2.1. LiF–Tetraamide Complexes, mLiF
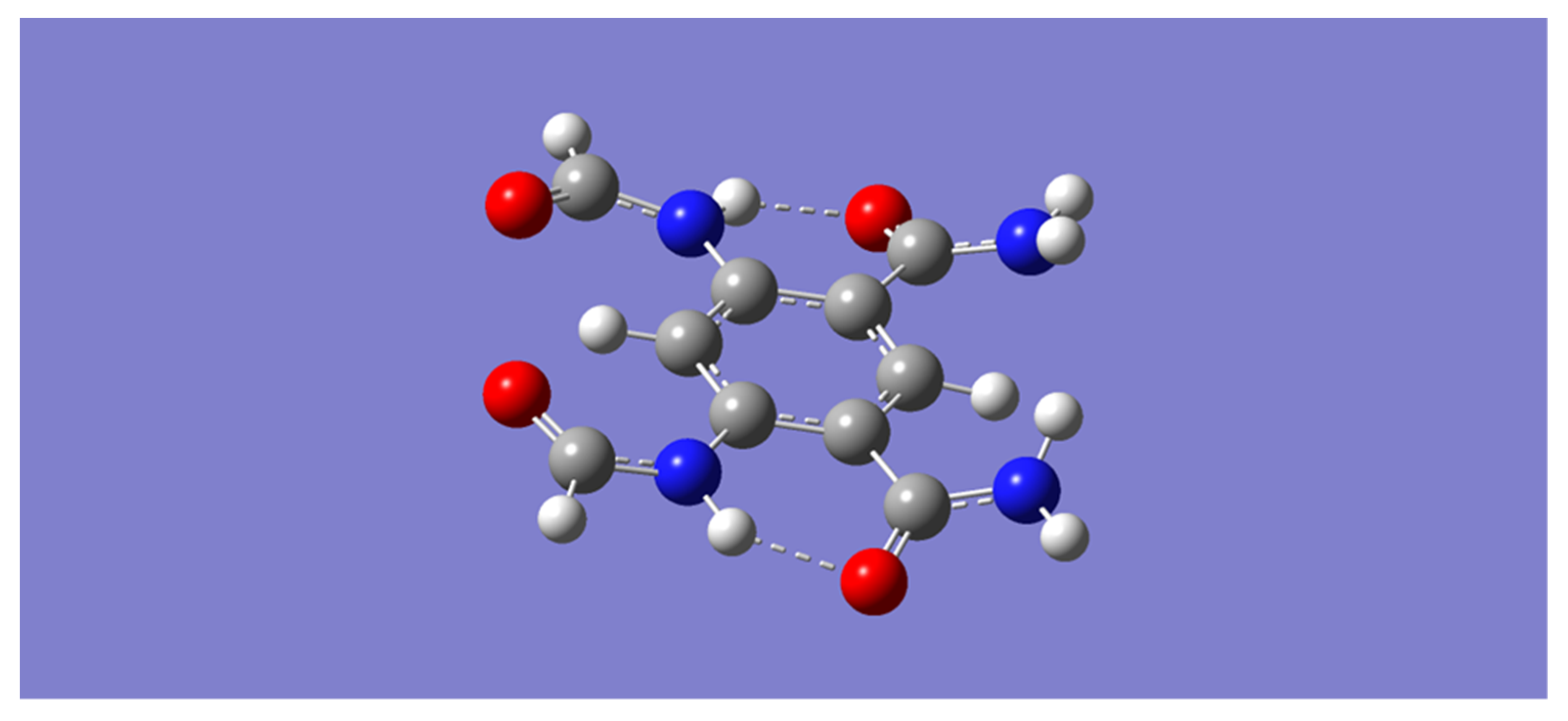
For convenience, the model aromatic tetraamide will be referred to with the letter “m”. All methods give a similar optimized geometry of the model tetraamide, m, as that displayed in
Figure 1. Inspection of
Figure 1 shows that two of the four amides connect to benzene via their carbonyl carbons, and that the other two amides connect via their nitrogen atoms. In this geometry, the two pairs of amides are connected through N-H⋯O=C hydrogen bonds. In addition, it is apparent in this geometry that the two pairs of amides possess complementary binding ability, which allows for the binding of an electron-rich species through hydrogen bonding with the N-H moieties of one pair of amides, and the binding of an electron-deficient species with the O=C moieties of the other pair of amides [
37,
43].
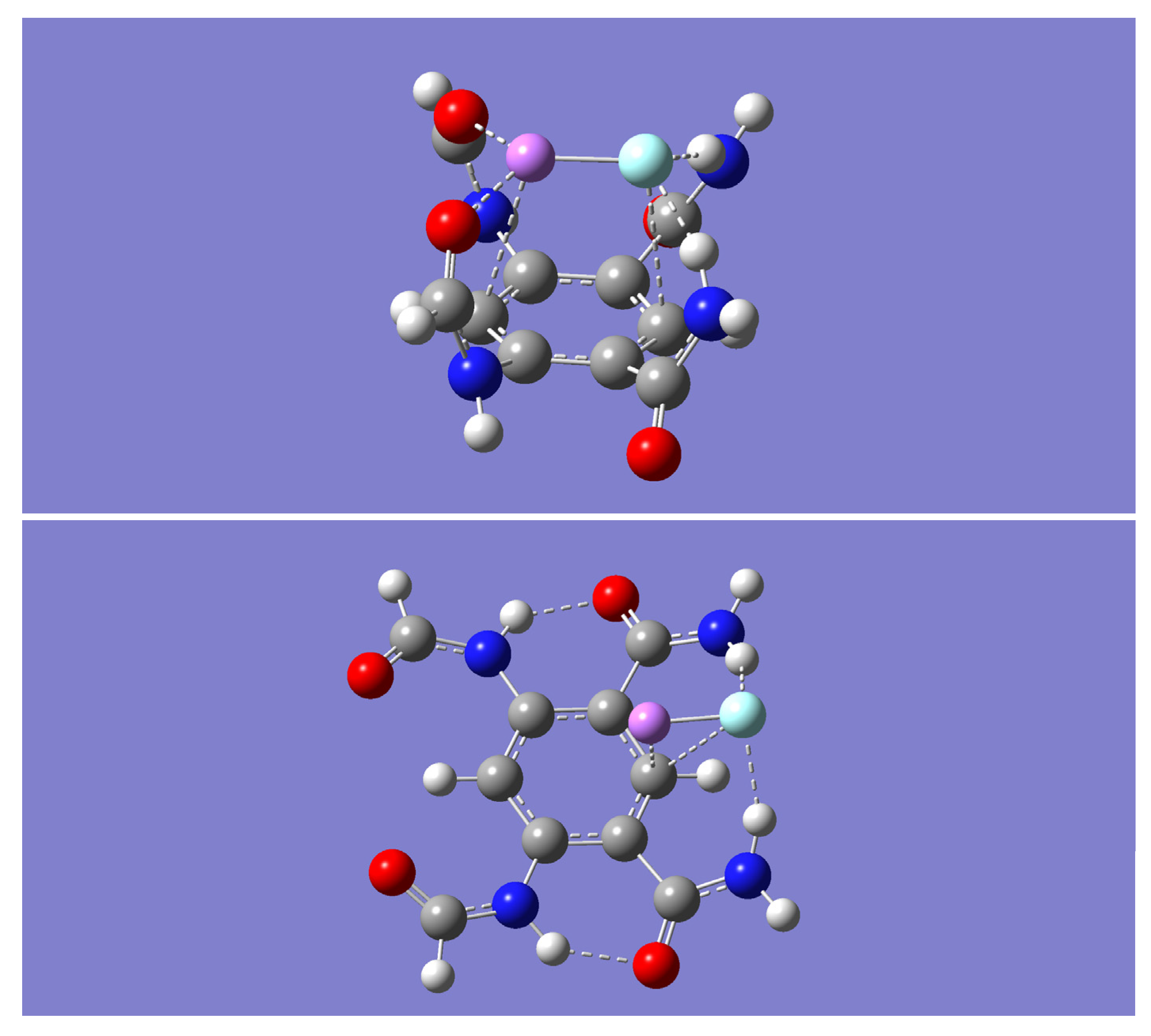
The extent of this complementary binding ability is investigated by optimizing the geometry resulting from bringing together the model tetraamide, m, and a molecule of lithium fluoride, LiF, from various intermolecular approaches. Three distinct minimum energy complexes (with zero imaginary frequencies), mLiF-A, mLiF-B, and mLiF-C, were obtained with the B3LYP method (see
Figure 2). These optimized dimer complexes were further optimized with the M05-2X method and both the B3LYP-D and the wB97XD methods. In all cases, frequency calculations confirm that the optimized geometries were minima (no imaginary frequencies). The most striking finding is that the mLiF-C complex, obtained with the B3LYP method, is converted into the mLiF-A complex upon geometry optimization with any of the other three methods.
Table 1 shows the relative electronic energies and zero-point energies in kcal/mol for all complexes. All methods predict that mLiF-A is lower in energy relative to mLiF-B. Including the small zero-point energy correction, the smallest relative energy is found with the B3LYP method, 10.91 kcal/mol. All the other methods give corresponding relative energies that are close to one another, with the largest value given by the B3LYP-D method, 14.77 kcal/mol. Interestingly, the mLiF-C complex is only 4.15 kcal/mol above mLiF-A even though it does not remain as a minimum with the other three methods but rather is converted into the mLiF-A complex.
The BSSE-corrected binding energies calculated with the various methods are given in
Table 2. The binding energies listed in
Table 2 as BE are calculated as the difference between the energy of the complex and the sum of the energies of the constituent monomers, assuming each monomer has the same geometry as in the corresponding complex. All methods agree that the binding energies for all complexes are large and that, except for the wB97XD method, the binding energy of the mLiF-A complex more than doubles that of the mLiF-B complex. The mLiF-A binding energy calculated with the wB97XD method is slightly less than twice that of the mLiF-B complex. Additionally, the B3LYP results show that the binding energy of the mLiF-A complex is more than the sum of the other two complexes, mLiF-B and mLiF-C, found with this method. Relative to the other three methods, B3LYP underestimates the binding energies for the mLiF-A and mLiF-B complexes. Lastly, the M05-2X binding energy of either mLiF-B or mLiF-A lies in between that calculated with the B3LYP-D and wB97XD methods. The binding energies listed in
Table 2 as BE-DE take into account the increase in energy of the monomers in the complex due to the geometrical deformations that each monomer suffers upon complex formation. Inspection of
Table 2 shows that the energy cost of the geometry deformation of the monomers, relative to their geometries when they are isolated and fully optimized, is much larger upon mLiF-A formation (27.59 to 32.26 kcal/mol) than upon mLiF-B formation (3.29 to 5.24 kcal/mol).
Pertinent geometrical parameters for the mLiF dimers optimized with the various theoretical methods are displayed in
Table 3 (distances) and
Table 4 (angles). In general, the covalent Li-F bond is elongated relative to the optimized Li-F monomer. For reference, the optimized Li-F bond distance is calculated to be 1.587, 1.583, 1.583, and 1.598 Å with the B3LYP, M05-2X, B3LYP-D, and wB97XD methods, respectively. In particular, the Li-F bond distance is longer in the mLiF-A complex than in the other two complexes. The larger elongation of the Li-F bond in mLiF-A results from the simultaneous chelate binding of Li and F by the aromatic tetraamide. In mLiF-B, the binding is primarily on the F end of the molecule, and in mLiF-C, the binding is on the Li end of the molecule, as revealed in the intermolecular Li⋯O=C and N-H⋯F distances and angles. Additional interactions of the Li-F molecule with the aromatic ring can be inferred from the Li or F distance to the closest carbon in the aromatic ring, as shown in the respective Li⋯C and F⋯C distances in
Table 3.
Complexation of LiF in mLiF-A produces a substantial weakening of the intramolecular N-H⋯O=C hydrogen bond, as observed in the respective N-H⋯O distances (
Table 3) and angles (
Table 4). As a reference, the N-H⋯O distance in the optimized tetraamide monomer, m, is calculated to be 1.822, 1.856, 1.827, and Å with the B3LYP, M05-2X, B3LYP-D, and wB97XD methods, respectively. In the same order of the DFT methods, the optimized N-H⋯O angle in the tetraamide monomer is calculated to be 138.6, 137.1, 138.1 and degrees, respectively. On the other hand, the distortion of the intramolecular N-H⋯O=C hydrogen bond is much smaller upon formation of the mLiF-B or the mLiF-C complexes. For example, the N-H ⋯O angle in mLiF-B is quite close to that in the optimized tetraamide monomer, and thus the distortion of the hydrogen bond is shown mostly in the N-H⋯O distance, which is somewhat lengthened in the complex.
The geometrical flexibility of the model tetraamide to bind LiF is demonstrated in the dihedral angles that relate the two amides forming the intramolecular N-H⋯O=C hydrogen bond with each other in relation to the aromatic ring carbons, namely, the (O=)C-N-C-C and the O=C-C-C dihedral angles listed in
Table 5. The values of these dihedral angles reflect the deviation of each amide from the aromatic ring plane. Accordingly, the more aligned the two amides are with the plane, the closer to 180 degrees and to 0 degrees the (O=)C-N-C-C and the O=C-C-C dihedral angles are, respectively. Inspection of
Table 5 shows that the amides are aligned with the aromatic plane the most in the uncomplexed tetraamide monomer, m. In contrast, the least alignment with the plane is seen in the mLiF-A complex.
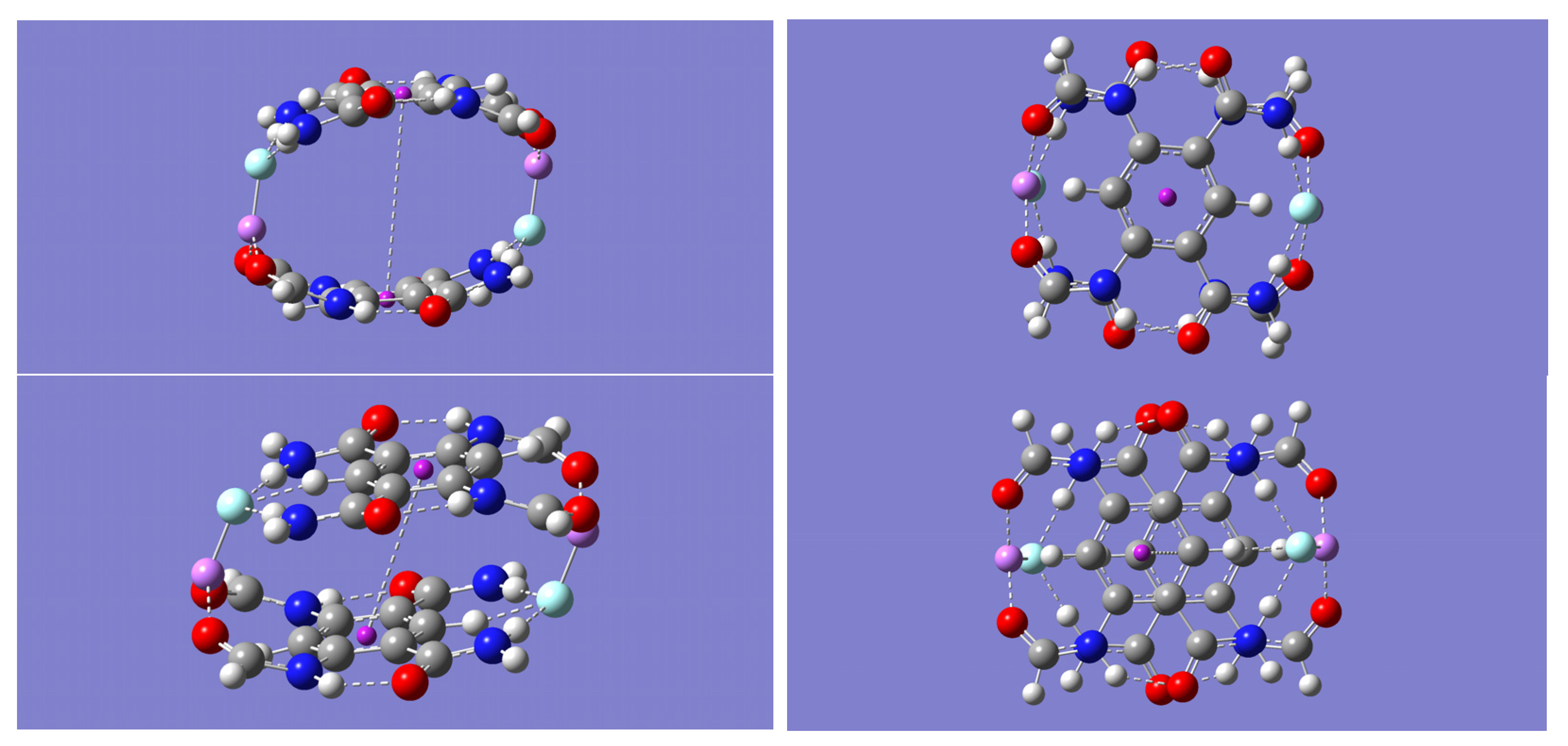
2.2. (m-LiF)2 Complexes
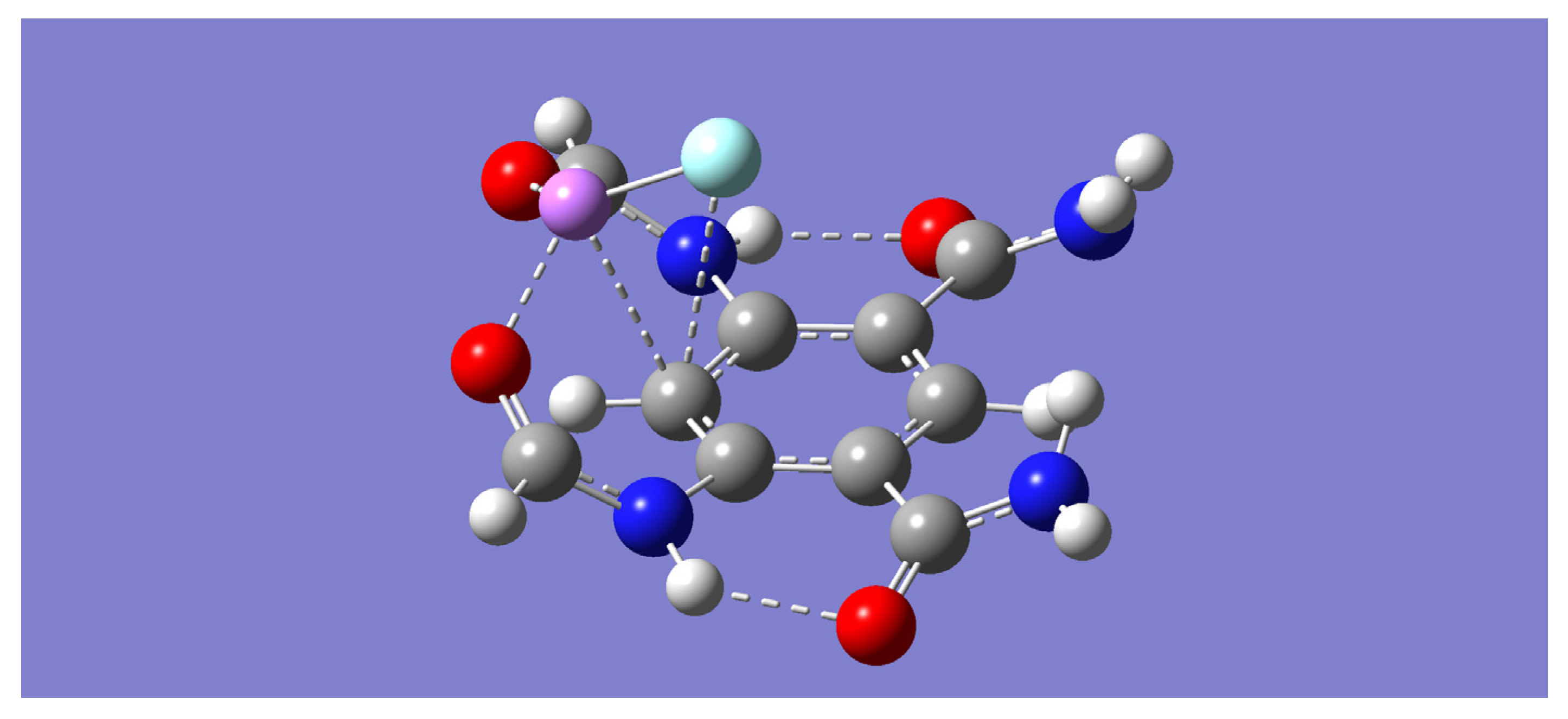
Each of the dimer complexes, mLiF-A or mLiF-B, was used as a motif to build a dimer of a dimer complex or a tetramer complex, (m-LiF)
2. Two distinct tetramer complexes, (m-LiF)
2-A and (m-LiF)
2-B, were found upon geometry optimization by all the computational methods employed in this work. The former has a C
2 point group symmetry (
Figure 3) while the latter has a bracelet-like geometry of C
2h symmetry (
Figure 4). The absence of any imaginary frequencies also verified that the optimized tetramers are indeed minima. Specifically, the complementary overlap of two mLiF-A complexes resulted in the (m-LiF)
2-A tetramer complex, while the corresponding overlap of two mLiF-B complexes resulted in the (m-LiF)
2-B tetramer. Incidentally, using the optimized ion-pair (Li
+ and F
−) complex of the model tetraamide reported previously [
37] also resulted in the (m-LiF)
2-B tetramer.
Table 6 shows the relative electronic and zero-point energies in kcal/mol for all tetramer complexes. All methods predict (m-LiF)
2-B to be lower (by more than 20 kcal/mol) in energy relative to its (m-LiF)
2-A counterpart. Including the small zero-point energy correction, the largest relative energy is found with the B3LYP method, which shows (m-LiF)
2-A above (m-LiF)
2-B by 27.67 kcal/mol. In turn, the M05-2X gives the smallest energy gap between these complexes, 20.67 kcal/mol. The transition state structure connecting the two tetramers was found by each of the DFT methods employed here.
Table 6 shows that the energy barrier, including zero-point energy, for the transition from the higher to the lower energy tetramer is relatively small, with the B3LYP method giving the lowest energy barrier (2.44 kcal/mol).
The BSSE-corrected binding energies for the two tetramers, calculated with the various methods, are given in
Table 7. Examination of
Table 7 indicates that the B3LYP consistently underestimates the binding energies relative to the other three methods, partly due to the inability of B3LYP to account for dispersion forces such as the π⋯π interactions resulting from the overlap of the two benzene ring units [
48,
49,
50]. The binding energy calculated for the (m-LiF)
2-B complex corresponds to the breaking of the complex into its four constituent units and is listed as (m-LiF)
2-B_4 in
Table 7. In this case, all methods predict binding energies that are substantially larger than twice the binding energy of the parent dimer mLiF-B. The result is not surprising, given that the Li end of each LiF unit is chelated by amide oxygens in the tetramer, an interaction which is missing in the parent dimer. That is, the much stronger Li⋯O=C interactions in (m-LiF)
2-B replace the weak Li⋯π interaction in mLiF-B. Regarding the (m-LiF)
2-A tetramer, two different binding energies were calculated. One shows the binding energy associated with all four units, the two LiF molecules and the two model tetraamides, (m-LiF)
2-A_4. In this case, all methods predict binding energies that are also more than twice the binding energy of the parent dimer mLiF-A. The difference can be traced down to the interaction between the LiF molecules in the LiF dimer mediating the formation of (m-LiF)
2-A. Consequently, the (m-LiF)
2-A complex can be thought of as a dimer of dimers mediated by the interaction of two LiF molecules, and the other binding energy, (m-LiF)
2-A_2, essentially shows the energy required to break the interaction of the two LiF moieties holding the two dimers together. In this case, the calculated binding energies are similar across the various methods (33.57 to 35.86 kcal/mol). Subtracting this binding energy from that calculated in (m-LiF)
2-A_4 gives results that are indeed close to twice the binding energies found for the parent dimer mLiF-A (
Table 3). Moreover, a comparison of the (m-LiF)
2-A_2 binding energy can be made with that of the breaking of the optimized LiF dimer into the two constituent LiF monomers. To this effect, the LiF dimer was first optimized yielding a cyclic structure of D
2h symmetry, and the corresponding BSSE-corrected binding energy was calculated next. These results agree with previously published results [
38,
39,
40,
41,
42]. The binding energies shown in
Table 7 indicate that the binding energy in the isolated LiF dimer (66.08 to 69.71 kcal/mol) is slightly less than twice that in the (m-LiF)
2-A_2. That is, the chelate binding, NH⋯F-Li⋯O=C, of each Li-F unit by the amide motifs weakens (by almost 50%) the strength of the interactions in the isolated LiF dimer. Consequently, the binding energy of the LiF dimer in the tetramer is decreased relative to that of the isolated LiF dimer, as demonstrated by all the methods employed.
Relevant geometrical parameters for the (m-LiF)
2-A tetramer are displayed in
Table 8. These parameters can be compared with those for the mLiF-A parent dimer listed in
Table 3 (distances) and
Table 4 (angles).
Table 8 shows two sets of different values for each of the Li-F, Li⋯O=C, and NH⋯F distances in (m-LiF)
2-A, which are in all cases longer than the corresponding values in
Table 3, regardless of the method used. The larger Li-F bond distances correspond to those of the LiF units in the same geometrical orientation as that in mLiF-A, while the smaller distances correspond to the separation of the adjacent LiF molecules holding together the (m-LiF)
2-A tetramer. For additional comparison, the smaller Li-F distances are still larger than those found in the isolated LiF dimer of D
2h symmetry. Specifically, the Li-F distance in the LiF dimer obtained by the B3LYP, B3LYP-D, M05-2X, and wB97XD methods are 1.735, 1.737, 1.726, and 1.745 Å, respectively. Two other parameters listed in
Table 8 are the symmetric distances from each Li or F to its closest aromatic carbon, Li⋯C or F⋯C, respectively. The former shows an elongation relative to that in the parent dimer, while the opposite is true for the latter. Lastly, the Li⋯O=C and N-H⋯F angles change slightly upon tetramer formation. For example, the B3LYP method shows a widening of about 3.6 degrees for the Li⋯O=C angles concomitant with a narrowing of 3.6 degrees for the N-H⋯F angles. The widening for the Li⋯O=C angles is smaller with the other three methods, with a slightly larger N-H⋯F narrowing.
Important geometrical parameters for the bracelet-like (m-LiF)
2-B tetramer are displayed in
Table 9. Comparable parameters for the mLiF-B parent dimer are listed in
Table 3 (distances) and
Table 4 (angles). In particular, the binding of each Li end of a LiF molecule by the oxygen atoms of the pertinent amides in each aromatic ring in the tetramer results in a substantial decrease in the Li⋯O=C distances relative to those in the parent dimer where these interactions are missing. The Li⋯O=C angles are in tandem widened greatly upon tetramer formation. Similar results are found for the N-H⋯F hydrogen bond distances and angles, indicating stronger hydrogen bond interactions in the tetramer. Even the intramolecular N-H⋯O=C hydrogen bond is strengthened in the tetramer, as demonstrated mostly by the sizeable decrease in the pertinent hydrogen bond distance. One striking interaction that is absent in the parent mLiF-B dimer but that emerges in the (m-LiF)
2-B tetramer is the intermolecular C-H⋯F hydrogen bond interaction. Except for the B3LYP method, all other methods consistently show relatively small hydrogen bond distances (1.842–1.863 Å) and quasilinear angles (168.2–172.6 degrees). The B3LYP method, however, shows a much larger C-H⋯F hydrogen bond distance (2.132 Å) and narrower angle (127.6 degrees), suggesting that the C-H⋯F hydrogen bond interactions make negligible contributions to the stability of the tetramer at the B3LYP level. Another interaction that is unique to the (m-LiF)
2-B tetramer complex is the π⋯π interaction resulting from the overlapping of the two aromatic rings.
Table 9 shows the distance separating the centers of each aromatic ring. Except for the B3LYP method, all the other methods predict relatively small distances between the aromatic rings (3.852–4.092 Å). The much larger distance between the aromatic ring centers predicted by the B3LYP method, 5.378 Å, is consistent with the inability of this method to account for dispersion forces, and thus any contribution to the stability of the tetramer due to the π⋯π interaction is essentially unaccounted for with the B3LYP method. It is worth noting that the B3LYP method predicts an almost perfectly eclipsed orientation of the two aromatic rings, while the other three methods predict a staggered orientation with the center of one ring directly above one of the carbon atoms in the other ring (see
Figure 3). Although not shown in
Table 9, the intermolecular Li⋯F separation is close to 7 Å with the B3LYP method and close to 8 Å with the other three methods.
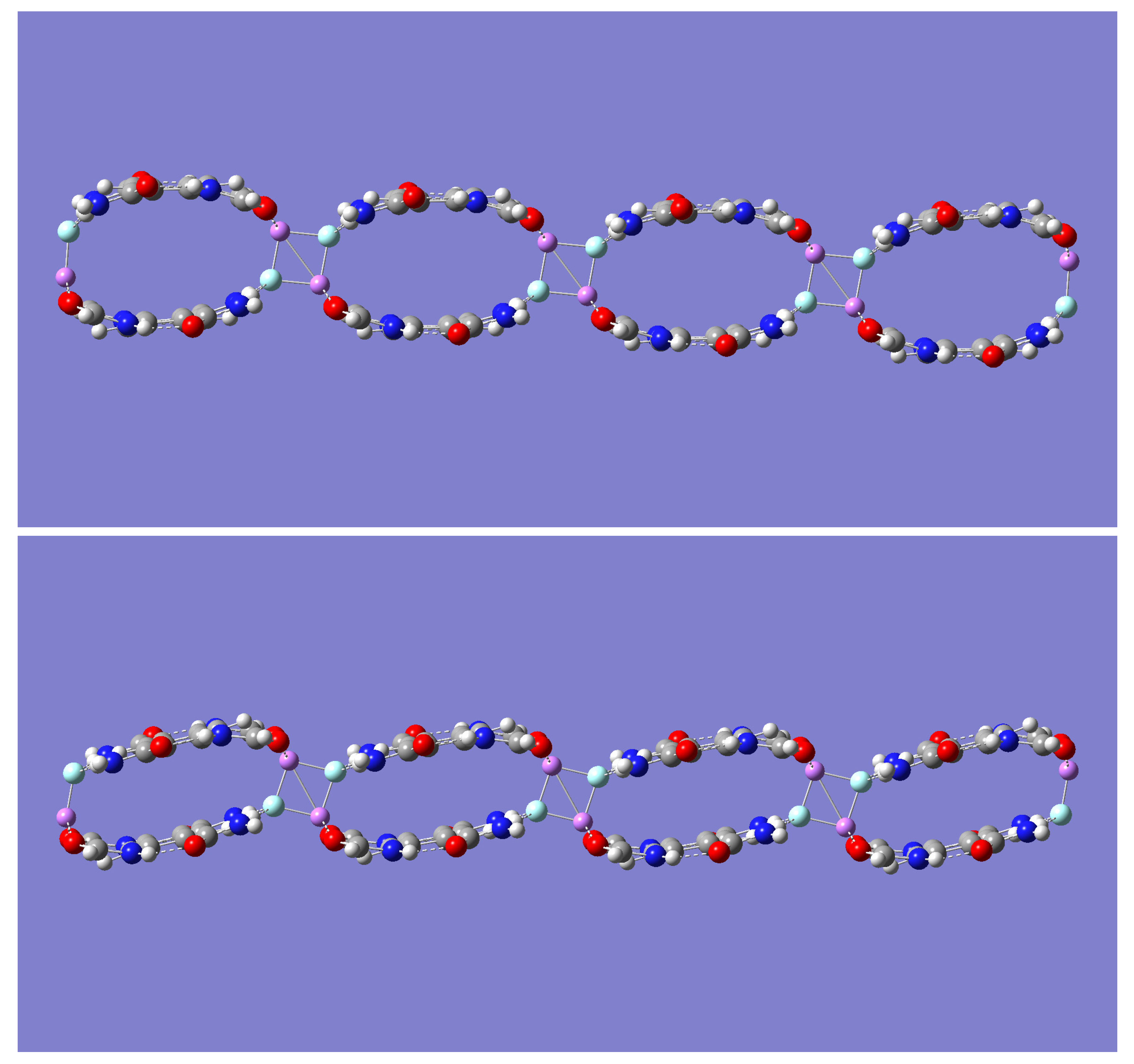
2.3. Self-Assembly: [(m-LiF)2-B]n Complexes
Although both (m-LiF)
2-A and (m-LiF)
2-B tetramer complexes exhibit large binding energies, it is the latter complex that offers a compelling possibility for self-assembly into a growing network of
n ≥ 1 tetrameric units, [(m-LiF)
2-B)]
n. Indeed, the potential for the interaction of adjacent tetrameric units via their LiF units seems plausible, as was seen in the formation of the (m-LiF)
2-A complex from two mLiF-A parent dimers. In the (m-LiF)
2-A complex, however, continuous self-assembly via the LiF dimer interaction is not possible. In the case of the (m-LiF)
2-B self-assembly, the possibility for continuous network growth remains after two or more tetrameric units are brought together. This possibility was explored first for the case of
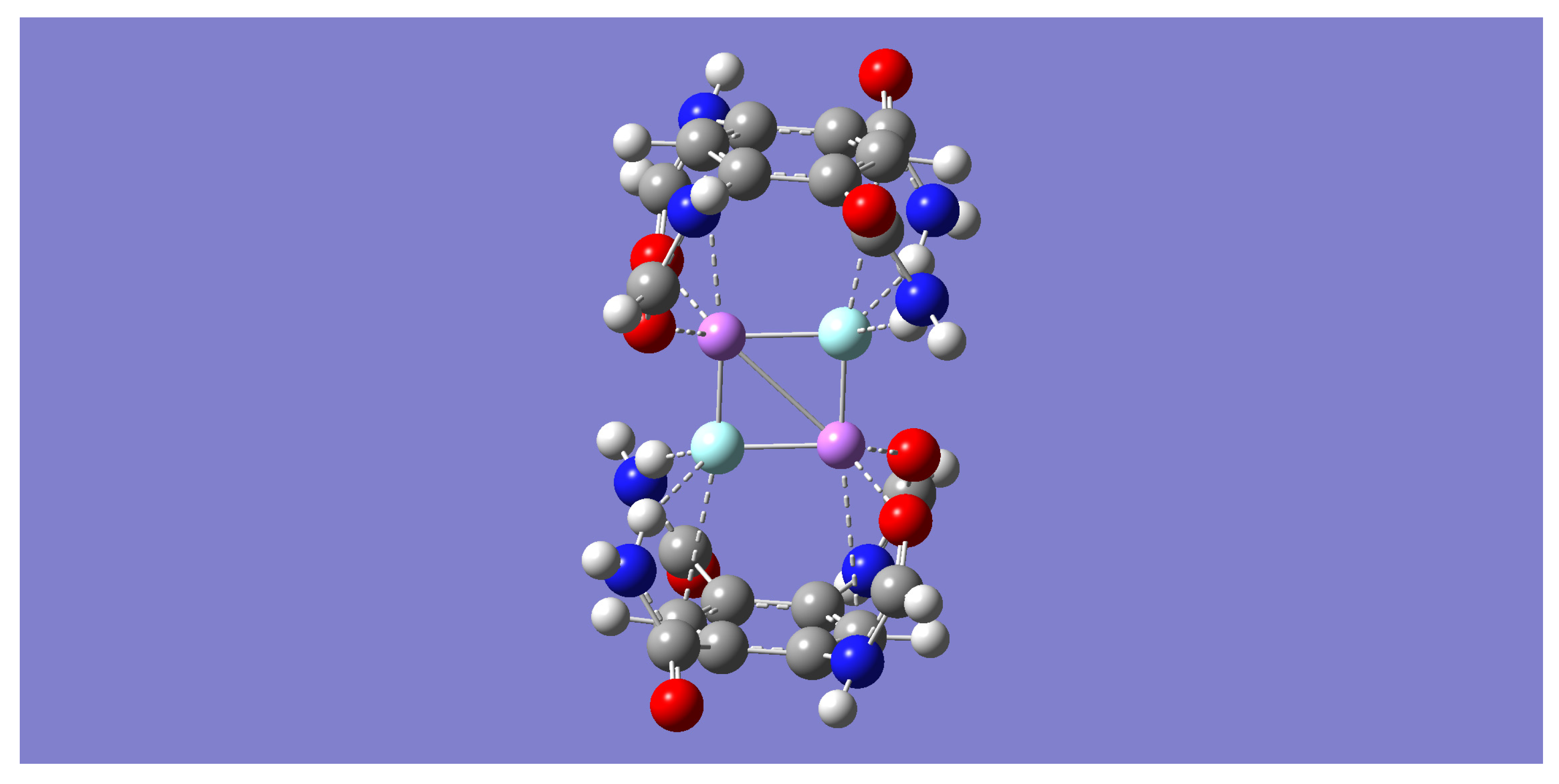
n = 2. Geometry optimization and frequency calculations with the B3LYP method resulted in a [(m-LiF)
2-B)]
2 complex of D
2h symmetry (See
Figure 5) whose nature as a minimum energy structure was confirmed by the absence of any imaginary frequencies. In this larger complex, each tetrameric unit is linked sideways with an adjacent tetramer in an interaction mediated by the formation of the LiF dimer in a way that resembles the D
2h symmetry of the isolated LiF dimer, as it did in the (m-LiF)
2-A complex. Similar results were also found with the other three methods.
The energy required to break the [(m-LiF)
2-B)]
2 complex into its two tetrameric units was calculated and the results are listed in
Table 10. This energy can be appropriately compared with the energy required to break the (m-LiF)
2-A complex into its constituent dimers, (m-LiF)
2-A_2 (
Table 7). In general, the calculated BSSE-corrected binding energies are just few kcal/mol smaller than those listed in
Table 7. More specifically, the largest decrease in the [(m-LiF)
2-B)]
2 complex binding energies occurs with the B3LYP method (−16%), and the smallest decrease occurs with the wB97XD method (−6%). The corresponding decreases with the other two methods B3LYP-D (−8%) and M05-2X (−9%) are close to each other. Despite the noted decrease relative to (m-LiF)
2-A_2, the calculated binding energies in the [(m-LiF)
2-B)]
2 complex are still large enough (in the range of 28.04 to 35.90 kcal/mol) to hold the two tetrameric units together.
To further examine the LiF-mediated self-assembly of the (m-LiF)
2-B complex, a geometry optimization of the much larger [(m-LiF)
2-B)]
4 complex was carried out with the less computationally demanding B3LYP and M05-2X methods. The optimized geometries are shown in
Figure 6. The energy required to break the self-assembled complex into its four tetrameric units was calculated as 85.59 and 97.51 kcal/mol with the B3LYP and M05-2X methods, respectively. After dividing each of these binding energies by the number of mediating LiF dimers holding the [(m-LiF)
2-B)]
4 complex, the results (28.53 and 32.50 kcal/mol) are essentially the same as those obtained for the [(m-LiF)
2-B)]
2 complex with each of these methods. Moreover, the energy required to break the [(m-LiF)
2-B)]
4 into two complexes of size
n = 3 and
n = 1 is 32.50 kcal/mol, while that to break it into two equivalent complexes of size
n = 2 is 32.65 kcal/mol with the M05-2X method. The corresponding results with the B3LYP method are, respectively, 28.47 and 28.85 kcal/mol. The results are important as they suggest that the binding energy for the LiF-mediated self-assembly in [(m-LiF)
2-B)]
n is independent of its size,
n.
Inspection of
Figure 4 reveals one striking geometrical distinction between the relative orientation of the tetrameric units, upon self-assembly, predicted by the B3LYP and M05-2X methods. The latter method predicts a geometry with the adjacent tetrameric units linked in a staircase manner, while the former method maintains the tetrameric units linked along the same line, one each for the top and the low end of the complex. Relevant geometric data are presented in
Table 11 (distances) and
Table 12 (angles) for both [(m-LiF)
2-B)]
2 and [(m-LiF)
2-B)]
4.
Table 11 lists two different sets of Li-F distances. One set refers to the distance within each of the Li-F monomers already present in the parent (m-LiF)
2-B parent (the outer LiF molecules), and the other set refers to the distance between the adjacent LiF molecules (the inner LiF molecules), one from each (m-LiF)
2-B parent, involved in the LiF-mediated self-assembly. For [(m-LiF)
2-B)]
2, cross-inspection of
Table 9 and
Table 11 shows that all methods predict the Li-F distance for each of the outer LiF monomer to remain essentially unchanged when compared with the value it has in the parent tetrameric unit. In contrast, the Li-F distance for each of the inner LiF monomers is significantly lengthened, with an Li-F distance that is closer to that between the LiF molecules in the mediating LiF dimer. The trends noted for the outer and inner Li-F distances are generally true for the other distances listed in
Table 11, Li⋯O=C, N-H⋯F, and C-H⋯F. One exception is the inner C-H⋯F distance for which the B3LYP method predicts an inner distance that is shorter than the outer one. Lastly, the distance between the centers of the aromatic rings decreases slightly in [(m-LiF)
2-B)]
2 relative to that in (m-LiF)
2-B. With respect to the angles,
Table 12 shows that while the outer Li⋯O=C, N-H⋯F, and C-H⋯F angles remain very close to their original values in the parent tetramer, the inner angles exhibit a small increase. It should be noted that the B3LYP method, in contrast to all other methods, predicts a sizeable increase for the inner C-H⋯F angles. Regarding the larger [(m-LiF)
2-B)]
4 self-assembled complex,
Table 11 shows that the listed outer and inner distances remain close to their values in [(m-LiF)
2-B)]
2. Interestingly, the distance between the centers of the inner aromatic rings still shows a slight decrease in [(m-LiF)
2-B)]
4. The trends in the Li⋯O=C, N-H⋯F, and C-H⋯F angles also mirror those found in [(m-LiF)
2-B)]
2.