2b)
3.4.4. Ethyl (1R*,2S*,5S*)-2-benzyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3b)
3.4.5. Ethyl (1R*,2R*,5S*)-2-(4-bromobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2c)
3.4.6. Ethyl (1R*,2S*,5S*)-2-(4-bromobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3c)
3.4.7. Ethyl (1R*,2R*,5S*)-1,5-dimethyl-2-(4-methylbenzyl)-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2d)
3.4.8. Ethyl (1R*,2S*,5S*)-1,5-dimethyl-2-(4-methylbenzyl)-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3d)
3.4.9. Ethyl (1R*,2S*,5S*)-1,5-dimethyl-2-(4-nitrobenzyl)-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2e)
3.4.10. Ethyl (1R*,2S*,5S*)-1,5-dimethyl-2-(4-nitrobenzyl)-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3e)
3.4.11. Ethyl (1R*,2S*,5S*)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2f)
3.4.12. Ethyl (1R*,2R*,5S*)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3f)
3.4.13. Ethyl (1R*,2S*,5S*)-2-ethyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2g)
3.4.14. Ethyl (1R*,2R*,5S*)-2-ethyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3g)
3.4.15. Ethyl (1R*,2S*,5S*)-2-butyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2h)
3.4.16. Ethyl (1R*,2R*,5S*)-2-butyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3h)
3.4.17. Ethyl (1R*,2S*,5S*)-2-hexyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2i)
3.4.18. Ethyl (1R*,2R*,5S*)-2-hexyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3i)
3.4.19. Ethyl (1R*,2R*,5S*)-2-allyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2j)
3.4.20. Ethyl (1R*,2S*,5S*)-2-allyl-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3j)
3.4.21. Ethyl (1R*,2S*,5S*)-2-(2-cyanoethyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (2k)
3.4.22. Ethyl (1R*,2R*,5S*)-2-(2-cyanoethyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octane-2-carboxylate (3k)
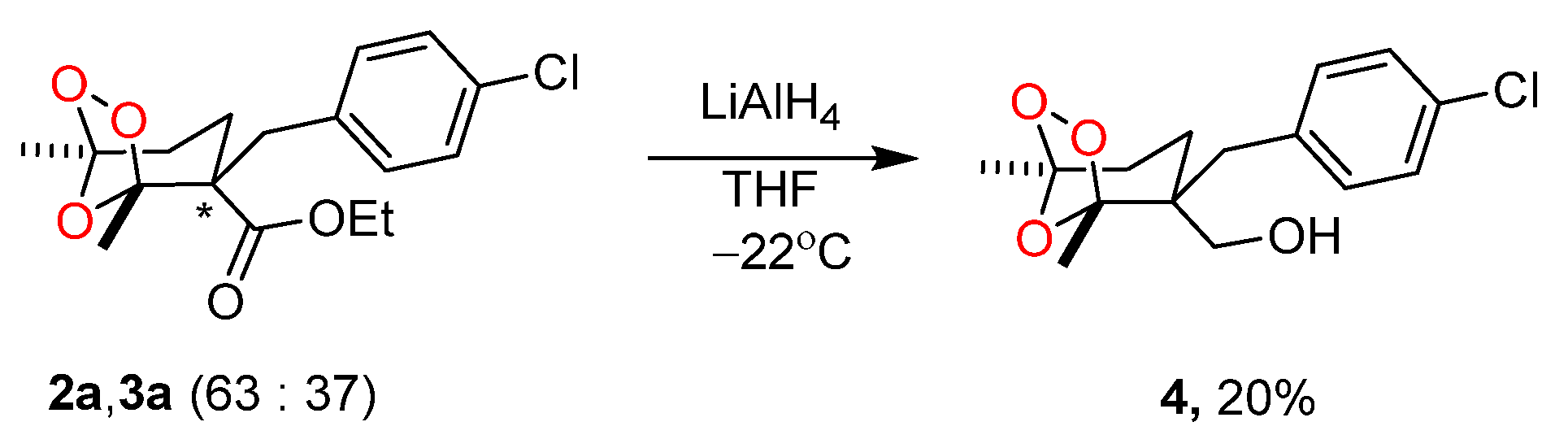
3.5. Synthesis of (2-(4-chlorobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)methanol (4)
(1R*,2S*,5S*)-2-(4-chlorobenzyl)-1,5-dimethyl-6,7,8-trioxabicyclo[3.2.1]octan-2-yl)methanol (4)
3.6. Maintenance of the Parasites at the Swiss TPH
3.7. In Vitro Compound Screening on S. mansoni NTS and Adult S. mansoni
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Giannangelo, C.; Fowkes, F.J.I.; Simpson, J.A.; Charman, S.A.; Creek, D.J. Ozonide Antimalarial Activity in the Context of Artemisinin-Resistant Malaria. Trends. Parasitol. 2019, 35, 529–543. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Amewu, R.K.; Charman, S.A.; Sabbani, S.; Gnädig, N.F.; Straimer, J.; Fidock, D.A.; Shore, E.R.; Roberts, N.L.; Wong, M.H.L.; et al. A tetraoxane-based antimalarial drug candidate that overcomes PfK13-C580Y dependent artemisinin resistance. Nat. Commun. 2017, 8, 15159. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, C.; Sharma, B.; Mishra, A.; Agarwal, D.; Kannan, D.; Held, J.; Singh, S.; Awasthi, S.K. N-sulfonylpiperidinedispiro-1,2,4,5-tetraoxanes exhibit potent in vitro antiplasmodial activity and in vivo efficacy in mice infected with P. berghei ANKA. Eur. J. Med. Chem. 2022, 244, 114774. [Google Scholar] [CrossRef]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Wu, J.N.; Zhang, R.L.; Ng, J.P.L.; Belyakova, Y.Y.; Law, B.Y.K.; Radulov, P.S.; Uthaipibull, C.; et al. Antimalarial and Anticancer Activity Evaluation of Bridged Ozonides, Aminoperoxides, and Tetraoxanes. ChemMedChem. 2022, 17, e202200328. [Google Scholar] [CrossRef]
- Cabral, L.I.L.; Pomel, S.; Cojean, S.; Amado, P.S.M.; Loiseau, P.M.; Cristiano, M.L.S. Synthesis and Antileishmanial Activity of 1,2,4,5-Tetraoxanes against Leishmania donovani. Molecules. 2020, 25, 465. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.; Armada, A.; Cabral, L.I.L.; Amado, P.S.M.; Campino, L.; Cristiano, M.L.S.; Cortes, S. 1,2,4-Trioxolane and 1,2,4,5-Tetraoxane Endoperoxides against Old-World Leishmania Parasites: In Vitro Activity and Mode of Action. Pharmaceuticals. 2022, 15, 446. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.E.M.; Krishna, S.; Greten, H.J.; Kremsner, P.G.; Efferth, T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 2016, 110, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-G.; Li, G.-X.; Zhao, S.-S.; Xu, F.-L.; Wang, Y.-H.; Wang, W. A review of dihydroartemisinin as another gift from traditional Chinese medicine not only for malaria control but also for schistosomiasis control. Parasitol. Res. 2014, 113, 1769–1773. [Google Scholar] [CrossRef]
- Abrams, R.P.; Carroll, W.L.; Woerpel, K.A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS. Chem. Biol. 2016, 11, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Coghi, P.; Prommana, P.; Qiu, C.; Radulov, P.S.; Qu, Y.; Belyakova, Y.Y.; Zanforlin, E.; Kokorekin, V.A.; Wu, Y.Y.J.; et al. Synthetic peroxides promote apoptosis of cancer cells inhibiting of P-glycoprotein ABCB5. ChemMedChem. 2020, 15, 1118–1127. [Google Scholar] [CrossRef]
- Gao, F.; Sun, Z.; Kong, F.; Xiao, J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020, 188, 112044. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’Ev, A.O. Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application. Chem.—Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and Unstable 1,2-Dioxolanes: Origin, Synthesis, and Biological Activities. Sci. Synth. Knowl. Updates. 2020, 38, 277–321. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules. 2020, 25, 1954. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, V.; Jaiswal, P.K.; Gaikwad, A.N.; Sinha, S.K.; Puri, S.K.; Sharon, A.; Maulik, P.R.; Chaturvedi, V. Stable aricyclic antitubercular ozonides derived from Artemisinin. Org. Lett. 2015, 17, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-W.; Lei, H.-S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.-M.; Xu, X.-R.; Chen, L.; Zhou, C.-H.; Zou, Y.-Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin–fluoroquinolone conjugates as a novel type of potential antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.W.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res. 2011, 92, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Frohlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtlander, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorgan. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnøe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef]
- Herrmann, L.; Yaremenko, I.A.; Çapcı, A.; Struwe, J.; Tailor, D.; Dheeraj, A.; Hodek, J.; Belyakova, Y.Y.; Radulov, P.; Weber, J.; et al. Synthesis and In Vitro Study of Artemisinin/Synthetic Peroxide Based Hybrid Compounds against SARS-CoV-2 and Cancer. ChemMedChem. 2022, 17, e202200005. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Miller, H.; Knox, K.; Kundu, M.; Henrickson, K.J.; Arav-Boger, R. Inhibition of Human Coronaviruses by Antimalarial Peroxides. ACS. Infect. Dis. 2021, 7, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Chiu, F.C.K.; Häberli, C.; Shackleford, D.M.; Ryan, E.; Kamaraj, S.; Bulbule, V.J.; Wallick, A.I.; Dong, Y.; et al. Structure–Activity Relationship of Antischistosomal Ozonide Carboxylic Acids. J. Med. Chem. 2020, 63, 3723–3736. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Terent’ev, A.O.; Vil, V.A.; Novikov, R.A.; Chernyshev, V.V.; Tafeenko, V.A.; Levitsky, D.O.; Fleury, F.; Nikishin, G.I. Approach for the Preparation of Various Classes of Peroxides Based on the Reaction of Triketones with H2O2: First Examples of Ozonide Rearrangements. Chem.—Eur. J. 2014, 20, 10160–10169. [Google Scholar] [CrossRef]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorgan. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- Hess, J.; Panic, G.; Patra, M.; Mastrobuoni, L.; Spingler, B.; Roy, S.; Keiser, J.; Gasser, G. Ferrocenyl, Ruthenocenyl, and Benzyl Oxamniquine Derivatives with Cross-Species Activity against Schistosoma mansoni and Schistosoma haematobium. ACS. Infect. Dis. 2017, 3, 645–652. [Google Scholar] [CrossRef]
- Ren, R.; Wang, X.; Leas, D.A.; Häberli, C.; Cal, M.; Dong, Y.; Kaiser, M.; Keiser, J.; Vennerstrom, J.L. Antischistosomal tetrahydro-γ-carboline sulfonamides. Bioorg. Med. Chem. Lett. 2022, 59, 128546. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, C.R.; El-Sakkary, N.; Mäder, P.; Krieg, R.; Becker, K.; Schlitzer, M.; Drewry, D.H.; Vennerstrom, J.L.; Grevelding, C.G. Drug Discovery and Development for Schistosomiasis. In Neglected Tropical Diseases: Drug Discovery and Development; Swinney, D., Pollastri, M., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 187–225. [Google Scholar] [CrossRef]
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; da Silva Filho, A.A.; de Moraes, J. Antischistosomal agents: State of art and perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef]
- Žmitek, K.; Zupan, M.; Stavber, S.; Iskra, J. Iodine as a Catalyst for Efficient Conversion of Ketones to gem-Dihydroperoxides by Aqueous Hydrogen Peroxide. Org. Lett. 2006, 8, 2491–2494. [Google Scholar] [CrossRef]
- Ghorai, P.; Dussault, P.H. Mild and Efficient Re(VII)-Catalyzed Synthesis of 1,1-Dihydroperoxides. Org. Lett. 2008, 10, 4577–4579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, H.-D.; Zhang, Q.; Wu, Y. A Broadly Applicable Mild Method for the Synthesis of gem-Diperoxides from Corresponding Ketones or 1,3-Dioxolanes. Org. Lett. 2009, 11, 1615–1618. [Google Scholar] [CrossRef]
- Bunge, A.; Hamann, H.-J.; Liebscher, J. A simple, efficient and versatile synthesis of primary gem-dihydroperoxides from aldehydes and hydrogen peroxide. Tetrahedron Lett. 2009, 50, 524–526. [Google Scholar] [CrossRef]
- Azarifar, D.; Khosravi, K.; Soleimanei, F. Mild and Efficient Strontium Chloride Hexahydrate-Catalyzed Conversion of Ketones and Aldehydes into Corresponding gem- Dihydroperoxides by Aqueous H2O2. Molecules. 2010, 15, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Deng, J.; Gao, J.-W.; Zhang, Z.-H. Triflic Acid-Functionalized Silica-Coated Magnetic Nanoparticles as a Magnetically Separable Catalyst for Synthesis of gem-Dihydroperoxides. Adv. Synth. Catal. 2012, 354, 441–447. [Google Scholar] [CrossRef]
- Surya Prakash, G.K.; Shakhmin, A.; Glinton, K.E.; Rao, S.; Mathew, T.; Olah, G.A. Poly(N-vinylpyrrolidone)–H2O2 and poly(4-vinylpyridine)–H2O2 complexes: Solid H2O2 equivalents for selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides. Green. Chem. 2014, 16, 3616–3622. [Google Scholar] [CrossRef]
- Bityukov, O.V.; Vil’, V.A.; Terent’ev, A.O. Synthesis of Acyclic Geminal Bis-peroxides. Russ. J. Org. Chem. 2021, 57, 853–878. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Kutkin, A.V.; Troizky, N.A.; Ogibin, Y.N.; Nikishin, G.I. Synthesis of Geminal Bisperoxides by Acid-Catalyzed Reaction of Acetals and Enol Ethers with tert-Butyl Hydroperoxide. Synthesis. 2005, 13, 2215–2219. [Google Scholar] [CrossRef]
- Ghorai, P.; Dussault, P.H. Broadly Applicable Synthesis of 1,2,4,5-Tetraoxanes. Org. Lett. 2008, 11, 213–216. [Google Scholar] [CrossRef]
- Novikov, V.L.; Shestak, O.P. Reactions of hydrogen peroxide with acetylacetone and 2-acetylcyclopentanone. Russ. Chem. Bull. 2014, 62, 2171–2190. [Google Scholar] [CrossRef]
- Amado, P.S.M.; Frija, L.M.T.; Coelho, J.A.S.; O’Neill, P.M.; Cristiano, M.L.S. Synthesis of Non-symmetrical Dispiro-1,2,4,5-Tetraoxanes and Dispiro-1,2,4-Trioxanes Catalyzed by Silica Sulfuric Acid. J. Org. Chem. 2021, 86, 10608–10620. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Stiasny, B.; Stierstorfer, J.; Winter, C.H. Energetic Organic Peroxides—Synthesis and Characterization of 1,4-Dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptanes. Eur. J. Org. Chem. 2015, 2015, 6237–6242. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Tsogoeva, S.B.; Terent’ev, A.O. Lewis Acids and Heteropoly Acids in the Synthesis of Organic Peroxides. Pharmaceuticals 2022, 15, 472. [Google Scholar] [CrossRef] [PubMed]
- Rieche, A.; Bischoff, C.; Prescher, D. Alkylperoxyde, XXXV. Peroxyde des Triacetylmethans “Triacetylmethanperoxyd”. Chem. Ber. 1964, 97, 3071–3075. [Google Scholar] [CrossRef]
- Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Nikishin, G.I.; Yaremenko, I.A.; Terent’ev, A.O. Selective synthesis of cyclic triperoxides from 1,1′-dihydroperoxydi(cycloalkyl)peroxides and acetals using SnCl4. Russ. Chem. Bull. 2019, 68, 1289–1292. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Glinushkin, A.P.; Nikishin, G.I. Synthesis of peroxides from β,δ-triketones under heterogeneous conditions. Russ. J. Org. Chem. 2016, 51, 1681–1687. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Medvedev, M.G.; Krivoshchapov, N.V.; Belyakova, Y.Y.; Korlyukov, A.A.; Ilovaisky, A.I.; Terent Ev, A.O.; Alabugin, I.V. How to Build Rigid Oxygen-Rich Tricyclic Heterocycles from Triketones and Hydrogen Peroxide: Control of Dynamic Covalent Chemistry with Inverse alpha-Effect. J. Am. Chem. Soc. 2020, 142, 14588–14607. [Google Scholar] [CrossRef]
- Vil’, V.A.; Barsegyan, Y.A.; Barsukov, D.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent’ev, A.O. Peroxycarbenium Ions as the “Gatekeepers” in Reaction Design: Assistance from Inverse Alpha-Effect in Three-Component β-Alkoxy-β-peroxylactones Synthesis. Chem.—Eur. J. 2019, 25, 14460–14468. [Google Scholar] [CrossRef]
- Vil’, V.A.; Barsegyan, Y.A.; Kuhn, L.; Ekimova, M.V.; Semenov, E.A.; Korlyukov, A.A.; Terent’ev, A.O.; Alabugin, I.V. Synthesis of unstrained Criegee intermediates: Inverse α-effect and other protective stereoelectronic forces can stop Baeyer–Villiger rearrangement of γ-hydroperoxy-γ-peroxylactones. Chem. Sci. 2020, 11, 5313–5322. [Google Scholar] [CrossRef]
- Hawkins, E.G.E. Reactions of organic peroxides. Part XI. Amino-peroxides from cyclic ketones. J. Chem. Soc. C. 1969, 2671–2677. [Google Scholar] [CrossRef]
- Hawkins, E.G.E. Reactions of organic peroxides. Part XVI. Amino-peroxides from autoxidation of imines. J. Chem. Soc. C. 1971, 160–166. [Google Scholar] [CrossRef]
- Kaminskii, V.A.; Alekseev, V.I.; Tilichenko, M.N. Aminoperoxidation of 2,2′-methylenedicyclohexanone. Chem. Het. Comp. 1972, 8, 1551–1552. [Google Scholar] [CrossRef]
- Shumakov, S.A.; Kaminskii, V.A.; Tilichenko, M.N. Hydroacridines and Related-Compounds. 22. Synthesis of Compounds with 2,6-Epidioxipiperidine Structures. Chem. Het. Comp. 1985, 21, 72–77. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent’ev, A.O. Marriage of Peroxides and Nitrogen Heterocycles: Selective Three-Component Assembly, Peroxide-Preserving Rearrangement, and Stereoelectronic Source of Unusual Stability of Bridged Azaozonides. J. Am. Chem. Soc. 2021, 143, 6634–6648. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent′ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Alabugin, I.V.; Terent’ev, A.O. Cascade Assembly of Bridged N-Substituted Azaozonides: The Counterintuitive Role of Nitrogen Source Nucleophilicity. Org. Lett. 2022, 24, 6582–6587. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Ishmukhametova, I.R.; Tyumkina, T.V.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis of N-aryl-hexaoxazadispiroalkanes using lanthanide catalysts. Tetrahedron Lett. 2018, 59, 3161–3164. [Google Scholar] [CrossRef]
- Makhmudiyarova, N.N.; Shangaraev, K.R.; Dzhemileva, L.U.; Tyumkina, T.V.; Mescheryakova, E.S.; D’Yakonov, V.A.; Ibragimov, A.G.; Dzhemilev, U.M. New synthesis of tetraoxaspirododecane-diamines and tetraoxazaspirobicycloalkanes. RSC. Adv. 2019, 9, 29949–29958. [Google Scholar] [CrossRef]
- Zvilichovsky, G.; Zvilichovsky, B. Ozonolysis. Hydroxyl, Ether and Peroxide Groups; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1993; pp. 687–784. [Google Scholar] [CrossRef]
- Griesbaum, K.; Liu, X.; Kassiaris, A.; Scherer, M. Ozonolyses of O-Alkylated Ketoximes in the Presence of Carbonyl Groups: A Facile Access to Ozonides. Liebigs Ann. 1997, 1997, 1381–1390. [Google Scholar] [CrossRef]
- Dos Passos Gomes, G.; Yaremenko, I.A.; Radulov, P.S.; Novikov, R.A.; Chernyshev, V.V.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Stereoelectronic Control in the Ozone—Free Synthesis of Ozonides. Angew. Chem. Int. Ed. 2017, 56, 4955–4959. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Gomes, G.d.P.; Radulov, P.S.; Belyakova, Y.Y.; Vilikotskiy, A.E.; Vil’, V.A.; Korlyukov, A.A.; Nikishin, G.I.; Alabugin, I.V.; Terent’ev, A.O. Ozone-Free Synthesis of Ozonides: Assembling Bicyclic Structures from 1,5-Diketones and Hydrogen Peroxide. J. Org. Chem. 2018, 83, 4402–4426. [Google Scholar] [CrossRef] [PubMed]
- Akimova, T.I.; Rybin, V.G.; Soldatkina, O.A. New Tetracyclic Spiro-1,2,4-trioxolanes (Ozonides). Synthesis and Mass Spectrometric Study. Russ. J. Org. Chem. 2019, 55, 101–107. [Google Scholar] [CrossRef]
- Akimova, T.I.; Soldatkina, O.A.; Savchenko, V.G.; Pilipenko, A.V.; Kapustina, A.A. Stereoisomerism in the representative tetracyclic dispiro ozonide derived from (methylene)dicyclohexanone with the 1,5-diketo arrangement. Mendeleev Commun. 2022, 32, 271–273. [Google Scholar] [CrossRef]
- Ingram, K.; Yaremenko, I.A.; Krylov, I.B.; Hofer, L.; Terent’ev, A.O.; Keiser, J. Identification of Antischistosomal Leads by Evaluating Bridged 1,2,4,5-Tetraoxanes, Alphaperoxides, and Tricyclic Monoperoxides. J. Med. Chem. 2012, 55, 8700–8711. [Google Scholar] [CrossRef]
- Saito, I.; Nagata, R.; Yuba, K.; Matsuura, T. Synthesis of α-silyloxyhydroperoxides from the reaction of silyl enol ethers and hydrogen peroxide. Tetrahedron. Lett. 1983, 24, 1737–1740. [Google Scholar] [CrossRef]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | Equiv. of H2O2 vs. 1a | Equiv. of SnCl4 vs. 1a | Solvent | Isolated yield of 2a + 3a, % | Ratio of 2a:3a |
| 1 | 1.5 | SnCl4 (1.0) | THF | 21 | 1.4:1.0 |
| 2 | 1.5 | SnCl4 (3.0) | THF | 64 | 1.2:1.0 |
| 3 | 3.0 | SnCl4 (3.0) | THF | 70 | 1:1 |
| 4 | 1.5 | SnCl4 (5.0) | THF | 83 | 1:1 |
| 5 | 3.0 | SnCl4 (5.0) | THF | 71 | 1:1 |
| 6 | 1.5 | SnCl4 (5.0) | 1,4-Dioxane | 81 | 2.6:1.0 |
| 7 | 1.5 | SnCl4 (5.0) | Et2O | 79 | 8.6:1.0 |
| 8 | 1.5 | SnCl4 (5.0) | СН3СN | - | - |
| 9 | 1.5 | SnCl4 (5.0) | СН2Сl2 | - | - |
| Compound | NTS | Adult | |
|---|---|---|---|
| (Effect %) at 33.3 µM | (Effect %) at 10 µM | (Effect %) at 10 µM | |
| 2b | 100 ± 0 | 74 ± 5 | 23 ± 4 |
| 2c | 100 ± 0 | 67 ± 20 | 70 ± 2 |
| 2d | 100 ± 0 | 100 ± 0 | 82 ± 0 |
| 2e | 100 ± 0 | 100 ± 0 | 14 ± 4 |
| 2f | 93 ± 11 | 30 ± 5 | ND |
| 2g | 36 ± 22 | 39 ± 5 | ND |
| 2h | 77 ± 38 | 17 ± 5 | ND |
| 2i | 100 ± 0 | 87 ± 5 | 55 ± 3 |
| 2j | 96 ± 5 | 23 ± 9 | ND |
| 2k | 90 ± 0 | 23 ± 0 | ND |
| 3b | 22 ± 0 | 12 ± 0 | ND |
| 3c | 100 ± 0 | 100 ± 0 | 76 ± 0 |
| 3d | 74 ± 11 | 22 ± 0 | ND |
| 3e | 22 ± 5 | 12 ± 0 | ND |
| 3f | 61 ± 6 | 11 ± 6 | ND |
| 3g | 73 ± 6 | 15 ± 6 | ND |
| 3h | 100 ± 0 | 42 ± 11 | ND |
| 3j | 100 ± 0 | 88 ± 0 | 23 ± 4 |
| 3k | 12 ± 0 | 3.2 ± 0 | ND |
| Artesunate | - | 63 ±0 | not active |
| Praziquantel | - | 77 ± 0 | 100 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulov, P.S.; Yaremenko, I.A.; Keiser, J.; Terent’ev, A.O. Bridged 1,2,4-Trioxolanes: SnCl4—Catalyzed Synthesis and an In Vitro Study against S. mansoni. Molecules 2023, 28, 4913. https://doi.org/10.3390/molecules28134913
Radulov PS, Yaremenko IA, Keiser J, Terent’ev AO. Bridged 1,2,4-Trioxolanes: SnCl4—Catalyzed Synthesis and an In Vitro Study against S. mansoni. Molecules. 2023; 28(13):4913. https://doi.org/10.3390/molecules28134913
Chicago/Turabian StyleRadulov, Peter S., Ivan A. Yaremenko, Jennifer Keiser, and Alexander O. Terent’ev. 2023. "Bridged 1,2,4-Trioxolanes: SnCl4—Catalyzed Synthesis and an In Vitro Study against S. mansoni" Molecules 28, no. 13: 4913. https://doi.org/10.3390/molecules28134913
APA StyleRadulov, P. S., Yaremenko, I. A., Keiser, J., & Terent’ev, A. O. (2023). Bridged 1,2,4-Trioxolanes: SnCl4—Catalyzed Synthesis and an In Vitro Study against S. mansoni. Molecules, 28(13), 4913. https://doi.org/10.3390/molecules28134913